Abstract
Background: elective cataract surgery is the most commonly performed surgical procedure in developed countries. However, it is unclear whether cataract surgery on the second eye provides enough incremental benefit to be considered cost-effective. This study conducted a cost-effectiveness analysis of second-eye cataract surgery in the UK.
Design: a cost-effectiveness analysis.
Methods: a decision-analytical model was developed to estimate the cost-effectiveness of second-eye cataract surgery, based on a comprehensive epidemiological and economic review to develop the parameters for the model. The model followed the clinical pathway of cohorts of patients receiving second-eye cataract surgery and included costs and health benefits associated with post-surgical complications.
Results: in the model, second-eye surgery generated 0.68 additional quality-adjusted life years (QALY) with an incremental cost-effectiveness ratio of £1,964 per QALY gained. In sensitivity analyses, model results were most sensitive to changes in the health-related quality of life (HRQoL) gain associated with second-eye surgery, but otherwise robust to changes in parameter values. The probability that second-eye surgery is cost-effective at willingness to pay thresholds of £10,000 and £20,000 was 100%.
Conclusion: second-eye cataract surgery is generally cost-effective based on the best available data and under most assumptions. However, there are only a small number of clinical trials for second-eye cataract surgery, and these have not been conducted in recent years.
Keywords: cataract, cost-effectiveness, second-eye surgery, older people
Introduction
Cataract is a very common eye condition in which clouding of the lens inside the eye can lead to blurred or reduced vision and, if left untreated, can lead to blindness in the affected eye(s). Cataract is responsible for ∼50% of world blindness and affects ∼20 million people [1]. Cataracts occur primarily in older people and are treated with elective cataract removal surgery, which is the most commonly performed surgical procedure in developed countries. Bilateral cataracts occur commonly [2], and patients with bilateral cataract may only have surgery on one eye. Surgery on the second eye may have additional benefits for patients in terms of improving vision and being able to perform everyday activities, for example being able to drive. However, there has been some uncertainty about whether second-eye surgery is financially worthwhile, as typically there is a larger gain in visual acuity in cataract surgery in the first operated eye than for the second.
Previous studies have assessed the cost-effectiveness of second-eye cataract surgery with results varying between the studies. Of the three identified studies, the US study by Busbee et al. [3] reported a cost-effectiveness estimate of US $2,495 per quality-adjusted life year (QALY) gained [3], while the UK study by Sach et al. [4] reported £44,263 per QALY gained over a 1-year time horizon. However, in the latter study, the estimate reduced to £17,299 per QALY when a lifetime horizon was used. A Finnish study by Räsänen et al. [5] found that second-eye cataract surgery was not associated with an improvement in HRQoL, reported at 6 months after second-eye surgery. To assess the most robust estimate of cost-effectiveness, we conducted a comprehensive review of the evidence. In this article, we describe a cost-effectiveness model developed for the UK Health Technology Assessment Programme to estimate the health and cost consequences of second-eye cataract surgery.
Methods
We developed a decision-analytical model, constructed in Microsoft Excel, to estimate the cost-effectiveness of second-eye cataract surgery in patients with bilateral cataract, compared with patients with bilateral cataract who receive only first-eye cataract surgery. The modelling was conducted following accepted standards for economic evaluation [6–8], and systematic searches were conducted to identify the data inputs for the model. The model evaluates costs (in UK pounds using a 2012 price base) from the perspective of the NHS and Personal Social Services. Outcomes in the model are expressed as QALYs by incorporating quality-of-life estimates using patient health state utility values. Cost-effectiveness is expressed in terms of incremental cost-effectiveness ratios (ICERs). Both costs and outcomes were discounted to give a time preference to costs and health outcomes that happen in the near rather than distant future, using a 3.5% annual discount rate in line with current guidance in the UK [6, 9].
Uncertainty with regard to model input parameters was investigated through deterministic and probabilistic sensitivity analyses (PSAs) and scenario analyses. One-way sensitivity analyses were performed by varying each parameter between its higher and lower estimates shown in Table 1. The estimates used for the sensitivity analysis were based upon the 95% confidence interval ranges for these parameters. Multi-parameter uncertainty was addressed using a PSA [10], in which probability distributions were assigned to all parameters used in the base case analysis. The model was run for 1,000 iterations, with a different set of parameter values for each iteration, by sampling parameter values at random from their probability distributions. The distributions used for sampling each parameter are reported elsewhere [11].
Table 1.
Input parameters used in the economic model
| Parameter | Base case | Upper estimate | Lower estimate | Source |
|---|---|---|---|---|
| Costs | ||||
| Cataract surgery (weighted average day-case and in-patient) | £862.66 | £1121.46 | £603.86 | UK NHS reference costs 2011–12 (HRG code BZ02Z) [12] |
| Ophthalmology out-patient visit | £85.12 | £110.66 | £59.58 | UK NHS reference costs 2011–12 (service code 130) [12] |
| GP visits | £43.00 | £55.90 | £30.10 | PSSRU 2012 [13] |
| PCO (YAG laser posterior capsulotomy) | £506.42 | £658.35 | £354.49 | UK NHS reference costs 2011–12 (HRG code BZ04Z lens capsulotomy) [12] |
| Retinal detachment (vitrectomy) | £1615.65 | £2100.35 | £1130.96 | UK NHS reference costs 2011–12 (HRG code BZ21Z major vitreous retinal procedures) [12] |
| Endophthalmitis (vitreous tap; vitrectomy) | £760.11 | £988.14 | £532.08 | UK NHS reference costs 2011–12 (HRG codes BZ21Z and BZ23Z) [12] |
| CMO (fluorescein angiogram and OCT)a | £313.30 | £407.29 | £219.31 | Colquitt et al. [14] |
| Lost lens fragments (vitrectomy) | £451.69 | £587.20 | £316.18 | UK NHS reference costs 2011–12 (HRG code BZ23Z minor vitreous retinal procedures) [12] |
| Resources | ||||
| Out-patient visits surgery | 6.94 | 7.98 | 5.90 | Sach et al. [4] |
| Out-patient visits no surgery | 2.81 | Sach et al. [4] | ||
| GP visits surgery | 4.40 | 5.21 | 3.59 | Sach et al. [4] |
| GP visits no surgery | 4.00 | Sach et al. [4] | ||
| Incidence of complications | ||||
| PCO Year 1 | 3.49% | 5.24% | 1.75% | ECCERT [15] |
| PCO Year 2 | 9.49% | 14.24% | 4.75% | ECCERT [15] |
| PCO Year 3 | 5.06% | 7.59% | 2.53% | ECCERT [15] |
| Retinal detachment Year 1 | 0.26% | 0.39% | 0.13% | Erie et al. [16] |
| Retinal detachment year 2+ | 0.14% | 0.21% | 0.07% | Erie et al. [16] |
| Endophthalmitis | 0.10% | 0.15% | 0.05% | UK National Cataract Survey [17] |
| CMO | 1.62% | 2.43% | 0.81% | UK Cataract National Dataset [18] |
| Lost lens fragments | 0.45% | 0.68% | 0.23% | UK Cataract National Dataset [18] |
| HRQoL utilities | ||||
| HRQoL no surgery | 0.70 | Hiratsuka et al. [19] | ||
| HRQoL gain for surgical group | 0.08 | 0.14 | 0.017 | Hiratsuka et al. [19] |
| Reduction in utility for non-second-eye surgery group, per year | 0.002 | 0.004 | 0.0001 | ECCERT [15] |
| Other parameters | ||||
| Discount rate, benefits/costs | 3.50% | 6.00% | 1.5% | UK NICE reference case [6] |
CMO, cystoid macular oedema; PCO, posterior capsule opacification; VA, visual acuity; OCT, optical coherence tomography; HRQoL, health-related quality of life; GP, general practitioner; NICE, National Institute of Health and Care Excellence.
aCosts for fluorescein angiogram and OCT inflated to current prices.
Model structure
The model structure is shown in Figure 1 and is similar to that developed by Busbee et al. [3]. A hypothetical cohort of patients receives second-eye cataract surgery compared with a cohort who receives no second-eye surgery (not shown). The patient cohort starting age is 75 years.
Figure 1.
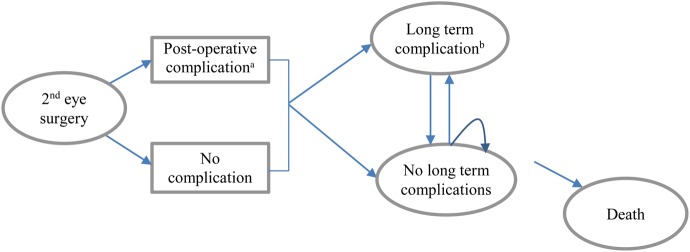
Schema of cataract model. aPostoperative complication: endophthalmitis, cystoid macular oedema, retained lens fragments. bLong-term complications: PCO, retinal detachment.
In the model, patients receiving surgery may experience short-term postoperative complications (endophthalmitis, cystoid macular oedema (CMO) and retained lens fragments). They may also experience longer term post-surgical complications and consequences (posterior capsule opacification (PCO) or retinal detachment). These post-surgical complications and consequences are associated with additional out-patient visits and additional remedial procedures. Patients remain in the short- or long-term complications health states (including PCO or retinal detachment) for one cycle and then are assumed to be successfully treated. Patients may die in any model cycle (based on general population mortality rates). The model has a lifetime (25 years) horizon, with a cycle length of 1 year.
HRQoL was included within the model by assigning a health state utility value, ranging from 0 (death) to 1 (perfect health) for patients in each cycle of the model, using values found in our systematic review. HRQoL provides a quantitative measure that allows comparison of health across different diseases [7]. HRQoL values for the second-eye cataract surgery are assumed to remain constant over patient lifetimes, unless patients have complications from the surgery. HRQoL values for the no second-eye surgery group are assumed to decline over time due to un-operated cataract progression and age-related visual acuity decline. Post-surgical complications are assumed to incur a disutility for 1 year. Mean life expectancy for all patients in the model was calculated to be 9.7 years, based on UK mortality tables [20].
With the exception of costs for long-term post-surgical complications, the costs for out-patient visits and further procedures were assumed to be the same after the first year for the second-eye cataract surgery and no-surgery groups. A summary of all the parameter values included in the economic model is given in Table 1.
Cataract complications
We based our estimates for cataract complications upon observational studies in the medical literature. We assumed that the incidence of post-surgical complications would not differ between first- or second-eye cataract surgery, and hence, the rates used were not specific to studies of second-eye surgical patients. It was assumed that short-term post-surgical complications occurred within the first year of surgery. Endophthalmitis was assumed to occur at a rate of 0.1% [17], CMO at a rate of 1.62% [18] and lost lens fragments at a rate of 0.45% [18]. Longer term post-surgical complications and consequences were assumed to occur in only the first three successive model cycles for PCO and in any model cycle for retinal detachment. Incidence of PCO was based on estimates from a meta-analysis, as reported in an economic evaluation by The Eye Care Comparative Effectiveness Research Team (ECCERT) [15]. Probabilities of PCO in surgical patients were 3.49, 9.49 and 5.06% for years 1, 2 and 3, respectively. The probability of retinal detachment was 0.26% in Year 1, 0.14% in Year 2 and all following years (Table 1) [16].
Utility value data
HRQoL utility estimates were taken from a Japanese economic evaluation by Hiratsuka et al. [19]. This study was chosen from a systematic review of HRQoL studies [11], because it estimated health utility using a generic preference-based HRQoL instrument (HUI-3) that included a visual utility sub-scale. The gain in utility was assumed to last the patient's lifetime, similarly to other economic evaluations [3], and is in accord with clinical consensus of a permanent improvement in clinical vision from cataract surgery.
In the base case, we have assumed that visual acuity declines with un-operated cataract progression and age for the no-second-eye surgery group. The decline was estimated using data from the Japanese ECCERT study [15], to be a mean annual utility decline of 0.002.
Medical costs
The healthcare costs associated with the cataract surgery and the cataract complications were derived from multiple sources (Table 1). Costs for cataract surgery (phacoemulsification) were taken from 2011–12 UK NHS reference costs [12]. Resources associated with cataract surgery for out-patient visits and general practitioner visits were based upon a UK economic evaluation of second-eye cataract surgery by Sach et al. [4]. In the base case, we have assumed that there is no difference in social care costs for patients who have second-eye surgery and those who do not.
The costs of procedures for treating post-surgical complications and consequences were estimated using 2011–12 UK NHS reference costs [12]. For all the treatment of post-surgical complications, we assumed an additional two ophthalmic out-patient appointments would be required. Longer term complication costs for PCO are incurred within the first 3 years, and retinal detachment may occur in any year.
We assumed that 80% of patients with endophthalmitis would receive a vitreous tap (biopsy) [12] and a further 18% of patients with severe cases would require vitrectomy [21]. Lost lens fragments in the postoperative period may require a day-case vitrectomy to remove the fragments, and we assumed this in 70% of cases. Where a small number of fragments are retained, these may dissolve spontaneously. Patients may receive topical anti-inflammatory drops and be monitored for intraocular pressure, CMO and retinal detachment. No surgical treatment was assumed to be necessary for CMO; however, patients would receive a fluorescein angiogram and optical coherence tomography (OCT). In addition, some cases may be treated with intra-vitreal injections of steroid, but this cost was assumed to be included within the non-surgical procedure costs, as the number of cases treated this way is small. Costs were taken from a previous Health Technology Assessment of treatment for macular degeneration [14], inflated to current prices using inflation indices [13]. PCO is most commonly treated using Nd:YAG laser capsulotomy [22]. Retinal detachment was assumed to be treated via day-case vitrectomy (Table 1).
Results
The cost-effectiveness results for second-eye cataract surgery compared with no second-eye cataract surgery are shown in Table 2 for a hypothetical individual of age 75 years and preoperative visual acuity in the surgical (second) eye of 6/12. In the base case analysis, patients receiving second-eye cataract surgery would have an additional cost of £1,341, an additional 0.68 QALY and an ICER of £1,964 per QALY gained compared with no second-eye cataract surgery. The results indicate that second-eye cataract surgery is likely to be cost-effective at conventional willingness-to-pay thresholds [6].
Table 2.
Summary of the discounted cost-effectiveness results
| QALYs | Costs | ICER (cost/QALY) | |
|---|---|---|---|
| No second-eye cataract surgery | 5.29 | £411 | |
| Second-eye cataract surgery | 5.97 | £1,752 | |
| Incremental | 0.68 | £1,341 | £1,964 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
One-way sensitivity analyses were performed for all model parameters. The model results were most sensitive to the utility gain, where the ICER varies between £1,185 and £6,342 per QALY gained. This reflects the uncertainty around the utility gain estimate from the Hiratsuka et al. study [19], which had a 95% confidence interval between 0.017 and 0.14. The model results were also sensitive to the cost of the cataract operation, where the ICER varies between £1,585 and £2,343. Other parameters had only a small effect on the model results, and the results for these are reported elsewhere [11]. For the PSA, the scatterplot of the results for the 1,000 iterations is shown in the Supplementary data, Appendix, available in Age and Ageing online. The PSA results indicate that for all analyses, second-eye surgery has a cost-effectiveness estimate <£20,000 per QALY.
Discussion
Based on economic modelling using the best available evidence, second-eye surgery would be considered generally cost-effective under conventional willingness-to-pay thresholds of £20,000–£30,000 per QALY gained used in the UK NHS [6]. The economic model was informed by previously published models, and their limitations were taken into account where possible. Our results are comparable with those from the Busbee et al. [3] study which reported an ICER of US $2,495 per QALY, but differ significantly from the other two cost-effectiveness studies [4, 5]. The reason for these differences is largely driven by the utility gain for second-eye surgery assumed in the studies, and the assumptions used for long-term utility for no-second-eye cataract surgery patients.
The results in our study are sensitive to the HRQoL gain from second-eye surgery which varies widely between and even within the source studies. There are several generic preference-based HRQoL measures to value health utility, including time-trade off, EQ-5D, SF-6D, HUI3 and 15D, and different measures can generate different values for a given disease or condition [7]. The study providing the utility data used in our model [19] had a HRQoL gain of 0.08 associated with second-eye cataract surgery using the HUI3 HRQoL measure. Other studies, such as those by Dolders et al. [23] and Räsänen et al. [5], showed a reduction in HRQoL associated with second-eye surgery, while Sach et al. showed a lower HRQoL gain of 0.02. In the base case, we considered that the study by Hiratsuka et al. [19] was the most appropriate estimate, as we considered the HUI3 provided the best estimate of HRQoL rather than EQ-5D as used by Sach et al. [4], 15D used by Räsänen et al. [5], or time-trade off, and standard gamble methods used by Dolders et al. [23] The EQ-5D does not include any sensory-related dimensions and may not be sensitive to improvements in vision following cataract surgery [4, 5, 24]. There was some uncertainty around the generalisability of the study by Hiratsuka et al. [19], which did not report the starting visual acuity or the visual acuity gained by second-eye cataract patients. It was unclear whether patients treated for second-eye surgery differed from UK patients who would have a visual acuity threshold for surgery of 6/24. However, in sensitivity analyses, second-eye cataract surgery remained cost-effective even with lower utility gains. Indeed, if we were to disregard the study by Hiratsuka et al. [19], and instead use utility values from the UK population by Sach et al. [4], second-eye cataract surgery remained cost-effective with an ICER of £5,734 per QALY.
Despite the strengths of our analysis, the economic evaluation has some limitations. It was necessary to make some simplified assumptions regarding resources, costs, surgical complications, patient characteristics and outcomes. However, these assumptions were tested extensively through scenario analysis and sensitivity analysis. We would have liked to stratify our base case analyses by age or baseline visual acuity, as these factors may predict outcome of surgery. However, this was not possible due to limited availability of the data that would have been required for all of the model parameters. The evidence base is limited to a small number of older clinical trials, based on patients with relatively good baseline clinical measures.
Although the mean visual acuity gain would be higher after first-eye cataract surgery [25], second-eye surgery appears to be cost-effective even in those with a relatively small deficiency of preoperative binocular visual acuity. Measuring visual acuity alone does not fully reflect patients' functional disability resulting from a cataract, as it refers to clearness of vision (e.g. the ability to read ordinary newsprint and recognise a friend on the other side of the street) rather than depth perception. After first-eye cataract surgery, patients may experience problems due to the different refractive powers between the eyes. A study by Mueleners et al. [26] of 28,396 individuals who underwent bilateral cataract surgery in Western Australia showed an increased risk of falls after first-eye cataract surgery, which was substantially reduced after second-eye cataract surgery. Commenting on that study, Harwood and Foss [27] suggested that the interval between first- and second-eye surgery should be reduced as much as possible. Our cost-effectiveness analysis focuses on the costs and benefits for patients attending hospital ophthalmology departments and does not include the wider benefits to the NHS and to society that would be made by the prevention of accidents and their sequelae that result from poor vision related to cataracts. In addition, as in the UK, social care and health budgets may be treated separately so that economic savings accrued from preventing accidents, and their sequelae are not fed back into the NHS healthcare budget. If this were the case, it would encourage health improving technologies such as second-eye cataract surgery.
In conclusion, our economic model shows that second-eye surgery would be considered cost-effective under conventional willingness-to-pay thresholds used in the NHS, tested under a range of scenarios and assumptions, using the best available evidence. The results specifically address clinical practice in England and Wales, but the results are likely to be generalisable within the UK and probably beyond. More detail studies that link combined changes in visual acuity and stereopsis to changes in utility values are needed to reduce the uncertainty of economic modelling.
Key points.
Elective cataract surgery is the most commonly performed surgical procedure in developed countries.
It is unclear whether cataract surgery on the second eye provides enough incremental benefit to be considered cost-effective.
This study found that second eye is generally cost-effective, although there are only a small number of trials that report second-eye cataract surgery.
Conflicts of interest
None declared.
Funding
This work was commissioned by the UK National Institute of Health Research Health Technology Assessment programme as project number 12/72/01. The funders played no role in the design, analysis and interpretation of data, or writing of the study.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Acknowledgements
We are grateful to Karen Welch, Prof Andrew Clegg and Jeremy Jones for their contribution to this research. The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health.
References
- 1.WHO. Global Data on Visual Impairments 2010. http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf (31 July 2013, date last accessed).
- 2.Acosta R, Hoffmeister L, Roman R, Comas M, Castilla M, Castells X. Systematic review of population-based studies of the prevalence of cataracts. Arch Soc Esp Oftalmol 2006; 81: 509–16. [DOI] [PubMed] [Google Scholar]
- 3.Busbee BG, Brown MM, Brown GC, Sharma S. Cost-utility analysis of cataract surgery in the second eye. Ophthalmology 2003; 110: 2310–7. [DOI] [PubMed] [Google Scholar]
- 4.Sach TH, Foss AJ, Gregson RM et al. Second-eye cataract surgery in elderly women: a cost-utility analysis conducted alongside a randomized controlled trial. Eye 2010; 24: 276–83. [DOI] [PubMed] [Google Scholar]
- 5.Rasanen P, Krootila K, Sintonen H et al. Cost-utility of routine cataract surgery. Health Qual Life Outcomes 2006; 4: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal. London: NICE, 2013. [PubMed] [Google Scholar]
- 7.Philips Z, Ginnelly L, Sculpher M et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004; 8: iii–xi, 1. [DOI] [PubMed] [Google Scholar]
- 8.Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. 3rd edition Oxford, UK: Oxford University Press, 2005. [Google Scholar]
- 9.Her Majesty's Treasury. The Green Book. Appraisal and Evaluation in Central Government. 2010. http://www.hm-treasury.gov.uk/data_greenbook_index.htm.
- 10.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford, UK: Oxford University Press, 2006. [Google Scholar]
- 11.Frampton G, Harris P, Cooper K, Lotery A, Shepherd J. The clinical and cost effectiveness of second-eye cataract surgery: a systematic review and economic evaluation. Health Technol Assess 2014; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health. NHS Reference Costs 2011/2012. 2012. https://www.gov.uk/government/publications/reference-costs-guidance-for-2011-12.
- 13.Curtis L. Unit Costs of Health and Social Care 2012. http://www.pssru.ac.uk/project-pages/unit-costs/2012/ (31 July 2013, date last accessed).
- 14.Colquitt JL, Jones J, Tan SC, Takeda AL, Clegg AJ, Price A. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2008; 12. [DOI] [PubMed] [Google Scholar]
- 15.Eye Care Comparative Effectiveness Research Team (ECCERT). Cost-utility analysis of cataract surgery in Japan: a probabilistic Markov modeling study. Jpn J Ophthalmol 2013; 57: 391–401. [DOI] [PubMed] [Google Scholar]
- 16.Erie JC, Raecker ME, Baratz KH, Schleck CD, Robertson DM. Risk of retinal detachment after cataract extraction, 1980–2004: a population-based study. Trans Am Ophthalmol Soc 2006; 104: 167–75. [PMC free article] [PubMed] [Google Scholar]
- 17.Desai P, Minassian DC, Reidy A. National cataract surgery survey 1997–8: a report of the results of the clinical outcomes. Br J Ophthalmol 1999; 83: 1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaycock P, Johnston RL, Taylor H et al. The Cataract National Dataset electronic multi-centre audit of 55,567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye (Lond) 2009; 23: 38–49. [DOI] [PubMed] [Google Scholar]
- 19.Hiratsuka Y, Yamada M, Murakami A et al. Cost-effectiveness of cataract surgery in Japan. Jpn J Ophthalmol 2011; 55: 333–42. [DOI] [PubMed] [Google Scholar]
- 20.Office of National Statistics. Death Registrations by Single Year of Age, United Kingdom 2010. http://www.ons.gov.uk (31 July 2013, date last accessed).
- 21.Kamalarajah S, Silvestri G, Sharma N et al. Surveillance of endophthalmitis following cataract surgery in the UK. Eye (Lond) 2004; 18: 580–7. [DOI] [PubMed] [Google Scholar]
- 22.The Royal College of Ophthalmologists. Cataract Surgery Guidelines. London: Scientific Department, 2010. [Google Scholar]
- 23.Dolders MG, Nijkamp MD, Nuijts RM et al. Cost effectiveness of foldable multifocal intraocular lenses compared to foldable monofocal intraocular lenses for cataract surgery. Br J Ophthalmol 2004; 88: 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosh J, Brazier J, Evans P, Longworth L. A review of generic preference-based measures of health-related quality of life in visual disorders. Value Health 2012; 15: 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott DB, Patla A, Bullimore MA. Improvements in clinical and functional vision and perceived visual disability after first and second eye cataract surgery. Br J Ophthalmol 1997; 81: 889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meuleners LB, Fraser ML, Ng J, Morlet N. The impact of first- and second-eye cataract surgery on injurious falls that require hospitalisation: a whole-population study. Age Ageing 2014; 43: 341–6. [DOI] [PubMed] [Google Scholar]
- 27.Harwood RH, Foss AJ. Second-eye cataract surgery: valuable investment or unaffordable luxury? Age Ageing 2014; 43: 310–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


