Abstract
We are developing a live-attenuated tetravalent dengue vaccine (TDV) candidate based on an attenuated dengue 2 virus (TDV-2) and 3 chimeric viruses containing the premembrane and envelope genes of dengue viruses (DENVs) -1, -3, and -4 expressed in the context of the attenuated TDV-2 genome (TDV-1, TDV-3, and TDV-4, respectively). In this study, we analyzed and characterized the CD8+ T-cell response in flavivirus-naive human volunteers vaccinated with 2 doses of TDV 90 days apart via the subcutaneous or intradermal routes. Using peptide arrays and intracellular cytokine staining, we demonstrated that TDV elicits CD8+ T cells targeting the nonstructural NS1, NS3, and NS5 proteins of TDV-2. The cells were characterized by the production of interferon-γ, tumor necrosis factor–α, and to a lesser extent interleukin-2. Responses were highest on day 90 after the first dose and were still detectable on 180 days after the second dose. In addition, CD8+ T cells were multifunctional, producing ≥2 cytokines simultaneously, and cross-reactive to NS proteins of the other 3 DENV serotypes. Overall, these findings describe the capacity of our candidate dengue vaccine to elicit cellular immune responses and support the further evaluation of T-cell responses in samples from future TDV clinical trials.
Keywords: CD8+ T cells, cytokines, dengue virus, multifunctional T cells, vaccine
Dengue viruses (DENVs) transmitted primarily by the Aedes aegypti mosquito cause infections that impact public health mainly in tropical and subtropical regions of the world [1, 2]. Recently, it was estimated that these viruses cause approximately 390 million DENV infections annually [3]. DENVs circulate in nature as 4 distinct serotypes (DENV-1 to DENV-4) that share a high degree of homology with each other [1]. Each serotype causes a spectrum of diseases, including subclinical infection, dengue fever (DF), and life-threatening dengue hemorrhagic fever or dengue shock syndrome [4]. Moreover, there appears to be a distinct clinical and epidemiological pattern for each serotype, suggesting that they exhibit variability in virulence and pathogenesis [5].
Currently, there is no dengue vaccine or antiviral therapy for DENV. Generally, infection with 1 dengue serotype will confer homologous, long-term protection [6]. However, subsequent reinfection with a heterologous serotype has the potential to cause severe disease, which could be mediated by antibodies (antibody-dependent enhancement) and/or T cells [7–9]. Therefore, vaccine development against DENV has focused on tetravalent formulations that simultaneously provide immunity to all 4 serotypes [10]. We have developed a live-attenuated tetravalent dengue vaccine (TDV) candidate that consists of an attenuated DENV-2 strain (TDV-2), and 3 chimeric viruses containing the premembrane (prM) and envelope (E) genes of DENV-1, -3, and -4 expressed in the context of the TDV-2 genome (TDV-1, TDV-3, TDV-4, respectively) [11–15]. TDV (under the previous name DENVax) has been extensively tested in preclinical studies [16–18], 2 completed Phase I clinical trials [19, 20], and is currently being tested in Phase II clinical trials. In the Phase I studies with healthy adult volunteers, the candidate vaccine was shown to be generally well tolerated, and induced neutralizing antibody responses to all 4 dengue serotypes [19, 20].
The humoral immune response to DENV primarily targets the prM and E structural proteins and is predominately composed of serotype-cross-reactive antibodies [21–23]. In contrast, the cellular immune response to DENV mainly targets the nonstructural (NS) proteins [24]. DENV vaccine candidates have been shown to elicit T-cell responses [17, 18, 25, 26], and in a mouse model the protective role of CD8+ T cells is well established [27]. More recently, a comprehensive analysis of DENV-specific T-cell responses provided evidence suggesting that a vigorous multifunctional CD8+ T-cell response is associated with protection from DENV disease [28].
In this study, we performed an analysis of the kinetics of CD8+ T-cell responses to the backbone of the TDV candidate vaccine in flavivirus-naive individuals that received 2 doses of the vaccine by intradermal (ID) or subcutaneous (SC) administration. Moreover, these responses were characterized in terms of cytokine profile produced, their multifunctional nature, and the targeted NS proteins they recognized.
MATERIALS AND METHODS
Vaccine
The construction and characterization of each TDV vaccine strain has been previously reported [15]. The clinical material used for vaccination in this Phase I trial consisted of 2 × 104 plaque-forming units (pfu) of TDV-1, 5 × 104 pfu of TDV-2, 1 × 105 pfu of TDV-3, and 3 × 105 pfu of TDV-4.
Ethics Statement
Ethical approval of the study protocol was granted by the Saint Louis University Institutional Review Board prior to initiation of a National Institutes of Health (NIH)–sponsored Phase I clinical trial at the university. Informed written consent was obtained from all study participants, and the study was registered with clinicaltrials.gov, identifier: NCT01110551.
Subjects
The development of DENV-specific CD8+ T-cell responses was measured in peripheral blood mononuclear cells (PBMCs) of 6 individuals randomly selected from 2 cohorts of flavivirus-naive, healthy adults. Five individuals per cohort were vaccinated with TDV via either the SC or ID route; 1 individual from each cohort received placebo and was included in this study as a control. In both cohorts, TDV or placebo was administered on days 0 and 90. The kinetics of T-cell responses were measured in PBMCs collected on days 0, 14, 90, 104, and 270 postprimary vaccination via the SC or ID routes. Standardized assays to measure vaccine virus RNA in serum samples postvaccination were performed as described by Osorio et al [19], and data from individuals who participated in this study as reported [20] are presented in Supplementary Table 1.
PBMCs
Blood from each subject was collected in sodium heparin cell preparation tubes at various time points pre- and postvaccination (see above), and PBMCs were separated by centrifugation at room temperature (18°C–25°C) in a horizontal rotor at 1800g (relative centrifugal force) for 25 minutes. Cells were washed twice with phosphate buffer saline (PBS), and cell viability was monitored by trypan blue exclusion test. The cell pellet was resuspended in fetal bovine serum (FBS) at a concentration of 1 × 107 cells/mL and then was mixed with an equal volume of freezing media (20% dimethyl sulfoxide [DMSO] (volume/volume) in heat-inactivated FBS) to yield a final concentration of 5 × 106 cells/mL. After gently mixing, 1 mL aliquots of cell suspension in prelabeled Nunc Cryo Tubes were placed into a room temperature isopropanol-filled cell freezer. The apparatus was stored at −70°C overnight, and the next day vials were transferred into liquid nitrogen for long-term storage.
Peptide Arrays
Peptide arrays representing the entire sequence of the NS1 (aa 1–352; 47 peptides), the NS3 (aa 1–618; 83 peptides), and the NS5 (pool 1; 80 peptides spanning residues 1–469, and pool 2; 76 peptides spanning residues 458–900) proteins from the DENV-2 New Guinea C (NGC) strain were used to measure T-cell responses to the DENV-2 backbone of TDV. Cross-reactive T-cell responses were measured using peptide arrays representing the entire sequence of NS1, NS3, and NS5 proteins from DENV-1 (strain; Singapore/S275/1990), DENV-3 (strain; Philippines/H87/1956), and DENV-4 (strain; Singapore/8976/1995). All peptide arrays used in this study were obtained from the National Institute of Allergy and Infectious Diseases Biodefense and Emerging Infections Research Resources Repository (BEI Resources). The peptide length ranged from 12–20 mers, and the amino acid overlap ranged from 10–13 amino acids and varied between peptide arrays. Detailed information about each array is available at http://www.beiresources.org/.
Intracellular Cytokine Staining Assay
Frozen PBMCs were thawed and washed twice with warm complete Roswell Park Memorial Institute (cRPMI)–10 (RPMI 1640, 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin, 100 µg/mL streptomycin). Cells were resuspended at 1–4 × 106/mL in cRPMI-10 and rested for 6–7 hours. Cells were then washed once and aliquoted for stimulation with peptide pools at 10 µM of final total peptide concentration. Staphylococcal enterotoxin B (SEB) (3 µg/mL) was used as positive control. Anti-CD28 (L293)/CD49d (L25) (1 µg/mL each) and protein transport inhibitors GolgiPlug (1 µg/mL) and GolgiStop (2 µM) were added at the beginning of peptide stimulation. After 16 hours’ stimulation, cells were surface-stained with Live/dead Fixable Blue dye (Life Technologies), followed with antihuman antibodies CD3 PerCP (SK7), CD4 PE-CF-594 (RPA-T4), and CD8 V500 (RPA-T8). Cells were then fixed and permeabilized before staining for intracellular cytokines interferon (IFN)–γ Alexa Fluor 700 (B27), interleukin (IL)–2 allophycocyanin (5344.111), and tumor necrosis factor (TNF)–α fluorescein isothiocyanate (6401.111). Stained cells were resuspended in 1% paraformaldehyde/PBS and acquired on a 5-laser BD LSRII flow cytometer within 24 hours. Compensation was performed using antimouse IgG BD CompBeads according to the manufacturer's instruction. For the majority of tested samples, at least 400 000 total events were collected and data were analyzed mainly with FlowJo V9.7.2. The viability of lymphocytes was >85% for all samples. Boolean gating was used to determine simultaneous cytokine production in cells. SEB-stimulated samples produced all 3 cytokines. Data are presented as the percentage of cytokine-producing CD8+ T cells after subtracting the background cytokine level in DMSO/medium control cultures from stimulated cells at all indicated time points. Multifunctional T-cell data were presented using SPICE v5.3033 (National Institute of Allergy and Infectious Diseases, NIH).
RESULTS
Kinetics and Characterization of Memory CD8+ T-cell Responses to the TDV Backbone
Because the backbone of our vaccine candidate is based on an attenuated DENV-2 strain, we predominantly focused our analyses on the NS proteins that constitute the main target of the CD8+ T-cell response [28]. To measure the magnitude of CD8+ T-cell responses over time, we employed peptide arrays spanning the NS1, NS3, and NS5 proteins of DENV-2, and an intracellular cytokine staining (ICS) assay to analyze PBMCs collected from flavivirus-naive individuals immunized with TDV. A representative gating for CD8+ T cells from NS3 peptide array or control DMSO-stimulated cells (subject 5, d270) is shown in Figure 1. Following vaccination via the SC or ID routes, an increased number of CD8+ T cells relative to day 0 were detected on days 90, 104, and 270 postprimary immunization targeting the NS1, NS3, and NS5 proteins of DENV-2 (Figures 2 and 3). In the majority of individuals tested, the NS3 protein was the main target of the CD8+ T-cell response followed by the NS5 protein represented by pool 1. CD8+ T cells mainly produced IFN-γ (Figure 2), and to a lesser extent TNF-α (Figure 3). Overall, percentages of cells producing IL-2 were low (Figure 4). CD8+ T-cell responses peaked on day 90 but were still detectable on day 270. A booster injection on day 90 had no detectable impact on the cellular responses measured on day 104, and none of the vaccinated individuals had detectable viremia after boosting (data not shown). The overall CD8+ T-cell response of subjects from both SC and ID cohorts is summarized in Table 1. Using the same peptide arrays, CD4+ T-cell responses to TDV backbone were inconsistent with very low magnitude of response (ranging from 0.051%–0.14%) for some individuals (data not shown). Just as all vaccinated individuals showed a positive response to 1 or more peptides, all individuals vaccinated with TDV had detectable neutralizing antibody response on day 90 (20; Supplementary Table 2). Seven of 10 subjects had detectable viral RNA from TDV-2 (days 7–14). Among these 7 individuals, 2 also had detectable viral RNA from TDV-3 on day 9 (Supplementary Table 1). In general, there were no strict associations between viral RNA, the magnitude of the antibody response, and the CD8+ T-cell response.
Figure 1.
Overview of the gating strategy that was used to identify the cytokine-producing CD8+ T cells upon restimulation with DENV peptide pools. Representative gating for CD8+ T cells from subject 5 collected on day 270 stimulated with DMSO (A) or DENV-2 NS3 peptide array (B). Abbreviations: DENV, dengue virus; DMSO, dimethyl sulfoxide; IFNg, interferon-γ; IL2, interleukin 2; NS3, nonstructural protein 3; TNF-a, tumor necrosis factor–α.
Figure 2.
Percentages of IFN-γ-producing CD8+ T cells at different time points postvaccination. SC cohort: subjects 1–5 and placebo 1 (P1); ID cohort: subjects 6–10 and placebo 2 (P2). Responses that are above the top level of the scale are displayed as numbers above their respective columns. Abbreviations: ID, intradermal; IFN-γ, interferon-γ; NS, nonstructural; SC, subcutaneous.
Figure 3.
Percentages of TNF-α-producing CD8+ T cells at different time points postvaccination. SC cohort: subjects 1–5 and placebo 1 (P1); ID cohort: subjects 6–10 and placebo 2 (P2). Abbreviations: ID, intradermal; NS, nonstructural; SC, subcutaneous; TNF-α, tumor necrosis factor–α.
Figure 4.
Percentages of IL-2-producing CD8+ T cells at different time points post vaccination. SC cohort: subjects 1–5 and placebo 1 (P1); ID cohort: subjects 6–10 and placebo 2 (P2). Abbreviations: ID, intradermal; IL-2, interleukin 2; NS, nonstructural; SC, subcutaneous.
Table 1.
Total CD8+ T-cell Responses From Subjects Vaccinated With TDV on Day 90
| SC |
ID |
Total Response | |||||
|---|---|---|---|---|---|---|---|
| Responsea | IFN-γ (%) | TNF-α (%) | Response | IFN-γ (%) | TNF-α (%) | ||
| NS1 | 3/5 | 0.43 | 0.21 | 2/5 | 0.71 | 0.47 | 5/10 |
| NS3 | 5/5 | 3.18 | 1.82 | 2/5 | 1.17 | 0.82 | 7/10 |
| NS5-1 | 2/5 | 1.66 | 0.93 | 3/5 | 0.86 | 0.62 | 5/10 |
| NS5-2 | 2/5 | 0.36 | 0.17 | 3/5 | 0.30 | 0.25 | 5/10 |
| Sum | 5/5 | 5.56 | 3.13 | 5/5 | 3.10 | 2.16 | 10/10 |
Abbreviations: DMSO, dimethyl sulfoxide; ID, intradermal; IFN-γ, interferon-γ; SC, subcutaneous; TDV, tetravalent dengue vaccine; TNF-α, tumor necrosis factor–α.
a A cytokine-positive response was defined by (1) ≥0.05% of cytokine-positive CD8+ T cells after medium/DMSO background subtraction; (2) a peptide-stimulated response to be at least 3-fold above that of d0 control.
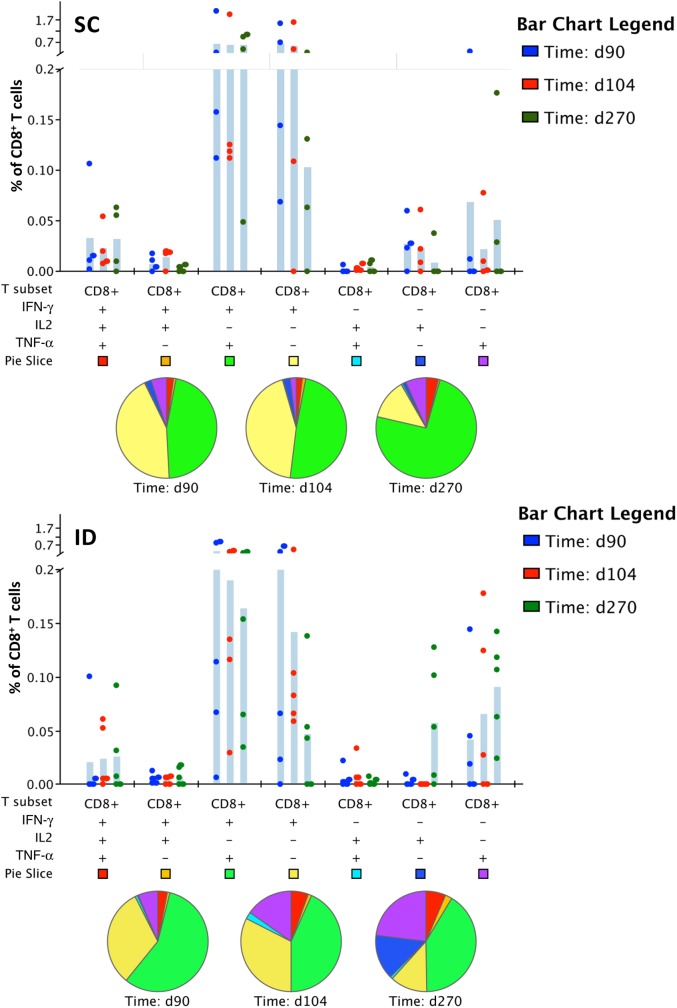
TDV Elicits Multifunctional CD8+ T Cells
To determine the breadth of CD8+ T-cell responses, we looked for production of specific combinations of cytokines 3 months postprimary immunization or 14 and 180 days postboost (days 104 and 270, respectively). Figures 5A and 5B show percentages of CD8+ T cells producing a particular combination of cytokines for the indicated test days by both routes of immunization. The most common CD8+ T-cell phenotypes were IFN-γ+ or IFN-γ+/TNF-α+ at all time points postvaccination. Pie charts illustrate the distribution of T-cell populations that produce any given combination of cytokines at on days 90, 104, and 270 postprimary immunization.
Figure 5.
Multifunctional CD8+ T-cell responses. The accumulated percentages of CD8+ T cells that produced IFN-γ, TNF-α, and IL-2 upon stimulation with DENV-2 NS1, NS3, NS5-1, or NS5–2 peptide pools. The shaded bars stand for the mean. Each dot represents an individual subject. Pie charts show the distribution of different CD8+ T-cell populations that produce 1 or more cytokines. Upper panel: SC cohort, subjects 2–5; Lower panel: ID cohort, subjects 6–10. Abbreviations: DENV, dengue virus; ID, intradermal; IFN-γ, interferon-γ; IL-2, interleukin 2; NS, nonstructural; SC, subcutaneous; TNF-α, tumor necrosis factor–α.
TDV Elicits Cross-reactive CD8+ T Cells Targeting the NS3 Protein of Heterologous DENVs
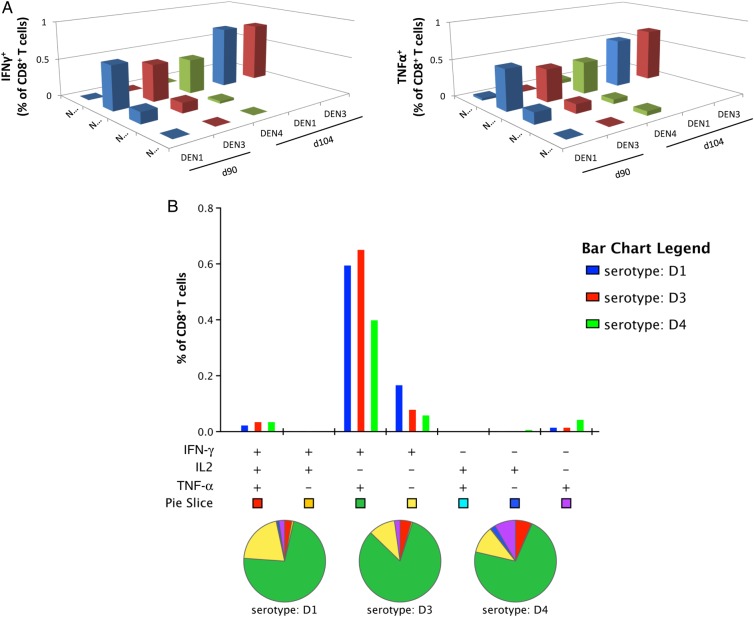
To determine whether the TDV-2 backbone elicits cross-reactive T-cell responses to the NS proteins of the other 3 DENV serotypes, we analyzed PBMCs from an individual from the SC cohort (subject 5). This subject gave higher IFN-γ and TNF-α response to NS3 and NS5 protein represented by pool 1 (NS5-1) at d90, d104, and d270 as compared to the other subjects (Figure 2 and 3). Cells from days 90 and 104 were stimulated with peptide arrays encompassing the sequences of NS1, NS3, and NS5 (pools 1 and 2) from DENV-1, DENV-3, or DENV-4. Our data indicate that TDV-2 elicits cross-reactive CD8+ IFN-γ- or TNF-α-producing T cells recognizing predominantly the NS3 protein of all 3 DENV serotypes (Figure 6A) and were predominantly multifunctional (IFN-γ+/TNF-α+) (Figure 6B).
Figure 6.
Cross-reactivity of TDV backbone–immune CD8+ T cells to the NS proteins of DENV-1, -3, and -4. A, PBMCs from subject 5 (SC cohort) collected on day 90 postprimary immunization were restimulated in vitro with NS1, NS3, or NS5 peptide pools of DENV-1, DENV-3, and DENV-4 and stained for intracellular IFN-γ, TNF-α (A), and IL-2 (data not shown). B, Multifunctional cross-reactive CD8+ T-cell responses. The percentages of CD8+ T cells that produced any combinations of IFN-γ, TNF-α, and IL-2 upon stimulation with NS3 peptide pools. Pie charts show the distribution of different CD8+ T-cell populations that produce 1 or more cytokines. Abbreviations: DENV, dengue virus; IFN-γ, interferon-γ; IL2, interleukin 2; NS, nonstructural; TNF-α, tumor necrosis factor–α.
DISCUSSION
In this study, we sought to measure and characterize the cellular responses to TDV in a subset of healthy flavivirus-naive individuals that participated in a Phase I clinical trial. The first key finding of our study was the demonstration that TDV elicits CD8+ T-cell responses characterized mainly by the production of IFN-γ and TNF-α cytokines. The CD8+ T-cell response was evident 3 months postprimary vaccination, was not affected by a booster vaccination, and was detectable 6 months after the last immunization. Generally, following DENV infection, T-cell response begins to develop when viremia levels start declining by day 5 or 6 postinfection. Sustained dengue-specific IFN-γ response correlates with protection against viremia in a human challenge study [29]. In the Phase I study, 79% of subjects that received the higher dosage form of TDV had detectable TDV viral RNA [20] and, of the subjects selected for this study, 7 of 10 had detectable viral RNA to TDV-2 that started on day 7 after prime and lasted up to day 14 postvaccination. There were no PBMC collections between days 14 and 90 (postprimary immunization) to precisely identify the timing of the onset of CD8+ T-cell response to TDV. Due to the attenuated nature of the vaccine viruses that constitute TDV, the CD8+ T-cell response may occur much later than in the case of a wild-type DENV infection. This is supported by our previous observations in cynomolgus macaques where T-cell responses to TDV were detectable much later following peak viremia [17]. A second key observation from our study was that the booster vaccination given on day 90 did not impact the magnitude of CD8+ T-cell responses. In our preclinical [17, 18] and Phase I clinical studies [19, 20], we have consistently observed that there is limited vaccine virus viremia after the boost when given 2 to 3 months after a primary immunization. This suggests that due to the short window between primary and secondary immunizations, circulating neutralizing antibodies are efficiently controlling vaccine virus replication, and hence boosting at the T-cell level is not apparent. When we analyzed PBMCs collected 180 days postbooster immunization, a strong and predominantly multifunctional IFN-γ/TNF-α memory CD8+ T-cell response was still detectable in most of the individuals tested from the SC or ID cohorts. This response profile may be beneficial since a rapid recall response may occur upon DENV infection as compared to naive individuals.
CD8+ T cells are thought to play a key role in controlling viral infections. They recognize viral epitopes in the context of human leukocyte antigen class I molecules on the surface of infected cells, and following activation they kill cells directly or via secretion of cytokines such as IFN-γ and TNF-α. In our studies, we employed peptide arrays encompassing the whole sequences of NS1, NS3, and NS5 of DENV-2 to identify the target proteins of the CD8+ T-cell response elicited by TDV. A key finding from our analysis was the demonstration that the NS3 protein was the main target of the CD8+ T-cell response followed by the NS5-1. Although the number of individuals tested from each cohort was small, our data suggest that the route of vaccination does not impact the specificity of the CD8+ T-cell response in terms of protein recognition. However, we cannot exclude the possibility that injection route might influence epitope selection due to differences in resident antigen-presenting cells. With reference to the target proteins that TDV-specific CD8+ T cells recognize, our findings are consistent with previous observations highlighting that NS3 and NS5 proteins of DENVs are among the main target proteins of the T-cell response [24, 28–30]. Differences in processing of these 2 NS proteins may account for preferential presentation of generated epitopes and their immunodominance over T-cell epitopes from other structural or NS proteins. However, our analysis was limited due to the availability of cells to screen peptide arrays from other NS proteins.
In our studies, we also provided evidence indicating that the vaccine backbone elicited CD8+ T cells that predominantly recognize the NS3 protein of DENV-1, -3, and -4 in 1 individual. This might be correlated with a highly conserved region in the NS3 protein of all 4 DENV serotypes containing motifs and charged residues that are essential for helicase activity and virus replication [31, 32]. More recently, an immunodominant CD8+ T-cell epitope encompassing residues 538–547 in the NS3 protein was shown to be conserved (100% identical) among the 4 DENV serotypes [33]. Therefore, one would predict that responses to conserved epitopes would be seen in humans [34]. Further studies are needed to characterize the breadth of cross-reactive T-cell responses to the backbone of the other DENV serotypes in more TDV-vaccinated individuals. The use of ICS assays permits us to evaluate simultaneously the presence of different cytokines that can delineate different subsets of T cells having multifunctional profiles. Multifunctional T-cells responses have been associated with protection in studies with various pathogens, including dengue [28, 30, 35, 36]. In this study, we found that all individuals from both cohorts produced TDV-specific multifunctional CD8+ T cells producing at least 2 cytokines. Although quantification of the magnitude and breadth of vaccine-induced CD8+ T-cell recall responses provides information regarding the immunogenicity of a given vaccine candidate, it is difficult to know what their impact would be on controlling viral replication. Several factors (such as host genetic determinants, immune status of the individual, kinetics and timing of antigen-specific immune responses, DENV serotype, etc.) could have a major impact on the protective efficacy of CD8+ T cells. Of particular interest would be to compare and contrast the T-cell responses to TDV with those elicited by natural infection. Overall, these findings highlight the immunogenic profile of the candidate dengue vaccine, TDV, and support the further evaluation of clinical samples from ongoing clinical trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), contract no. HHHSN27201300021I3C.
Potential conflicts of interest. H. C., D. T. S., C. D. P., and J. E. O. are affiliated with Takeda Vaccine, Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland, 2009. [PubMed] [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010; 8(12 suppl):S7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine 2011; 29:7221–8. [DOI] [PubMed] [Google Scholar]
- 5.Endy TP. Human immune responses to dengue virus infection: lessons learned from prospective cohort studies. Front Immunol 2014; 5:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabin AB. The dengue group of viruses and its family relationships. Bacteriol Rev 1950; 14:225–32. [PubMed] [Google Scholar]
- 7.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 2003; 60:421–67. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis 2010; 10:712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev 2008; 225:300–13. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol 2007; 5:518–28. [DOI] [PubMed] [Google Scholar]
- 11.Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J Virol 2000; 74:3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CY-H, Butrapet S, Pierro DJ et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol 2000; 74:3020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CY-H, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol 2003; 77:11436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinney RM, Butrapet S, Chang GJ et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 1997; 230:300–8. [DOI] [PubMed] [Google Scholar]
- 15.Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine 2011; 29:7251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewoo JN, Kinney RM, Powell TD et al. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine 2012; 30:1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osorio JE, Brewoo JN, Silengo SJ et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am J Trop Med Hyg 2011; 84:978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambuel Y, Young G, Brewoo JN et al. A rapid immunization strategy with a live-attenuated tetravalent dengue vaccine elicits protective neutralizing antibody responses in non-human primates. Front Immunol 2014; 5:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio JE, Velez ID, Thomson C et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) administered to flavivirus-naive healthy adults in Colombia: a randomized, placebo-controlled Phase 1 study. Lancet of Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George SL, Wong MA, Dube TJ et al. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naïve adults: a randomized, double-blind phase I clinical trial. J Infect Dis 2015; 212:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejnirattisai W, Jumnainsong A, Onsirisakul N et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Alwis R, Beltramello M, Messer WB et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLOS Negl Trop Dis 2011; 5:e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltramello M, Williams KL, Simmons CP et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010; 8:271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiskopf D, Sette A. T cell immunity to infection with dengue virus in humans. Front Immunol 2014; 5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy B, Nougarede N, Begue S et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008; 26:5712–21. [DOI] [PubMed] [Google Scholar]
- 26.Lindow JC, Borochoff-Porte N, Durbin AP et al. Primary vaccination with low dose live dengue 1 virus generates a proinflammatory, multifunctional T cell response in humans. PLOS Negl Trop Dis 2012; 6:e1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yauch LE, Zellweger RM, Kotturi MF et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol 2009; 182:4865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskopf D, Angelo MA, de Azeredo EL et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci USA 2013; 110:E2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunther VJ, Putnak R, Eckels KH et al. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 2011; 29:3895–904. [DOI] [PubMed] [Google Scholar]
- 30.Duangchinda T, Dejnirattisai W, Vasanawathana S et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci USA 2010; 107:16922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matusan AE, Kelley PG, Pryor MJ, Whisstock JC, Davidson AD, Wright PJ. Mutagenesis of the dengue virus type 2 NS3 proteinase and the production of growth-restricted virus. Gen Virol 2001; 82:1647–56. [DOI] [PubMed] [Google Scholar]
- 32.Matusan AE, Pryor MJ, Davidson AD, Wright PJ. Mutagenesis of the dengue virus type 2 NS3 protein within and outside helicase motifs: effects on enzyme activity and virus replication. J Virol 2001; 75:9633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piazza P, Campbell D, Marques E et al. Dengue virus infected human dendritic cells reveal hierarchies of naturally expressed novel NS3 CD8 T cell epitopes. Clin Exp Immunol 2014; 177:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiskopf D, Angelo MA, Bangs DJ et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol 2014; 88:11383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akondy RS, Monson ND, Miller JD et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 2009; 183:7919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betts MR, Nason MC, West SM et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107:4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.