Abstract
A prospective observational study of 176 men coinfected with human immunodeficiency virus and herpes simplex virus type 2 (HSV-2) was conducted to assess whether their sexual partners may be at an increased risk of HSV-2 from male circumcision (MC) wounds. Preoperative and weekly penile lavage samples were tested for penile HSV-2 shedding. Prevalence risk ratios (PRRs) were estimated using Poisson regression. Detectable penile HSV-2 shedding was present in 9.7% of men (17 of 176) before MC, compared with 12.9% (22 of 170) at 1 week (PRR, 1.33; 95% confidence interval [CI], .74–2.38) and 14.8% (23 of 155) at 2 weeks (PRR, 1.50; 95% CI, .86–2.62) after MC. HSV-2 shedding was lower among men with healed MC wounds (adjusted PRR, 0.62; 95% CI, .35–1.08). Men undergoing MC should be counseled on sexual abstinence and condom use.
Keywords: human immunodeficiency virus (HIV), herpes simplex virus type 2 (HSV-2), male circumcision, viral load, penile viral shedding, Uganda
Herpes simplex virus type 2 (HSV-2) is one of the most common sexually transmitted infections acquired by contact with an individual shedding the virus in their genital secretions or skin [1]. HSV-2 seropositivity is also associated with a 3-fold increased risk of acquiring human immunodeficiency virus (HIV) in observational studies [1]. In addition to breaches of the mucosal barrier due to genital ulceration, it has been proposed that the increased risk of HIV acquisition associated with HSV-2 infection may be due to recruitment of CD4+ T cells expressing CCR5 and immature dendritic cells into areas of HSV replication [1].
Randomized trials have shown that male circumcision (MC) significantly decreases male heterosexual acquisition of HIV, human papillomavirus, and HSV-2 [2, 3]. MC of HIV-negative men also provides benefits to female partners, including reduced rates of bacterial vaginosis and Trichomonas vaginalis and human papillomavirus infection [4, 5]. However, MC had no impact on HIV or HSV-2 transmission to female partners in a randomized trial [6, 7]. Penile HIV shedding is increased immediately after MC [8, 9]. Both the number of HIV shedding events and the quantity of virus shed from the MC wound were significantly elevated in HIV-infected men who did not report antiretroviral therapy (ART) use at baseline in the first 3 weeks after MC [8]. The increase in HIV shedding may be associated with increased HIV transmission among couples who resumed sexual intercourse before wound healing [6].
It is unknown whether MC also increases the frequency and intensity of HSV-2 shedding after surgery. Prior research on HSV-2 transmission suggests that higher HSV-2 load increases the risk of HSV-2 transmission irrespective of whether or not the infecting partner is symptomatic [10]. A better understanding is needed of whether MC might affect HSV-2 transmission to sexual partners. Here we report the prevalence and levels of HSV-2 shedding after MC in men coinfected with HSV-2 and HIV.
METHODS
Study Population and Design
A prospective study was conducted between June 2009 and April 2012 in Rakai, Uganda, to assess HIV (primary outcome) and HSV-2 (secondary outcome) shedding from MC wounds, as described elsewhere [8]. Briefly, all uncircumcised HIV-infected men (defined as aged ≥15 years) who requested free MC services and had no contraindication to surgery were invited to participate. They provided written informed consent and were also referred for HIV care. HIV-negative men were enrolled in a concurrent parallel study of MC wound healing to avoid stigmatization of the HIV-infected participants.
All men were offered free voluntary HIV counseling and testing; education on HIV prevention, risks and benefits of MC, wound care, and the need to abstain from sexual intercourse until complete wound healing was certified; and treatment for sexually transmitted infection. At enrollment, information on sociodemographics, self-reported ART use, and sexual behavior was collected using structured questionnaires. Venous blood was collected before surgery for HIV and HSV-2 serology, plasma HIV load testing, and CD4+ T-cell counts. MC was performed using the dorsal slit method under aseptic conditions. All participants were followed up weekly for 6 weeks and then at 8 and 12 weeks. Information on sexual behaviors was obtained at each follow-up visit.
Lavage samples to assess viral shedding at the coronal sulcus before MC and at the MC surgical site after MC were collected at all study visits, using nontraumatic washing with 5 mL of phosphate-buffered saline (pH 7.2); samples were stored at −80°C until DNA extraction [8]. Men were included in the study if they were HSV-2 seropositive at enrollment and had a lavage sample obtained at the presurgical visit and ≥1 postsurgical visit.
The study was reviewed by the Higher Degrees, Research and Ethics Committee of Makerere University School of Public Health, the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, Western Institutional Review Board, and the Johns Hopkins University School of Medicine Institutional Review Board.
HIV, HSV-2, and CD4+ T-Cell Count Testing
HIV status, plasma HIV load, and baseline CD4+ T-cell count were assayed as described elsewhere [8]. Men were considered virally suppressed if their HIV load was <400 copies/mL at enrollment. HSV-2 serostatus was determined by an HSV-2 enzyme-linked immunosorbent assay (Kalon Biological), with a positive result defined as an optical density index value ≥1.5 [2].
MC lavage samples were assessed for penile HSV-2 shedding using a total nucleic acid extraction followed by real-time quantitative polymerase chain reaction assay with primers to glycoprotein B to detect HSV DNA [11]. HSV-2 shedding was defined as positive polymerase chain reaction results of >50 copies of HSV-2 DNA/mL in 2 separate assays. Samples obtained beyond week 6 were not evaluated because preliminary data showed essentially no HSV-2 shedding from MC wounds at this point, and 100% of men were certified as healed by 6 weeks.
Statistical Analysis
Increased risk for detectable HSV-2 shedding in lavage samples was assessed at each weekly visit after MC relative to the presurgical visit. The relative risks of HSV-2 shedding at postsurgical visits were reported as prevalence risk ratios (PRRs) estimated by Poisson regression with generalized estimating equations and a robust variance estimator. In separate analyses, we also assessed whether presurgical HSV-2 shedding, self-reported ART status, CD4+ T-cell count, plasma HIV load, penile HIV shedding, resumption of sex, and MC wound healing were associated with postsurgical HSV-2 shedding. Associations were estimated using unadjusted and adjusted Poisson regression models with generalized estimating equations and robust variance estimators. Finally, significant differences in absolute levels of lavage sample HSV-2 load (log10 copies/mL) among those with detectable lavage sample HSV-2 were tested using Wilcoxon–Mann–Whitney tests stratified by study visit. All analyses were conducted using the “geepack” package in R software (version 3.01).
RESULTS
A total of 176 HIV/HSV-2–coinfected men who agreed to participate in the study and had lavage samples before and after MC available for HSV-2 testing. Their median age was 33 years (interquartile range [IQR], 28–38 years), and 77% (136 of 176) of men were married. Of the 176 men, 145 (83%) self-reported being ART naive, 22 (12%) self-reported ART use and had an undetectable plasma HIV load (<400 copies/mL), and 9 (5%) self-reported ART use but had a detectable plasma HIV load. Among men with a detectable plasma HIV load, the median load was 39 240 copies/mL (IQR, 12 310–168 200 copies/mL). The overall median CD4+ T-cell count was 402/µL (IQR, 276–610/µL). Though all men were HSV-2 seropositive, only 26 (15%) reported a genital ulcer within the 30 days before enrollment. The retention rates were 97% (n = 170) at week 1, 88% (n = 155) at week 2, 87% (n = 154) at week 3, 90% (n = 159) at week 4, and 82% (n = 144) at week 6.
The median time to a healed MC wound was 4 weeks, and all participants' wounds were healed by week 6. Nine percent (n = 15) of men reported resumption of sex by week 4, and 68% by week 6. Only 4 men reported resuming sex before week 3. Twelve (80%) of the 15 men who resumed sex by week 4 did not have a healed MC wound before sex.
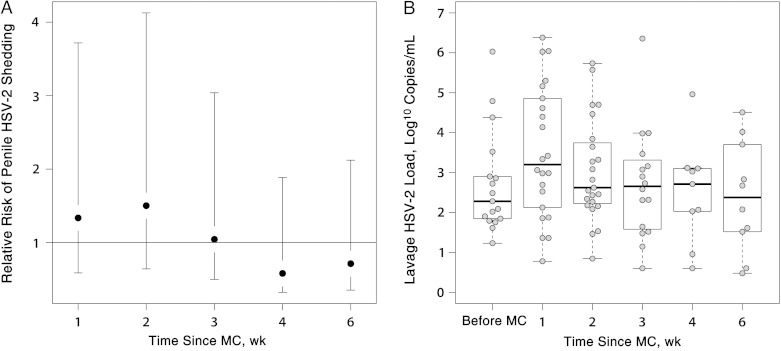
Before MC, penile HSV-2 shedding was detected in 9.7% of men (17 of 176). After MC, penile HSV-2 shedding was detected at 10.5% of postsurgical visits (80 of 761) in 27.3% of men (48 of 176). The prevalence of detectable HSV-2 shedding was nonsignificantly increased after MC relative to baseline at weeks 1 (12.9% [22 of 170]; PRR, 1.33; 95% confidence interval [CI], .75–2.38) and 2 (14.8% [23 of 155]; PRR, 1.50; 95% CI, .86–2.62) (Figure 1A), 10.4% (16 of 154; PRR, 1.04; 95% CI, .55–2.00) at week 3, and then nonsignificantly lower than baseline by weeks 4 (5.7% [9 of 158]; PRR, 0.58; 95% CI, .26–1.31) and 6 (6.9% [10 of 144]; PRR, 0.71; 95% CI, .36–1.41).
Figure 1.
Penile herpes simplex virus type 2 (HSV-2) shedding after male circumcision (MC) in 176 men (aged ≥15 years) with human immunodeficiency virus infection in Rakai, Uganda. A, Relative risk of any detectable HSV-2 shedding in penile lavage sample after MC through 6 weeks using the presurgical visit at the referent period. B, Lavage sample HSV-2 loads among men with detectable HSV-2 shedding before MC and at weekly postsurgical visits through 6 weeks.
In univariate and multivariate analyses, penile HSV-2 shed- 175 ding after MC was not associated with age, plasma HIV load, penile HIV shedding, CD4+ T-cell count, or resumption of sex (Table 1). Prevalence of HSV-2 shedding was non-significantly higher in those with recent genital ulcer disease (aPRR, 1.57; 95% CI, .89–2.74; P = .13) or HSV-2 shedding before MC (aPRR, 1.77; 95% CI, .95–3.32; P = .07). IT was nonsignificantly lower in men with self-reported ART use and suppressed HIV load (aPRR, 0.64; 95% CI, .22–1.87) and those with healed MC wounds (aPRR, 0.62; 95% CI, .35–1.08).
Table 1.
Factors Associated With Detectable Penile HSV-2 Shedding Through 6 Weeks After MC Among 176 HIV-Infected Men in Rakai, Ugandaa
| Factor | Post-MC Visits With HSV Shedding/ Total Post-MC Visits, No. (%) | PRR (95% CI) | P Value | aPRR (95% CI) | P Value |
|---|---|---|---|---|---|
| Overall | 80/781 (10.2) | … | … | … | … |
| Age, y | |||||
| 15–29 | 29/238 (12.2) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| 30–39 | 34/378 (10.3) | 0.73 (.39–1.35) | .31 | 0.76 (.42–1.40) | .38 |
| ≥40 | 17/165 (9.0) | 0.84 (.39–1.85) | .67 | 0.90 (.40–2.40) | .80 |
| Genital ulcer disease within 30 d before MC | |||||
| No | 63/671 (9.4) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| Yes | 17/110 (15.5) | 1.60 (.88–2.94) | .13 | 1.57 (.89–2.74) | .13 |
| Certified healed wound | |||||
| No | 57/475 (12.0) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| Yes | 22/288 (7.6) | 0.61 (.36–1.06) | .08 | 0.62 (.35–1.08) | .09 |
| Resumed sexb | |||||
| No | 65/627 (10.4) | 1.00 (Ref) | … | … | … |
| Yes | 15/136 (11.0) | 0.91 (.51–1.63) | .76 | … | … |
| Presurgical penile HSV-2 shedding | |||||
| No | 68/707 (9.6) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| Yes | 12/74 (16.2) | 1.75 (.86–3.62) | .12 | 1.77 (.95–3.32) | .07 |
| Postsurgical penile HIV shedding | |||||
| No | 69/674 (10.2) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| Yes | 11/104 (10.6) | 1.07 (.61–1.88) | .82 | 0.89 (.49–1.60) | .82 |
| Presurgical CD4+ T-cell count, cells/µL | |||||
| >600 | 28/300 (9.3) | 1.00 (Ref) | … | … | … |
| 200–500 | 43/378 (11.4) | 1.19 (.64–2.18) | .58 | 1.20 (.63–2.27) | .58 |
| <200 | 9/103 (8.7) | 0.92 (.41–2.05) | .84 | 0.96 (.41–2.22) | .84 |
| Plasma HIV load, copies/mLb | |||||
| <400 (undetectable) | 16/175 (9.1) | 1.00 (Ref) | … | … | … |
| 400–9999 | 22/144 (15.3) | 1.35 (.63–2.90) | .44 | … | … |
| 10 000–49 999 | 10/168 (6.0) | 0.63 (.24–1.63) | .34 | … | … |
| >50 000 | 31/259 (12.0) | 1.25 (.61–2.57) | .55 | … | … |
| Self-reported ART and plasma HIV load | |||||
| ART naive | 70/643 (10.9) | 1.00 (Ref) | … | 1.00 (Ref) | … |
| ART but detectable HIV load | 4/41 (9.8) | 0.87 (.31–2.45) | .80 | 1.04 (.38–2.79) | .94 |
| ART and suppressed HIV load | 6/97 (6.2) | 0.57 (.21–1.54) | .27 | 0.64 (.22–1.87) | .41 |
Abbreviations: aPRR, adjusted prevalence risk ratio; ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HSV-2, herpes simplex virus type 2; MC, male circumcision; PRR, prevalence risk ratio; Ref, reference standard.
a Wound healing, resumption of sex, plasma HIV load, and postsurgical penile HIV shedding were assessed as time-varying factors at the same visit as penile HSV-2 shedding. All other factors were measured at the presurgical visit.
b Resumption of sex and plasma HIV load were not included in the adjusted model because of collinearity (ie, high correlation) with wound healing and ART/plasma HIV load status variables, respectively.
Among men with detectable HSV-2 shedding, the median log10 HSV-2 load was elevated at week 1 (median, 3.2; IQR, 2.2–4.8) compared with before surgery (median, 2.3; IQR, 1.8–2.9) (Figure 1B), although differences were not statistically significant (P = .09). The levels of HSV-2 shedding were similar to baseline at all other postoperative visits.
DISCUSSION
Penile HSV-2 shedding from MC wounds was detected among 27% of HIV/HSV-2–coinfected men within 6 weeks of MC. HSV-2 shedding was lower among men with certified healed MC wounds than at baseline, but this difference was of borderline statistical significance. These data support findings in observational studies of MC performed before sexual debut suggesting that MC may have long-term benefits for reducing HSV-2 transmission to female partners [12, 13]. Postsurgical penile HSV-2 shedding was not significantly associated with preoperative penile HIV shedding, plasma HIV load, self-reported ART status, CD4+ T-cell count, or resumption of sex after MC. To our knowledge, this is the first study to evaluate HSV-2 shedding from penile lavage samples either before or after MC.
We have previously shown that MC reduces risk of HSV-2 acquisition in HSV-2 uninfected men [2]; however we observed no similar impact of MC on HSV-2 transmission to female partners [7]. The increased levels of HSV-2 shedding that we observed in the first 2 weeks after MC may partially explain why there was no short-term benefit of MC to female partners of HSV-2–infected men. Although few men in this study resumed sex before wound healing, in another similar setting 65% of married men and approximately 30% of all men reported resuming sexual intercourse before wound healing after MC [14]. Our findings further underscore the importance of abstaining from sex before wound healing and counseling to promote abstinence after MC. These data also highlight a need for interventions to reduce HIV and HSV-2 shedding during the postsurgical period among men undergoing MC.
In a prior study, we found a greater proportion of men shedding HIV and a higher quantity of HIV shedding in the first 3 weeks after MC relative to the presurgical visit [8]. Similarly, we observed an increase in the number of men shedding HSV-2 immediately after MC and higher HSV-2 loads in the current study, although the results were not statistically significant. The HSV-2 shedding and higher viral loads may be due to inflammation during wound healing [15].
The current study was limited in part because HSV-2 shedding was assessed among HIV-coinfected men only. Given that HSV-2 shedding was detected among a minority of men, we had limited power to detect statistically significant associations between risk factors and postsurgical HSV-2 shedding. We also do not know the impact of penile HSV-2 shedding from MC wounds on transmission to sexual partners. However, the increased quantity of shedding from genital ulcers has been shown to increase HSV-2 transmission [1]. Thus, it is plausible that HSV-2 shedding from a post-MC wound could increase the risk of transmission to partners. Finally, weekly sampling may also have obscured important observations or trends occurring over a shorter time frame. We cannot rule out the possibility the quantity and intensity of HSV-2 shedding during the first few days after surgery was significantly higher than before MC because shedding was assessed only at weekly intervals.
HIV/HSV-2–coinfected men undergoing MC, and their partners, should be counseled on the possible risk of HIV and HSV-2 transmission. MC programs should promote voluntary HIV counseling and testing, sexual abstinence during wound healing, and condom use thereafter.
Notes
Acknowledgments. We are most grateful to the study participants, the Rakai Community Advisory Board, and the Uganda Virus Research Institute, whose commitment and cooperation made this study possible.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant 22006.03 for sample collection); the Doris Duke Charitable Foundation (grant 2011036 for laboratory work and Clinician Scientist Development awards to M. K. G. and A. A. R. T.); the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, and the Fogarty International Center (grant 1D43TWOO9578-01); and the National Institutes of Health (grants T32AI102 supported M. K. G. and 1K23AI093152-01A1 to A. A. R. T.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant supported the content of the manuscript have been disclosed.
References
- 1.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr 2004; 35:435–45. [DOI] [PubMed] [Google Scholar]
- 2.Tobian AA, Serwadda D, Quinn TC et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009; 360:1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobian AA, Gray RH. The medical benefits of male circumcision. JAMA 2011; 306:1479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RH, Kigozi G, Serwadda D et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2009; 200:42.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wawer MJ, Tobian AA, Kigozi G et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet 2011; 277:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wawer MJ, Makumbi F, Kigozi G et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet 2009; 374:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobian AA, Kigozi G, Redd AD et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J Infect Dis 2012; 205:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobian AA, Kigozi G, Manucci J et al. HIV shedding from male circumcision wounds in HIV-infected men: a prospective cohort study. PLoS Med 2015; 12:e1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odoyo-June E, Rogers JH, Jaoko W, Bailey RC. Changes in plasma viral load and penile viral shedding after circumcision among HIV-positive men in Kisumu, Kenya. J Acquir Immune Defic Syndr 2013; 64:511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 2014; 11:20140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol 2002; 40:2609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray RH, Wawer M, Thoma M et al. Male circumcision and the risks of female HIV and sexually transmitted infections acquisition in Rakai, Uganda [abstract 128]. Presented at: 13th Conference on Retroviruses and Opportunistic Infections; 2006. [Google Scholar]
- 13.Mujugira A, Magaret AS, Celum C et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis 2013; 208:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman-Roloff A, Bailey RC, Agot K. Factors associated with the early resumption of sexual activity following medical male circumcision in Nyanza province, Kenya. AIDS Behav 2012; 16:1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KE, Redd AD, Quinn TC et al. Effects of HIV-1 and HSV-2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis 2011; 203:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]