Abstract
Mortality from pneumococcal pneumonia remains high despite antibiotic therapy, highlighting the pathogenic potential for host inflammation. We demonstrate that macrophage migration inhibitory factor (MIF), an innate immune mediator, is detrimental for survival and associated with lung pathology, inflammatory cellular infiltration, and bacterial replication in a mouse model of pneumococcal pneumonia, despite being necessary for clearance from the nasopharynx. Treatment of animals with a small-molecule inhibitor of MIF improves survival by reducing inflammation and improving bacterial control. Our work demonstrates that MIF modulates beneficial versus detrimental inflammatory responses in the host-pneumococcal interaction and is a potential target for therapeutic modulation.
Keywords: pneumococcus, pneumonia, macrophage migration inhibitory factor
Mortality from invasive pneumococcal infection remains at 10% today. Even with appropriate antibiotic administration, mortality is still a concern in the treatment of pneumonia, implicating the presence of a pathogenic component of the host response [1, 2]. Development of novel, antibiotic-independent, therapeutic strategies are needed to address the disease burden associated with pneumococcal lung infections.
The pathogenesis of pneumococcal pneumonia is governed by the interplay of inflammatory responses: some are required to control bacterial growth, whereas others are dispensable or detrimental and result in inflammatory pathology. After infection, sensing of the pneumococcus by epithelial and resident immune cells leads to the production of cytokines and chemokines, which recruit innate effector cells, neutrophils and monocytes, to the lung. Neutrophils and monocytes/macrophages are agents of pneumococcal killing, but their role in the regulation of inflammation has recently come to light. Depletion of neutrophils in pneumonia improves survival and lung pathology without compromising bacterial control [3]. Additionally, interleukin 1 (IL-1) signaling in monocytes and epithelial cells promotes expression of the neutrophil chemoattractants KC/CXCL1 and MIP-2/CXCL2, resulting in increased pulmonary neutrophilia and exacerbated disease [4]. The importance of cooperation between monocytes and neutrophils is highlighted by findings that mice lacking either macrophages or components of the macrophage apoptosis machinery demonstrate increased neutrophil recruitment and impaired pneumococcal control [4, 5].
We recently reported that macrophage migration inhibitory factor (MIF), a cytokine expressed abundantly by both immune and epithelial cells at sites of host-pathogen interaction, retains macrophages in the nasopharynx in response to pneumococcal colonization and is required for bacterial clearance [6]. This supports the observation that older adults who express low levels of MIF, by way of a commonly occurring genetic polymorphism, are at greater risk for community-acquired pneumonia [7]. However, given that host-pathogen interactions may vary in different regions of the respiratory tract, we next sought to define the role of MIF in pulmonary immunity during pneumococcal pneumonia.
METHODS
Mice
Adult C57BL/6 (wild-type [WT]) mice were age/sex matched with MIF-deficient (Mif−/−) mice in the C57BL/6 background for all experiments [6]. Procedures were performed in accordance with the Institutional Animal Care and Use Committee protocols of the University of Pennsylvania.
Model of Pneumococcal Colonization and Pneumonia
An animal-passaged serotype 3 strain of Streptococcus pneumoniae (ATCC 6303) was used for all experiments. Cultures were grown in tryptic soy broth (TS; Life Technologies) at 37°C to mid-log phase (OD620, approximately 1.0).
For colonization experiments, 107 mid-log phase bacteria in 10 µL of phosphate-buffered saline (PBS) were delivered to the nares of awake mice [6]. At the indicated time points, mice were euthanized, their tracheae cannulated, and lavage fluid collected from the nares for bacterial enumeration.
Pneumonia was modeled by administering 104 mid-log phase bacteria in 80 µL of PBS to the nares of isoflurane-anesthetized mice (Santa Cruz Biotechnology). During survival experiments, animals were monitored twice daily for signs of illness, such as decreased activity and labored breathing, and were euthanized if they were in extremis. Otherwise, they were euthanized by CO2 asphyxiation at the indicated times.
For MIF replacement experiments, PBS alone or 100 ng of endotoxin-free recombinant MIF (rMIF) dissolved in 80 µL of PBS was administered to the nares of anesthetized mice [6]. For MIF inhibition, vehicle alone or 40 mg/kg of 3-(3-hydroxybenzyl)-5-methylbenzooxazol-2-one, designated MIF098, dissolved in PEG 400 plus (2-hydroxypropyl)-β-cyclodextrin vehicle was administered intraperitoneally twice daily beginning on the day of infection [8]. MIF098 did not directly inhibit pneumococcal growth, even at 10 times the concentration used in vivo.
Bronchoalveolar lavage (BAL) was performed with 1 mL of cold PBS for protein analysis or with 3 mL for flow cytometry. Fluid was centrifuged to pellet cells prior to protein analysis (performed using the bicinchoninic acid assay; Thermo Scientific) and enzyme-linked immunosorbent assays (eBioscience). Blood specimens were obtained by cardiac puncture and diluted 1:10 in PBS before plating. For bacterial enumeration, the right lung lobes were collected in PBS and mechanically disrupted, and serial dilutions were plated. Histologic examination was performed on hematoxylin-eosin–stained sections of the left lung. Pathology was scored as follows: 0, no involvement; 1, localized infiltrates of neutrophils in alveoli, no bacteria; 2, dense infiltrates of neutrophils in airways with involvement of adjacent alveoli, no bacteria; 3-consolidation of neutrophil inflammation in bronchioles and alveoli, lobar pneumonia, with intact visible bacteria; and 4, overwhelming infection, with greater numbers of bacteria than inflammatory cells in alveoli or bronchioles.
Flow Cytometry
Single-cell suspensions were prepared by incubating minced tissue specimens (left lung) with collagenase IV (Worthington Biochemicals) and DNaseI (Sigma-Aldrich) for 1 hour at 37°C. The digestion was disrupted by passing the materials through a cell-strainer (BD Bioscience). Red blood cells were lysed, and cells were washed in Dulbecco's modified Eagle's medium/fetal bovine serum (Life Technologies). Cells were counted after trypan blue staining, using an automated counter (Life Technologies).
Cells were stained with CD11b-PerCP, Gr-1/Ly6G-PE, Ly6C-APC-Cy7, Siglec F-BV421, MHCII-AF700, CD11c-PE-Cy7, CD45–650NC (BD Biosciences), Annexin V-APC, and Zombie yellow fixable viability dye (BioLegend) according to the manufacturers' protocol. Samples were fixed prior to analysis, except in apoptosis studies. Data were acquired using the LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star).
RESULTS
MIF Is Detrimental for Survival and Associated With Lung Injury in Pneumococcal Pneumonia
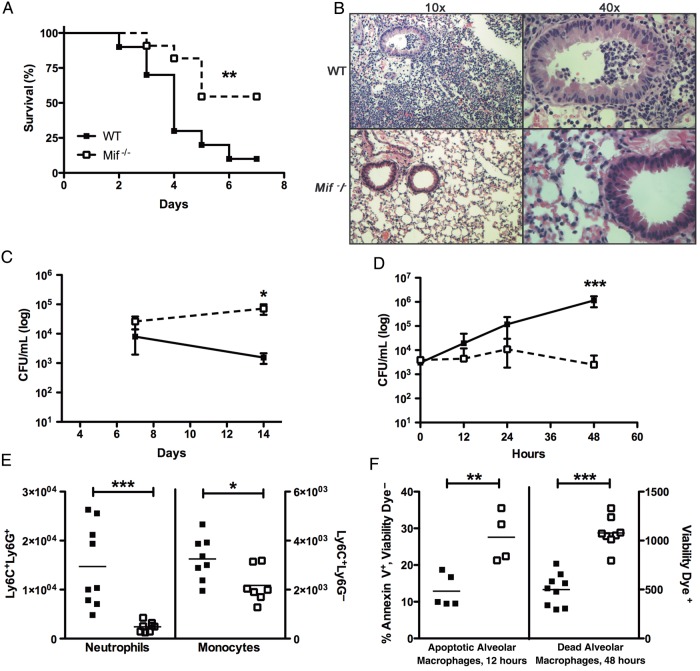
In our serotype 3 pneumonia model, mice developed severe illness that resulted in death beginning at 2 days after infection, with 90% of the WT animals dying by 7 days (Figure 1A). As is the case in human pneumococcal disease, bacteremia was noted in a majority (60%) of ill-appearing mice. They also had significant pulmonary pathology, with alveolar and bronchial inflammation (overall pathology score, 3; Figure 1B).
Figure 1.
Macrophage migration inhibitory factor (MIF) is detrimental to survival and increases lung pathology in a murine model of pneumococcal pneumonia. A, Pneumonia was induced in wild-type (WT; closed squares) and Mif−/− (open squares) mice by intranasally inoculating anesthetized mice with 1 × 104 colony-forming units (CFU) of type 3 pneumococci in 80 µL of phosphate-buffered saline (PBS). WT mice demonstrated reduced survival than their Mif−/− counterparts. B, When lungs from the mice were examined by hematoxylin-eosin staining 48 hours after infection, WT animals demonstrated greater lung pathology (ie, cellular infiltrates of the parenchyma and airways) than Mif−/− mice. C and D, While Mif−/− mice have impaired ability to clear type 3 pneumococcal colonization from the nasopharynx (modeled by inoculating awake mice with 1 × 107 CFU of pneumococci in 10 µL of PBS) (C), they have reduced lung bacterial burdens during pneumococcal pneumonia (D). E, WT mice demonstrate increased numbers of pulmonary neutrophils and monocytes, compared with their Mif−/− counterparts, during pneumonia (48 hours). Cellular composition was assessed by flow cytometry, in which Ly6C+Ly6G+ cells are designated as neutrophils and Ly6C+Ly6G− cells as monocytes, per 105 CD45+ live cells counted. F, Mif−/− mice have an increased ratio of alveolar macrophages undergoing early apoptosis at 12 hours, which correlates with increased numbers of dead alveolar macrophages at 48 hours. Cellular composition of bronchoalveolar lavage fluid was assessed by flow cytometry, in which Siglec F+CD11c+ cells are designated as alveolar macrophages, with Annexin V and Zombie yellow viability dye used to determine early apoptosis and cell death. There were ≥10 mice per group in survival experiments, and ≥4 mice per group in all others. *P < .05, **P < .01, and ***P < .001, by the log-rank test for analysis of survival curves and the Mann–Whitney U test for CFU and cell number comparisons.
Mif−/− animals had improved survival during pneumococcal pneumonia, with 46% mortality and no bacteremia (Figure 1A). Overall, the Mif−/− animals demonstrated fewer clinical signs of illness and lost less weight than their WT counterparts. The lungs of the Mif−/− mice had reduced parenchymal infiltration, and their airways remained free of inflammatory cells (overall pathology score, 1; Figure 1B).
MIF-Related Pathology Is Associated With Greater Pulmonary Bacterial Load, Inflammatory Cell Infiltrate, and Cytokine/Chemokine Production
There was 100-fold bacterial growth over 2 days in WT animals. Mif−/− animals, in contrast, were better able to control bacterial proliferation, correlating with their increased survival and attenuated lung pathology (Figure 1B and 1D). Since our observations seemed to indicate opposing roles for MIF in pneumonia versus colonization, we repeated carriage experiments with the same serotype 3 isolate. This strain colonized the murine nasopharynx effectively and did not produce symptomatic illness. While the WT mice began clearing the bacteria after 7 days, Mif−/− mice showed no change in burden by 14 days (Figure 1C).
To better understand the role of inflammation in pneumonia, we next characterized the pulmonary cellular infiltrate in WT and Mif−/− mice over the course of disease. WT and Mif−/− animals demonstrated a progressive infiltration of neutrophils into the lung parenchyma, but Mif−/− mice had approximately 5-fold fewer neutrophils than WT mice at 2 days (Figure 1E). Additionally, Mif−/− animals had approximately 1.5-fold fewer monocytes/macrophages in the lung than WT mice. While the total numbers of alveolar macrophages in BAL remained similar between groups (data not shown), we observed a significant increase in early apoptosis in these cells in Mif−/− mice, which correlated with a later increase in the number of dead cells (Figure 1F). These differences in apoptosis were not observed among neutrophils or monocytes/macrophages.
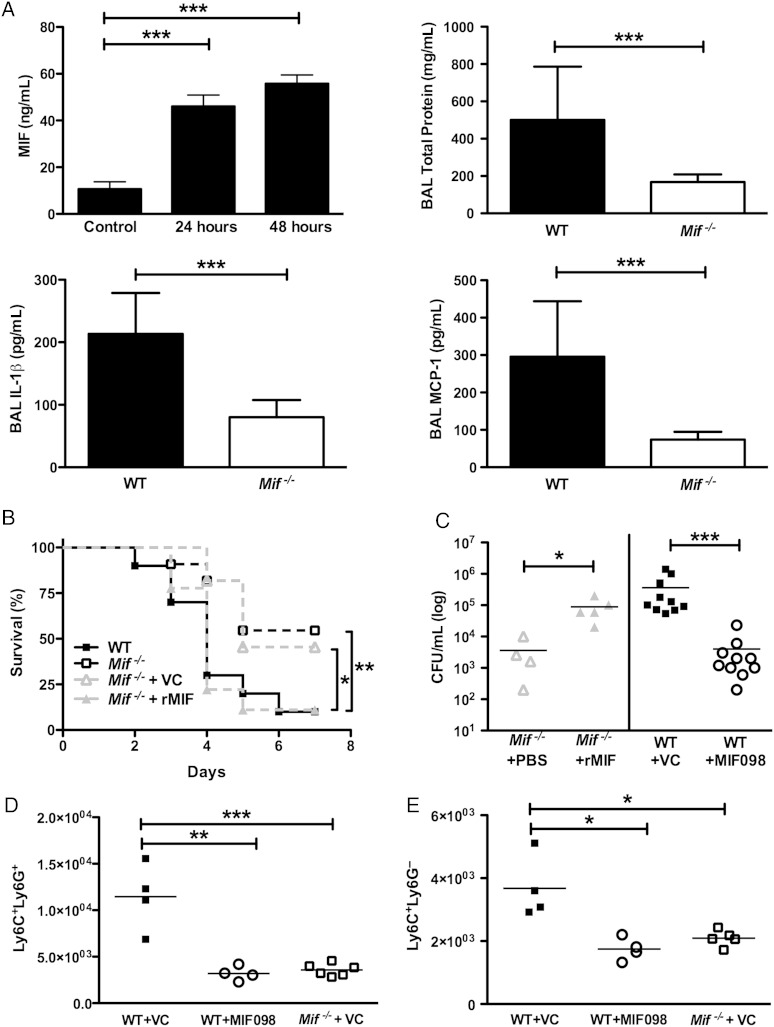
Next, we compared the chemoattractant microenvironment of the lungs between WT and Mif−/− mice. Similar to our observations in nasal colonization, local MIF production in lungs was induced early during pneumococcal infection and sustained over the course of the experiment (Figure 2A) [6]. Higher protein levels were present in the BAL fluid obtained from the WT animals as compared to the Mif−/− animals, indicating higher levels of inflammation in the WT group. Finally, levels of the specific chemoattractants of neutrophils and monocytes/macrophages, IL-1β and monocyte chemotactic protein 1 (MCP-1)/CCL2, respectively, were significantly higher in BAL obtained from WT mice, compared with that from Mif−/− mice.
Figure 2.
Macrophage migration inhibitory factor (MIF) regulates inflammation, influencing both bacterial load and survival in pneumococcal pneumonia. A, MIF was produced locally in the lung during the course of pneumococcal pneumonia. An increased level of MIF was associated with greater general inflammation in the lungs, as assessed by the total protein level in bronchoalveolar lavage (BAL) fluid, in the wild-type (WT) vs Mif−/− animals. Levels of specific cytokine attractants of both monocytes and neutrophils (monocyte chemotactic protein 1 [MCP-1] and interleukin 1β [IL-1β], respectively) were greater in the WT mice as compared to their Mif−/− counterparts. MIF, MCP-1, and IL-1β levels were assessed by specific enzyme-linked immunosorbent assays, and the total protein level was determined by the bicinchoninic acid assay. B, Mif−/− mice receiving local MIF supplementation (by delivery of 100 ng of recombinant MIF [rMIF] in 80 µL of phosphate-buffered saline [PBS] to the airways, beginning on the day prior to infection and continuing daily thereafter) had decreased survival than their counterparts, which received PBS alone. C, Additionally, they had higher bacterial burdens than their PBS-receiving Mif−/− counterparts. Conversely, when MIF was inhibited in the WT mice by using a small-molecule inhibitor (40 mg/kg MIF-098 delivered intraperitoneally, twice daily, beginning on the day of infection), the mice had greater survival than those that received vehicle control (VC; B). Additionally, greater survival in the MIF098-treated animals was associated with reduced pulmonary bacterial load, compared with VC-treated WT animals (C). D and E, Pharmacologic inhibition of MIF also reduced both neutrophil (Ly6C+Ly6G+) and monocyte (Ly6C+Ly6G−) infiltration, compared to VC treatment. All experiments were performed at 48 hours, unless otherwise stated. There were ≥10 mice per group in survival experiments and ≥4 mice per group in all others. *P < .05, **P < .01, and ***P < .001, by the log-rank test for analysis of survival curves and the Mann–Whitney U test for CFU and cell number comparisons. Abbreviaiton: CFU, colony-forming units.
Modulation of MIF Regulates Outcome in Pneumococcal Pneumonia
To investigate whether MIF was both necessary and sufficient to induce detrimental inflammation in pneumococcal pneumonia, we delivered rMIF to the lungs of Mif−/− mice. We found that local administration of rMIF decreased survival over the course of pneumonia and increased the pulmonary bacterial burden 48 hours after infection, compared with PBS administration (Figure 2B and 2C). Next, to assess the role of MIF inhibition in pneumococcal pneumonia, along with its therapeutic potential, we treated WT mice with either the small-molecule receptor antagonist for MIF, MIF098, or vehicle control over the course of infection [8]. Survival in the vehicle-treated WT animals mirrored that seen in the untreated mice (Figures 1A and 2B). Treatment with MIF098 both delayed initiation of mortality and improved overall survival from 10% to >50%, identical to that observed in Mif−/− mice. When we examined pulmonary bacterial loads 2 days after infection, we found a 100-fold reduction in burden in the MIF098-treated animals, compared with vehicle control (Figure 2C). The bacterial burden in MIF098-treated mice was similar to that in Mif−/− animals treated with vehicle control. Finally, we demonstrated a reduction in the numbers of infiltrating neutrophils and inflammatory monocytes/macrophages with MIF098 treatment (Figure 2D and 2E).
DISCUSSION
We show herein that MIF is detrimental for survival in pneumococcal pneumonia. Mice deficient in MIF have improved survival with reduced lung pathology, more effective bacterial control, and decreased infiltration of the lungs by innate immune cells, compared with their WT counterparts, which may be mediated by enhanced apoptosis of alveolar macrophages in the absence of MIF. These findings are in contrast to pneumococcal colonization of the nasopharynx, where MIF is required for bacterial clearance. Taken together, our experiments suggest that MIF-mediated inflammation plays a defining role in the outcome of the host-pneumococcal interaction in the upper versus lower respiratory tract. Additionally, we demonstrate that modulation of inflammation by a small-molecule antagonist of MIF improves outcomes in pneumococcal pneumonia.
There are parallels between the innate immune responses elicited by the presence of pneumococci in the nasopharynx and the lung. While neutrophils are recruited early to both sites, their relative contribution to bacterial control has been shown to be limited [3, 9]. Mice lacking IL-1 receptor have decreased pulmonary neutrophilia and bacterial dissemination in pneumococcal disease [4]. Previous work in a model of Toxoplasma gondii infection confirms our observation of reduced IL-1β production in the setting of MIF deficiency, but its mechanism remains to be determined [10]. Additionally, neutrophil-mediated lung pathology is associated with failure of pneumococcal control; whether the predominance of neutrophils or enhanced inflammation in the WT animals may be a cause of the greater bacterial growth in these animals as compared to Mif−/− mice remains to be investigated [11].
During colonization, MCP-1/CCL2 upregulation in WT mice correlates with the sustained presence in the nasopharynx of macrophages that are required for pneumococcal clearance, all of which are impaired in MIF deficiency [6, 12]. In pneumonia, CCL2/MCP-1 levels are also higher in the BAL of WT mice compared to Mif−/− mice and are associated with the increased number of monocytes/macrophages in the tissue. MIF may promote the migration and retention of circulating monocytes into tissues in a MCP-1/CCL2-dependent manner, setting up a positive-feedback loop as more cells are recruited [13]. Additionally, MIF is known to prolong the survival of inflammatory cells by inhibiting p53-dependent apoptosis [14]; the enhanced apoptosis we observed in alveolar macrophages may underlie improved outcomes in Mif−/− mice by promoting the resolution of inflammation.
Few host factors have been examined systematically in both pneumococcal colonization and disease. But studies examining the response to the pneumococcal cytolysin, pneumolysin, in host-bacterial interaction provide another example of the differing roles of inflammation in the nasopharynx versus the lung. Pneumolysin-deficient pneumococci are better able to persist in models of carriage because of an attenuated inflammatory response, but they are less virulent in pneumonia, during which they recruit fewer innate immune cells [6, 11].
Our observations of the detrimental role of MIF implicate it as a target for immunomodulation in pneumococcal pneumonia. While therapeutic potential of MIF inhibition has been evaluated for regulating inflammatory pathology in rheumatologic disease, this is the first examination of the use of a small-molecule inhibitor of the cytokine in response to bacterial infection [15]. Future work is needed to determine the timing and duration of MIF inhibition necessary for an optimal impact on the excess morbidity and mortality caused by inflammation in pneumococcal pneumonia.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R01AI05168 [to J. N. W.], R01AI042310 [to R. B.], and K08AI097223 [to R. D.]).
Potential conflicts of interest. W. L. J. and R. B. are coinventors on a patent application describing MIF098 and its therapeutic application. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med 1964; 60:759–76. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Active Bacterial Core Surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae. http://www.cdc.gov/abcs/reports-findings/survreports/spneu10.html Accessed November 2013.
- 3.Marks M, Burns T, Abadi M et al. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect Immun 2007; 75:1586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marriott HM, Gascoyne KA, Gowda R et al. Interleukin-1beta regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages. Infect Immun 2012; 80:1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rijneveld AW, de Vos AF, Florquin S, Verbeek JS, van der Poll T. CD11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. J Infect Dis 2005; 191:1755–60. [DOI] [PubMed] [Google Scholar]
- 6.Das R, LaRose MI, Hergott CB, Leng L, Bucala R, Weiser JN. Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J Immunol 2014; 193:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yende S, Angus DC, Kong L et al. The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia. Faseb J 2009; 23:2403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun H, Choo-Wing R, Fan J et al. Small molecular modulation of macrophage migration inhibitory factor in the hyperoxia-induced mouse model of bronchopulmonary dysplasia. Respir Res 2013; 14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol 2008; 180:6246–54. [DOI] [PubMed] [Google Scholar]
- 10.Flores M, Saavedra R, Bautista R et al. Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J 2008; 22:3661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernatter J, Pirofski LA. Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis 2013; 26:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest 2011; 121:3666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory JL, Morand EF, McKeown SJ et al. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J Immunol 2006; 177:8072–9. [DOI] [PubMed] [Google Scholar]
- 14.Sauler M, Leng L, Trentalange M et al. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2014; 306:L487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol 2013; 33(suppl 1):S72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]