Abstract
The association between oral human papillomavirus 16 (HPV16) DNA load and infection clearance was evaluated among 88 individuals with oral HPV16 infection who were identified within a prospective cohort of 1470 HIV-infected and uninfected individuals. Oral rinse specimens were collected semiannually for up to 5 years. The oral HPV16 load at the time of the first positive test result was significantly associated with the time to clearance of infection (continuous P trends <.01). Notably, clearance rates by 24 months were 41% and 94% in the highest and lowest HPV16 load tertiles (P = .03), respectively. High oral HPV16 load warrants consideration as a biomarker for infection persistence, the presumed precursor of HPV16-associated oropharyngeal cancer.
Keywords: oral HPV, viral load, persistence, oropharyngeal cancer, HIV
Oral human papillomavirus 16 (HPV16) infection causes >85% of HPV-positive oropharyngeal cancers [1], the incidence of which has been increasing over the past several decades in developed countries [2]. Case-control studies estimate oral HPV16 infection to confer a 50-fold increase in the odds of HPV-driven oropharyngeal cancer [3]. In the United States, oral HPV16 infection is uncommon (1% prevalence) in the general population [4], and our preliminary natural history studies indicate that a majority of oral HPV infections clear within 1–2 years [5]. Thus, a single, qualitative, positive test for oral HPV16 infection may be a poor predictor of disease risk.
The persistence of cervical HPV infection is an established surrogate for the risk of cervical dysplasia and cancer, and a high HPV16 load is associated with both the persistence of cervical infection and the risk of cervical dysplasia [6–8]. Oral high-risk HPV load was significantly associated with 6-month infection persistence in a short-term natural history study of infection in HIV-infected individuals [9]. Here we evaluate the association between oral HPV16 load and longer-term infection persistence within a prospective cohort study of individuals with or at risk for HIV infection [5].
METHODS
The study population for the Persistent Oral Papillomavirus Study (POPS) was nested within 2 prospective, multicenter cohort studies of individuals with or at risk for HIV infection in the United States: the Women's Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS) [5]. A total of 1470 enrolled participants were prospectively followed every 6 months for a maximum of 4 years. The study protocol was approved by the MACS/WIHS executive committee and the institutional review board of each participating site. All participants provided written informed consent.
Oral samples were collected semiannually from all POPS participants by use of a 30-second oral rinse and gargle with Scope mouthwash [5]. Oral DNA was isolated by use of a magnetic bead-based automated platform (QIAsymphony SP, Qiagen) as previously described [10]. HPV DNA of 37 types (including HPV16) was detected by polymerase chain reaction (PCR) with PGMY09/11 primer pools that targeted the L1 gene, followed by type specification with the Roche linear array. Samples positive for β-globin were considered evaluable.
Included in this analysis were all 88 POPS participants with at least 1 oral rinse sample positive for HPV16 DNA who also had 1 or more oral rinse samples collected at visits subsequent to the visit at which the HPV16-positive sample was obtained. The HPV16 load in the first positive sample was estimated by TaqMan quantitative PCR (qPCR) targeted to the HPV16 E6 open reading frame as previously described [9, 11, 12]. Reported values are the mean of duplicate samples. To account for possible variations in cellularity between oral rinse samples, the HPV16 load in each sample was standardized by the number of human epethelial cells in that sample as measured by number of a single copy human gene (human endogenous retrovirus ERV-3), as previously described [13], as previously described [9, 12]. Seventeen of the 88 samples that were positive for HPV16 by linear array were negative by qPCR. Differences in sample volume used for linear array (12 µL) and qPCR (2 µL) likely resulted in different assay sensitivities, as previously reported [12]. We assigned a viral load of 1 copy per 100 000 cells for these 17 samples [12].
The relationship between demographic, behavioral, and biological characteristics and HPV16 load were evaluated using Wilcoxon–Mann–Whitney test for medians. In addition, Poisson regression with robust variance was used to evaluate predictors of a higher oral HPV16 load. Variables that were statistically significant (P < .05) in bivariable models and variables considered relevant based on previous literature were included in the multivariable models [5].
Time to oral HPV16 clearance was defined as the time from collection of the first HPV16-positive sample to collection of the first HPV16-negative sample, with tests performed using the Roche linear array. An alternate definition of time to 2 consecutive HPV16-negative test results was also considered. For these analyses, oral HPV16 load was evaluated as a continuous variable and as a categorical variable in tertiles. Clearance rates were estimated using the Kaplan–Meier method and compared by the log-rank test. Hazard ratios were estimated by Cox proportional-hazard models. Approximately 20% of the 88 participants had a missing intermittent visit during the 5 years of follow-up, and we assumed that the HPV test result for the missing visit was the same as that from the previous visit, as previously described [5]. The association between oral HPV16 load and clearance was also considered when stratified by sex, HIV status, and incident/prevalent status of the HPV16 infection, since these variables were suggested to be effect modifiers for some risk factors in previous studies [5]. All statistical tests were 2-sided and considered significant, using an α level of 0.05. Analyses were performed in Stata MP 12.0.
RESULTS
Among the 88 HPV16-positive participants included in this analysis, 53 had oral HPV16 infections that were prevalent at baseline, and 35 had oral HPV16 infections that were incident. The study population included 66 HIV-infected individuals (75.0%) and 22 HIV-uninfected individuals (25.0%). The median and mean (±SD) oral HPV16 load at the first positive visit was 50.2 copies per 100 000 cells (interquartile range [IQR], 0.70–435.14) and 39 760 ± 277 694, respectively.
Associations between demographic and behavioral factors and oral HPV16 load are presented in Table 1. Oral HPV16 loads were significantly higher among prevalent versus incident HPV16 infections (P = .03) and among older individuals (Ptrend =.02). With each 10-year increase in age, the HPV16 load significantly increased by 10.6 HPV16 copies per 100 000 cells. In multivariable analysis, prevalent infection status and older age remained significantly associated with oral HPV16 load above the median (Supplementary Table 1). Other factors that were nonsignificantly associated with a higher oral HPV16 load in multivariable analysis included HIV positivity (adjusted prevalence ratio, 1.64; 95% confidence interval [CI], .91–2.94) and current cigarette use (adjusted prevalence ratio, 1.50; 95% CI, .98–2.28; Supplementary Table 1).
Table 1.
Median Oral Human Papillomavirus Type 16 (HPV16) Load Across Study Characteristics Among the 88 Individuals With Oral HPV16 Infection in the Persistent Oral Papillomavirus Study
| Characteristics of Participants | Subjects, No. | Copies Per 100 000 Cells, No., Median | P for Higher HPV Loada |
|---|---|---|---|
| Type of infection | .03 | ||
| Incident | 35 | 3.1 | |
| Prevalent | 53 | 76.6 | |
| Age, yb | .02 | ||
| ≤45 | 31 | 8.6 | |
| 45–54 | 35 | 74.1 | |
| ≥55 | 22 | 101.0 | |
| Current cigarette smoker | .21 | ||
| No | 37 | 37.3 | |
| Yes | 51 | 65.2 | |
| Sex | .54 | ||
| Male (MACS) | 40 | 66.5 | |
| Female (WIHS) | 48 | 34.8 | |
| Ever had a tonsillectomy | .35 | ||
| No | 62 | 32.3 | |
| Yes | 21 | 48.8 | |
| Not sure | 5 | 28571.4 | |
| Oral sex partners in past 6 months, no. | .56 | ||
| 0 | 57 | 48.4 | |
| 1 | 14 | 15.0 | |
| ≥2 | 17 | 88.9 | |
| HIV status | .11 | ||
| Negative | 22 | 17.0 | |
| Positive | 66 | 57.4 | |
| HIV status, CD4+ T-cell count | .22 | ||
| Negative | 22 | 17.0 | |
| Positive, >500 cells/µL | 31 | 73.8 | |
| Positive, 200–499 cells/µL | 24 | 42.8 | |
| Positive, ≤ 200 cells/µL | 11 | 547.3 |
Abbreviations: HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; WIHS, Women's Interagency HIV Study.
a Calculated using the Mann–Whitney test for medians. Variables were considered as continuous, when possible (ie, age and number of oral sex partners).
b With each 10-year increase in age, the HPV16 load significantly increased by 10.6 HPV16 copies per 100 000 cells.
Among the 88 individuals in this analysis, 57 (65%) had ≥7 follow-up visits. The median number of visits was 8 (IQR, 5–9 visits). The median follow-up time for participants with oral HPV16 infections in this study was 35.7 months (IQR, 17.4–48.5 months; 42.3 months for prevalent infections, and 25.0 months for incident infections). Sixty-three of the 88 infections were observed to clear at any point during the 5 years of observation. The 12-month clearance rate for all HPV16 infections was estimated to be 57% (95% CI, 47%–68%), with a higher clearance rate for incident than prevalent infections (77% vs 44%, respectively; P = .01). When clearance was defined by 2 consecutive negative test results, the 12-month clearance rate declined to 45% (95% CI, 35%–57%).
The Oral HPV16 load for the first specimen that tested positive was significantly associated with the time to clearance of infection (continuous Ptrend <.01). Kaplan–Meier curves for clearance, stratified by oral HPV16 infection tertile, are shown in Figure 1. Clearance rates were significantly higher for oral HPV16 infections in the lower viral load tertiles (log-rank P < .001). Estimated 12-month clearance rates were 26% (95% CI, 12%–44%), 67% (95% CI, 50%–82%), and 83% (95% CI, 65%–94%) for the highest, intermediate, and lowest tertiles, respectively. This trend continued: 41% of infections in the highest viral load tertile, compared with 94% in the lowest tertile, cleared within 24 months (P = .03). After adjustment for other factors, infections in the highest HPV16 load tertile were 4 times less likely to clear than infections in the lowest tertile (adjusted hazard ratio, 0.24; 95% CI, .11–.56; Supplementary Table 2). Results were also similar when defining clearance on the basis of 2 negative test results (Supplementary Table 1) and when restricting to the 71 samples that were detectable by qPCR (continuous Ptrend <.01; Supplementary Table 2). Among the 17 individuals who were HPV16 positive by linear array but negative by qPCR, 14 (82%) had HPV16 infections that cleared within 12 months, which was similar to the clearance rate among those who had a lower viral load (1.0–6.0 copies per 100 000 cells), from whom 73% had infections that cleared within 12 months. Analysis within subgroups revealed increasing tertile of oral HPV16 load to be consistently associated with lower clearance among incident and prevalent infections, among men and women, and among HIV-infected individuals and HIV-uninfected individuals (all Ptrend <.05).
Figure 1.
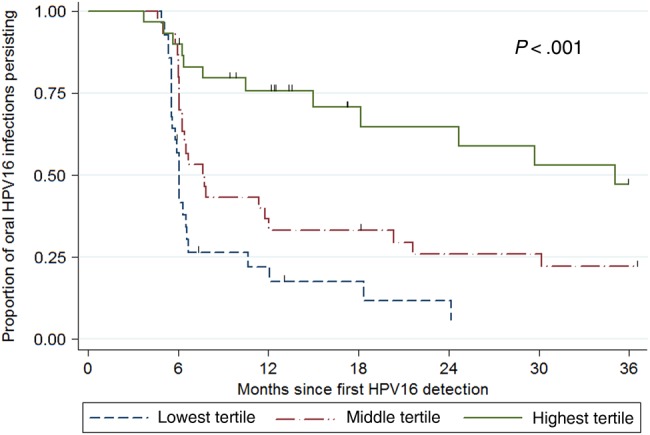
Kaplan–Meier curves for clearance of human papillomavirus virus type 16 (HPV16), by oral HPV16 infection tertile. *Oral HPV clearance defined at first visit when oral HPV16 DNA was not detected. ˆTertile ranges: Lowest tertile 1.0–5.9 copies per 100 000 cells; Middle tertile: 6.0–155.3 copies per 100 000 cells; Highest tertile: >155.3 copies per 100 000 cells. The P-trend was calculated using the log-rank test.
DISCUSSION
In this study of the long-term natural history of oral HPV16 infection in higher-risk HIV-infected adults and HIV-uninfected adults, we observed oral HPV16 load to be strongly associated with infection persistence, as over half of oral HPV16 infections in the highest tertile of viral load persisted for at least 24 months. Oral HPV load therefore warrants further investigation as a biomarker for longer-term persistence and the risk of HPV16-positive oropharyngeal cancer.
This study supports and helps elucidate previous findings from oral HPV natural history studies that suggested older age, prevalent infection, and current cigarette smoking to increase the risk of oral HPV16 persistence [5, 14]. Our study, along with another from the US-based National Health and Nutrition Examination Survey (NHANES) [12], suggest that these factors are associated with higher oral HPV16 load. Thus, we hypothesize that aging and cigarette smoking may lead to poorer immunological control and greater replication of oral HPV16 infections, which in turn leads to longer oral HPV16 persistence. We previously suggested that males are also potentially at increased risk of oral HPV persistence, compared with females [5], but unlike the NHANES analysis [12], we did not observe a higher oral HPV load in males. However, we should note that men and women in this study came from different study populations that represent higher-risk communities and that most of the men in the study were men who have sex with men [5].
While a majority of the study participants with a higher oral HPV16 load had HPV16 infections that persisted for multiple years, no participants in this study received a diagnosis of oropharyngeal cancer. Preliminary studies of the oral region have been unable to identify an HPV-positive precancerous lesion, limiting the potential for developing screening modalities [15]. At the same time, this lack of identifiable oropharyngeal precancerous lesions increases the need for biomarkers capable of identifying HPV infections that are most likely to progress to cancer. The relatively low incidence of HPV-positive oropharyngeal cancer (approximately 4 cases per 100 000) [2] suggests that very sensitive and specific markers would be needed for any potential screening modality to be beneficial. So while a high viral load appears to be a considerably better predictor of oral HPV16 persistence than oral HPV16 DNA, viral load alone is likely too limited in specificity, given that approximately 40% of the infections with a higher viral load cleared within 2 years. Given this limited specificity, biomarkers such as HPV16 methylation and HPV16 E6 seropositivity should be further evaluated to potentially complement oral HPV16 load.
In summary, a higher oral HPV16 load is strongly associated with multiyear oral HPV16 persistence in a population of individuals with or at risk for HIV infection. While this is the first long-term study to evaluate oral HPV16 load and oral HPV16 persistence, this study is limited by a 1-time viral load measurement, a limited number of oral HPV16 infections, and a lack of assessment for oral precancerous lesions. Further investigation is necessary to better understand the role of the oral HPV16 load in the natural history of oral HPV infection and the risk of clinical progression, particularly in HIV-uninfected individuals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The Multicenter AIDS Cohort Study (MACS) centers in the Persistent Oral Papillomavirus Study (POPS) are funded by U01-AI35042 (Johns Hopkins University–Margolick), U01-AI35039 (Northwestern University–Wolinsky), and U01-AI35041 (University of Pittsburgh–Rinaldo). The MACS DMAC at Johns Hopkins University is funded by UM1-AI35043 (Jacobson). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). The Women's Interagency HIV Study (WIHS) centers in the POPS are funded by U01-AI-031834 (Brooklyn–Minkoff and Gustafson), U01-AI-034993 (Chicago–Cohen), and U01-AI-034993 (Washington–Young). The WIHS DMAC is funded by U01-AI-042590 (Gange and Golub).
Financial support. This work was supported by the National Institute of Dental and Craniofacial Research (R01 DE021395 and related supplement to G. D. [principal investigator]), the National Institute of Allergy and Infectious Disease (U01-A1-35043), and the National Cancer Institute (T32 CA CA009314 and cancer prevention fellowship to D. C. B.).
Potential conflicts of interest. G. D., R. D. C., and D. J. W. have/had research support from Merck. D. J. W. is a member of the speakers bureau for Merck. M. L. G. has been a consultant for Merck and GSK. R. D. C. also reports institutional grant funding and royalties from UptoDate (on HPV-related topics). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14:467–75. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, D'Souza G, Westra W et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100:407–20. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Broutian T, Pickard RK et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012; 307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beachler DC, Sugar EA, Margolick JB et al. Risk factors for oral HPV infection acquisition and clearance among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2015; 181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravitt PE, Kovacic MB, Herrero R et al. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J Cancer 2007; 121:2787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi LF, Hughes JP, Castle PE et al. Viral load in the natural history of human papillomavirus type 16 infection: a nested case-control study. J Infect Dis 2011; 203:1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontaine J, Hankins C, Money D et al. Human papillomavirus type 16 (HPV-16) viral load and persistence of HPV-16 infection in women infected or at risk for HIV. J Clin Virol 2008; 43:307–12. [DOI] [PubMed] [Google Scholar]
- 9.Fakhry C, Sugar E, D'Souza G, Gillison M. Two-week versus six-month sampling interval in a short-term natural history study of oral HPV infection in an HIV-positive cohort. PLoS One 2010; 5:e11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol 2011; 50:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt PE, Burk RD, Lorincz A et al. A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev 2003; 12:477–84. [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Graubard BI, Pickard RK, Xiao W, Gillison ML. High-risk oral HPV load in the US population, NHANES 2009–2010. J Infect Dis 2014; 210:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods 2001; 91:109–17. [DOI] [PubMed] [Google Scholar]
- 14.Pierce Campbell CM, Kreimer AR, Lin HY et al. Long-term persistence of oral human papillomavirus type 16: the HPV infection in men (HIM) study. Cancer Prev Res (Phila) 2015; 8:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhry C, Rosenthal B, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “Pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila) 2011; 4:1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


