Abstract
Background. Data on effectiveness of preexposure prophylaxis (PrEP) for human immunodeficiency virus (HIV)–uninfected women attempting conception with HIV-infected male partners are limited to observational studies.
Methods. To explore the benefits of PrEP for conception, we developed a model to estimate the average annual probability of a woman remaining HIV-uninfected and having a child (“successful” outcome) via condomless sex with an HIV-infected male. The outcome likelihood is dependent upon parameters defining HIV-1 infectivity. We simulated 2 scenarios: optimal (condomless sex acts limited to the ovulation window), and suboptimal (acts not limited to ovulation).
Results. In the optimal scenario when the male is on antiretroviral therapy (ART), the average annual probability of the successful outcome is 29.1%, increasing to 29.2% with the addition of PrEP (P = .45). In the suboptimal scenario, the probability is 26.8% with ART alone versus 27.3% with ART/PrEP (P < .0001). Older maternal age reduces the probability of success in both scenarios, particularly after age 30.
Conclusions. In our model, PrEP provides little added benefit when the HIV-infected male partner is on ART, condomless sex is limited to the ovulation window, and other modifiable transmission risks are optimized. Older female age decreases the probability of success by increasing the number of condomless sex acts required for conception.
Keywords: antiretroviral therapy, mathematical model, preexposure prophylaxis, safer conception
(See the editorial commentary by Ciaranello and Matthews on pages 1525–8.)
Between 20% and 75% of human immunodeficiency virus (HIV)–infected individuals desire children [1–4] and serodiscordant couples engage in condomless sex in order to conceive, placing the HIV-uninfected partner at substantial risk of HIV acquisition [5]. For couples in which the female partner is HIV-infected, self-insemination during the window of ovulation can be utilized at low cost, with no risk of HIV transmission to the uninfected male partner. However, for HIV-infected men wishing to conceive with a negative female partner, the most effective risk reduction method of sperm washing plus intrauterine insemination or in vitro fertilization is often cost prohibitive or may be unavailable, depending on the setting [6]. A less costly menu of risk reduction options has been proposed, including screening and treating sexually transmitted infections (STIs) in both partners, limiting condomless sex to the window of ovulation, placing the HIV-infected partner on antiretroviral therapy (ART), and using preexposure prophylaxis (PrEP) for the HIV-uninfected female partner [7–9].
While PrEP with the fixed-dose formulation of tenofovir disoproxil fumarate (tenofovir-DF) and emtricitabine has proven to be efficacious in a variety of clinical studies, including heterosexual women and men in Africa [10–12], data on the use of PrEP for conception have been limited to small observational studies and expert opinion [13–15]. The Department of Health and Human Services Panel on Treatment of HIV-Infected Pregnant Women lists PrEP as an option for conception with a CIII rating (optional recommendation based on expert opinion) [16] and the Centers for Disease Control has released guidance on PrEP for conception, but raises concern about lack of safety data in HIV-uninfected women in early pregnancy [17]. In randomized clinical trials of PrEP, women becoming pregnant have been immediately taken off study drug [11, 12], limiting available data on safety [18]. Data on the benefit of PrEP for conception when other evidence-based prevention interventions are also used would be helpful for clinicians counseling couples about safer conception, as well as for resource-limited countries developing policies around the availability of PrEP.
In response to the lack of published data about transmission risk in HIV-infected men trying to conceive with HIV-uninfected female partners, we developed a mathematical model to explore the relative benefits of ART and PrEP each in isolation and in combination across different simulated clinical scenarios. Our objective was to determine how the addition of PrEP to ART influences 2 key outcomes of interest to clinicians and serodiscordant couples: (1) the average annual probability of the “successful” outcome of the female partner remaining HIV-uninfected and having a child, and (2) the annual probability of the “unsuccessful” outcome of HIV acquisition by the female partner, without a successful pregnancy. We sought to understand the probabilities of these 2 outcomes in an “optimal” scenario defined by condomless sex limited to the ovulation window versus a “suboptimal” scenario defined by condomless sex acts distributed indiscriminately over an average 1-month menstrual cycle. We also explore the role of maternal age on the outcomes of interest.
METHODS
We developed a probabilistic model to estimate the likelihoods of possible outcomes defined in terms of HIV infection, pregnancy, and delivery when an HIV-uninfected woman engages in condomless sex with an HIV-infected male partner. The likelihoods depend on biological parameters that define HIV infectivity, age-based female fertility (eg, conceiving and delivering a child), and the frequency of condomless sex over an average menstrual cycle (28 days). We implemented the probabilistic model in both the statistical software R [19] and Microsoft Excel. The implementation in R automates the performance of many simulations, whereas the Excel tool is interactive such that a user can select desired parameter values and intervention options.
Model Outcomes
The mathematical model examined outcomes for women aged 18 to 49 across multiple different outcomes related to the infection status of the woman and the infection status of the child if the female becomes HIV-infected. The model produces 5 possible outcomes:
Female remains HIV-uninfected and has a child.
Female becomes HIV-infected and does not have a child.
Female becomes HIV-infected and has an HIV-uninfected child.
Female becomes HIV-infected and has an HIV-infected child.
Female remains HIV-uninfected but does not have a child.
The majority of the results and discussion are focused on 2 outcomes based on their relevance to clinicians counseling serodiscordant couples desiring safer conception: (1) the “successful” outcome of a female remaining HIV-uninfected and having a child, and (2) the “unsuccessful” outcome of a female becoming HIV-infected and not conceiving. For outcome equations, please refer to Section 3.2 of the Supplementary Technical Appendix.
We represented the inputs for the outcome probabilities on a per-sex-act basis and obtained a monthly probability by raising the per-sex-act inputs to the number of coital acts over a 30-day period (an approximate 1-month menstrual cycle). We converted the monthly outcomes to average annual probabilities as outlined in section 3.3 of the Supplementary Technical Appendix.
Transmission Risk Scenarios Based on Frequency of Condomless Sex
We estimated the model outcomes under 2 different scenarios based on opposite ends of the spectrum of condom use behavior: (1) “optimal” transmission risk scenario based on an adherent serodiscordant couple exhibiting ideal behavior after counseling by a clinician, limiting condomless sex to an approximate 3-day window (ovulation day and 2 previous days) when conception would be most likely to occur [20, 21] (range of 1–12 sex acts, with peak sampling clustered around 3); and (2) “suboptimal” scenario in which a couple, despite counseling by a clinician about timing ovulation, does not adhere to the strategy and engages in condomless sex uniformly across an approximate menstrual cycle (1–60 sex acts sampled most frequently around 15).
Model Parameters
The model inputs define overall HIV transmissibility and the probability of successfully conceiving and delivering a child (Table 1). Additional parameter details can be found in Section 4.3 of the Supplementary Technical Appendix.
Table 1.
Model Input Parameters
| Estimate (per condomless sex act) | Range | Distribution | Notes | References | |
|---|---|---|---|---|---|
| Base Transmission Rate | |||||
| Male-female transmissibility per condomless sex act | 0.0022 | 0.0010–0.0031 | Beta-PERT | Assumes chronic infection | [22–24] |
| Multiplicative factors | |||||
| Transmissibility in late stage/advanced HIV infection, characterized by uncontrolled viral replication | 1.82 | 1.29–2.35 | Uniform | Only applies if male not on ART | [25, 26] |
| Male on antiretroviral therapy | 0.04 | 0.01–0.27 | Beta-PERT | Assumes male is adherent and suppressed | [27] |
| Female on preexposure prophylaxis | 0.34 | 0.16–0.72 | Beta-PERT | Assumes female is adherent to PrEP | [11] |
| MTCTa | |||||
| Probability of MTCT when the female becomes HIV-infected while trying to conceive | 0.26 | 0.18–0.33 | Uniform | Only applies if female becomes HIV-infected during attempts at conception and the pregnancy results in a child | [28, 29] |
| Multiplicative factor applied to MTCT when the female becomes pregnant while trying to conceive and is started on ART at the time of HIV diagnosis and for the duration of her pregnancy | 0.33 | 0.18–0.59 | Uniform | Only applies if female becomes HIV-infected during attempts at conception and the pregnancy results in a child | [30–34] |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MTCT, mother-to-child transmission; PrEP, preexposure prophylaxis.
a To obtain the probability of MTCT while the mother is on ART, the MTCT probability is multiplied by the multiplicative factor.
The reference condition is a male with chronic HIV infection who is not on ART and an HIV-uninfected woman not on PrEP, with both partners screened and treated for STIs. The calculation of the overall HIV transmissibility, , begins with the base male-to-female HIV transmissibility per condomless sex act, α, during chronic HIV infection [22–24]. Three conditions can increase or decrease the base transmissibility:
The male is not on ART and has late-stage/advanced HIV infection, characterized by uncontrolled viral replication, hL [25, 26].
The male is on ART and adherent with suppressed viral load, hART [27].
The female is on PrEP and adherent, hPrEP [11].
These parameters represent binary variables (either “off” or “on”), and when “on,” either multiplicatively increase (hL) or decrease (hART and hPrEP) the base transmissibility, such that the overall transmissibility is: . A nonapplicable condition turns the associated parameter “off” (ie, equaling “1”) and does not affect the overall transmissibility. For evaluating the role of ART with and without PrEP, we assumed all STIs were treated (ie, hSTIs = 1). Untreated STIs have a wide range of effect on model outcomes and are discussed separately in the Supplementary Technical Appendix (Section 5.2).
The likelihood of conceiving and successfully delivering a child depends on age-based female fertility. In the model, this was represented by the probability of successfully delivering a child pd(a), given that pregnancy occurs pc(a). To be consistent with the overall HIV transmissibility parameter, the probability of successfully conceiving and delivering a child (ie, pc(a) * pd(a)) is represented on a per-condomless sex-act basis (conversion outlined in Section 4.4 of the Supplementary Technical Appendix).
The likelihood that HIV is transmitted from the mother to child (MTCT) if the female becomes HIV-infected during conception is represented by pMTCT [28, 29]. While attempting conception, we assume HIV testing will be performed monthly, and therefore infection will be diagnosed expeditiously and ART initiated early in pregnancy. The probability that MTCT is reduced when the female is started on ART is represented in the model by the multiplicative factor hTxMTCT [30–34].
Uncertainty Analysis
To assess the uncertainty inherent in the model inputs, we identified a range of reasonable values and an appropriate distribution for each parameter and simulated over many parameter combinations. We obtained 10 000 sets of parameter combinations using Latin hypercube sampling, in which we sampled parameters within their ranges as specified in Table 1. We then evaluated all 10 000 parameter sets across permutations of the model inputs, resulting in 160 000 independent scenarios. The independent scenarios generated a range of probabilities for the outcomes of interest.
Clinical Interventions Modeled
We compared averages of the outcome ranges for the clinical interventions as follows:
No PrEP or ART: Female not taking PrEP and male not taking ART (hPrEP off, hART off).
PrEP only: Female taking PrEP and male not taking ART (hPrEP on, hART off).
ART only: Female not taking PrEP and male taking ART (hPrEP off, hART on).
PrEP and ART: Female taking PrEP and male taking ART (hPrEP on, hART on).
We compared the range of annual outcome probabilities in 2 ways: (1) comparing a single clinical strategy to all other strategies, and (2) within a single strategy (eg, PrEP only) for the 2 transmission risk scenarios defined by the frequency of condomless sex (optimal vs suboptimal). Pairwise testing between clinical strategies as well as between the optimal and suboptimal scenarios was performed.
RESULTS
Overall Model Results
Table 2 shows average annual probabilities across all simulations for the 5 model outcomes where the sum of the outcomes for a given intervention is 100%. Across all interventions and scenarios, the most likely outcome is the female remaining HIV-uninfected and not having a child (ranging from 53.8% to 72.0%), while the successful outcome is next most common, with the female remaining HIV-uninfected and having a child (17.8% to 29.2%). Outcomes in which the female becomes HIV-infected are uncommon, with the exception of the unsuccessful outcome in the suboptimal scenario when no ART or PrEP is used (annual probability 29.5%).
Table 2.
Aggregates of the Five Model Outcomes Presented by Intervention for the Optimal and Suboptimal Scenarios. Values Represent Average Annual Probabilities (0%–100%) Across All Simulations
| Outcome | Optimal |
Suboptimal |
||||||
|---|---|---|---|---|---|---|---|---|
| No ART or PrEP | PrEP | ART | ART + PrEP | No ART or PrEP | PrEP | ART | ART + PrEP | |
| Female remains HIV-uninfected and has a child (successful outcome) | 26.9 | 28.3 | 29.1 | 29.2 | 17.8 | 23.1 | 26.8 | 27.3 |
| Female becomes HIV-infected and does not have a child (unsuccessful outcome) | 7.1 | 2.7 | 0.4 | 0.1 | 29.5 | 13.2 | 2.1 | 0.8 |
| Female becomes HIV-infected and has an HIV-uninfected child | 2.0 | 0.8 | 0.1 | <0.1 | 8.0 | 3.6 | 0.6 | 0.2 |
| Female becomes HIV-infected and has an HIV-infected child | 0.4 | 0.2 | <0.1 | <0.1 | 1.7 | 0.8 | 0.1 | <0.1 |
| Female remains HIV-uninfected but does not have a child | 66.3 | 69.0 | 70.5 | 70.6 | 53.8 | 64.2 | 71.2 | 72.0 |
Values are rounded to the tenth and are the averages across all simulations. While the 5 outcomes sum to 100% for each individual simulation, over all simulations (at the aggregate level), the outcomes will not sum exactly to 100%.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MTCT, mother-to-child transmission; PrEP, preexposure prophylaxis.
Modeling the Optimal Transmission Risk Scenario for the Successful and Unsuccessful Outcomes
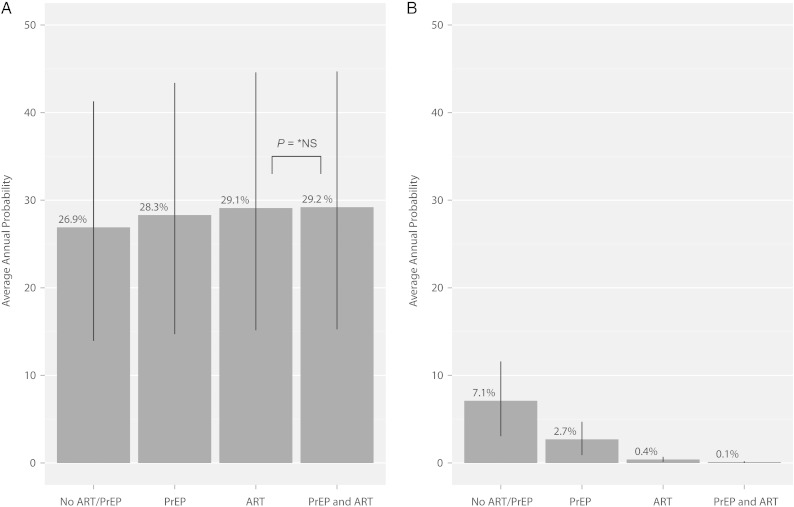
Figure 1A illustrates the average annual probability of the “successful” outcome in which the female partner remains HIV-uninfected and has a child in the optimal transmission risk scenario where the serodiscordant couple is able to adhere to the strategy of limiting condomless sex to the 3-day window when conception would be most likely to occur. With no intervention, the average annual probability of the successful outcome is 26.9%. With female PrEP use alone, the probability increases to 28.3%, while with male ART use alone it increases to 29.1%. In the most intensive scenario with the male on ART and the female on PrEP, the probability increases to 29.2%, with no significant difference between the ART versus the ART/PrEP combination approach (P = .45). All other pairwise comparisons are statistically significant at the P < .0001 level, with absolute differences in probabilities ranging from 0.8% to 2.3%.
Figure 1.
Average annual probabilities of successful and unsuccessful outcomes in the optimal scenario (condomless sex is limited to the window of ovulation) for each of the 4 clinical interventions modeled. A, Probability of a successful outcome (female remains HIV-uninfected and has a child). B, Probability of an unsuccessful outcome (female becomes HIV-infected and does not have a child). Within each panel, all pairwise comparisons of means are statistically different at the P < .0001 level except for ART compared to PrEP and ART in the successful outcome (29.1% versus 29.2%) Lines represent one standard deviation above and below the mean. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; *NS, nonsignificant; PrEP, preexposure prophylaxis.
We also evaluated the average annual probability of the “unsuccessful” outcome of the female becoming HIV-infected in attempts at conception but not having a child in the optimal scenario (Figure 1B). The average annual probability of the unsuccessful outcome is 7.1% with neither ART nor PrEP, 2.7% with PrEP alone, 0.4% with ART alone, and 0.1% with ART plus PrEP. All pairwise comparisons in this scenario are statistically significant at the P < .0001 level, with the absolute difference between ART alone and ART plus PrEP of 0.3%.
Modeling the Suboptimal Risk Transmission Scenario for the Successful and Unsuccessful Outcomes
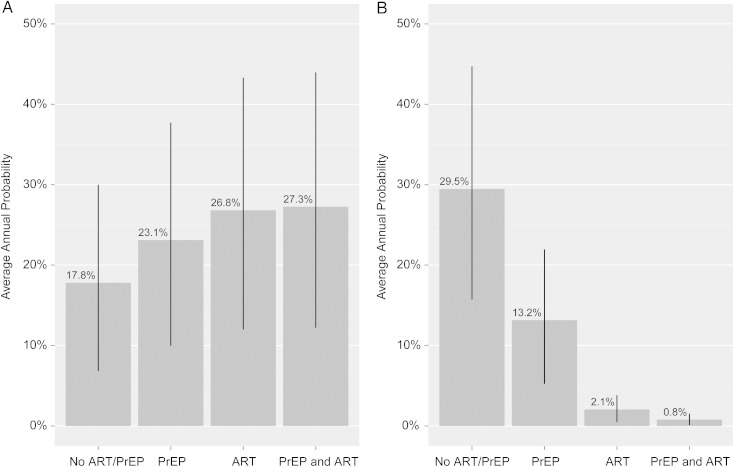
Figure 2A shows the average annual probability of the “successful” outcome in the suboptimal transmission risk scenario when the serodiscordant couple is not adherent to the safer conception strategy of timing ovulation and condomless sex occurs throughout the approximate 28 days of the menstrual cycle. When the male partner is not on ART and the female is not on PrEP, the average annual probability of the successful outcome is 17.8%. This increases to 23.1% with the use of female PrEP alone and further increases to 26.8% when the male is on ART alone. With the combination of PrEP and ART, the probability is increased to 27.3%. All pairwise comparisons between clinical strategies are statistically significant (P < .0001) with a difference between ART alone and PrEP plus ART of 0.5%.
Figure 2.
Average annual probabilities of successful and unsuccessful outcomes in the suboptimal scenario (condomless sex occurs throughout the month and is not limited to the window of ovulation) for each of the 4 clinical interventions modeled. A, Probability of a successful outcome (female remains HIV-uninfected and has a child). B, Probability of an unsuccessful outcome (female becomes HIV-infected and does not have a child). Within each panel, all pairwise comparisons of mean are statistically different at the P < .0001 level. Lines represent one standard deviation above and below the mean. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis.
We also evaluated the average annual probability of the “unsuccessful” outcome of the female becoming HIV-infected in attempts at conception but not ultimately having a child in the suboptimal transmission risk scenario (Figure 2B). The annual probability of this outcome is 29.5% with neither ART nor PrEP, 13.2% with PrEP alone, 2.1% with ART alone, and 0.8% with ART plus PrEP. All pairwise comparisons are statistically significant at the level of P < .0001.
Comparing Optimal and Suboptimal Scenarios: The Role of Adherence to the Safer Conception Strategy in Determining Model Outcomes
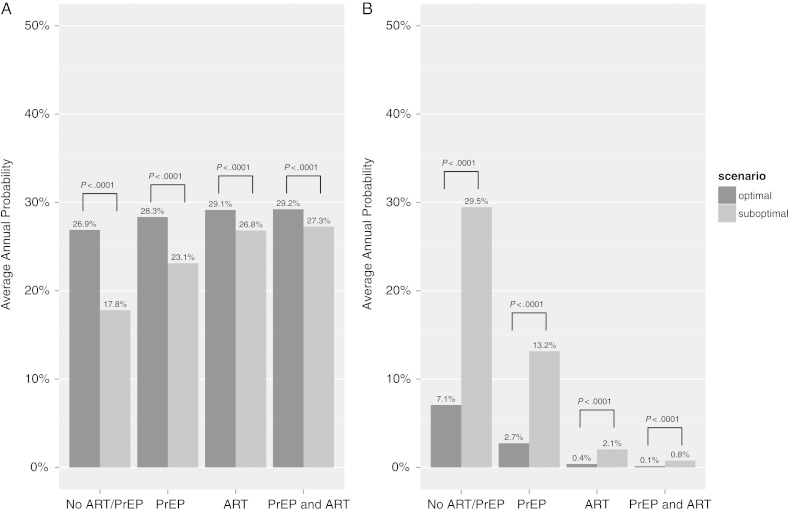
When comparing probabilities in the optimal versus suboptimal scenario for the “successful” outcome (Figure 3A), the probability is lower in the suboptimal scenario across all clinical interventions, with most notable differences when neither ART nor PrEP is utilized (26.9% in the optimal vs 17.8% in the suboptimal; P < .0001) and for PrEP alone (28.3% in the optimal vs 23.1% in the suboptimal; P < .0001). With the ART strategy, there is a small absolute difference in the probability of the successful outcome regardless of time-limited condomless sex (2.3% higher outcome probability in the optimal scenario; P < .0001). A slightly smaller absolute difference is seen for the ART/PrEP combination strategy (1.9% higher in the optimal scenario; P < .0001).
Figure 3.
Comparison of average annual probabilities in the optimal (condomless sex limited to the window of ovulation) versus suboptimal (condomless sex occurs throughout the month) scenarios for each of the 4 clinical interventions modeled. A, Comparison of optimal (dark gray bars) versus suboptimal (light gray bars) scenarios for the annual probability of the successful outcome of the female remaining HIV-uninfected and having a child. B, Comparison of optimal (dark gray bars) versus suboptimal (light gray bars) scenarios for the annual probability of the unsuccessful outcome of the female becoming HIV-infected and not having a child. Within each panel, the comparison between optimal and suboptimal for each strategy is statistically significant at the P < .0001 level. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis.
We used this same strategy to compare optimal and suboptimal scenarios for the “unsuccessful” outcome (Figure 3B). Compared to the “successful” outcome, there are larger differences between no intervention (7.1% in the optimal vs 29.5% in the suboptimal; P < .0001) and PrEP alone (2.7% in the optimal vs 13.2% in the suboptimal; P < .0001). The absolute difference for ART alone is 1.7% higher in the optimal scenario, and for ART plus PrEP is 0.7% higher in the optimal scenario.
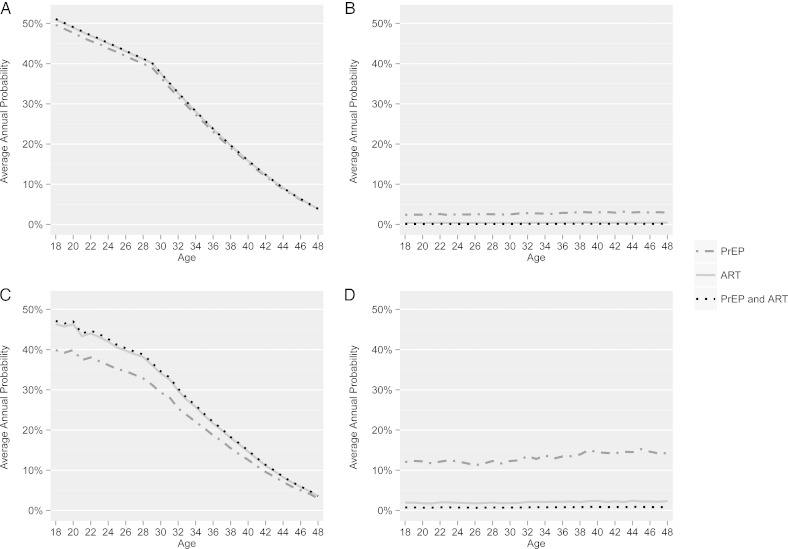
The Role of Maternal Age on Outcomes
In the optimal scenario, the probability of the “successful” outcome declines steadily by age, with an inflection point at age 30, dropping to approximately 15% by age 40 (Figure 4A). In this optimal scenario/successful outcome, the additive benefit of PrEP for the female when the male partner is on ART is minimal, as represented by almost entirely overlapping trajectories. Within the same optimal scenario for the “unsuccessful” outcome (Figure 4B), the probabilities of the female becoming HIV-infected and not having a child remain very low throughout the age spectrum when ART is used alone and when PrEP is added to ART (<1% for both clinical interventions).
Figure 4.
The annual probability of each outcome (successful and unsuccessful) for optimal and suboptimal scenarios when different clinical interventions are modeled (PrEP vs ART vs PrEP and ART), by maternal age. A, Optimal scenario, successful outcome. B, Optimal scenario, unsuccessful outcome. C, Suboptimal scenario, successful outcome. D, Suboptimal scenario, unsuccessful outcome. Abbreviations: ART, antiretroviral therapy; PrEP, preexposure prophylaxis.
For the suboptimal scenario, the same strong association of age on the “successful” outcome is shown (Figure 4C), however with more marked differences between the PrEP alone versus ART alone and ART plus PrEP groups. Similarly, for the “unsuccessful” outcome, the average annual probabilities of the female becoming HIV-infected and not having a child increase, with a marked risk for women on PrEP alone over all ages (Figure 4D), especially as compared to the optimal scenario (Figure 3B).
DISCUSSION
Given the magnitude of benefit of ART in prevention of HIV transmission in serodiscordant couples in HIV Prevention Trials Network 052 (96% risk reduction) [27], we sought to characterize the additive benefit of PrEP for safer conception when all other modifiable risk factors are optimized, including the male partner on suppressive ART, STIs treated in both partners, and condomless sex limited to the ovulation window. Our model was designed to highlight outcomes we felt would be of greatest interest to clinicians and serodiscordant couples who desire natural conception. We had a specific interest in the composite outcome of HIV status of the mother and successful pregnancy and delivery because of the importance of age in these determining outcomes.
The model was also utilized to determine outcomes based on real-world behaviors of serodiscordant couples adhering versus not adhering to the safer conception strategy of condom use with timed ovulation. Ultimately, the model highlights that limiting condomless sex to the window of ovulation drastically reduces the risk of HIV transmission such that the added benefit of PrEP for the female partner is negligible (0.1%) when this behavioral intervention is used, the partner is suppressed on ART, and STIs are treated in both partners. When condomless sex occurs continuously, the model demonstrates the added benefit of PrEP when the male partner is on ART; however, in this scenario, condomless sex throughout an average 1-month menstrual cycle increases the risk of HIV transmission without increasing the probability of conception. This framework may be helpful for patient–provider discussions to illustrate how the number of condomless sex acts drives the ultimate outcome for couples desiring natural conception, and may help serodiscordant couples understand the importance of adherence to timing ovulation. This context may also be helpful to modify the transmission risk behavior that occurs outside of periods of attempted conception. As a result of our modeling exercise, we are developing a tool that can be used during the patient encounter to illustrate how different clinical scenarios change the risk for HIV transmission. Adjusting the number of condomless sex acts and utilizing the tool to show a “dashboard-style” risk assessment may help to motivate behavior change and/or adherence to prevention strategies.
When considering PrEP as a safer conception strategy, serodiscordant couples should be counseled on the existing safety data of tenofovir-DF/emtricitabine in the setting of conception and pregnancy. While experience in HIV-infected women does not suggest an increased risk of congenital anomalies, the conversation should specifically weigh the possible risks in light of any benefits of PrEP in an otherwise optimized scenario. In settings where PrEP is not available or may be available only with considerable financial hardship, the model outputs are helpful to show that inexpensive interventions can have a substantial impact on risk reduction. In these resource-limited settings, consideration should be given to investment in ART for HIV-infected men who desire to conceive with their partners, even if their CD4 lymphocyte cell count and clinical stage would not otherwise qualify these individuals for treatment initiation based on country-specific guidelines.
Our analysis highlights the importance of younger maternal age on the annual probability of the desired outcome of remaining HIV-uninfected and successfully having a child. In our model, older maternal age reduced the probability of a successful outcome largely by increasing the number of condomless sex acts required for conception, particularly above age 30. However, additional factors play a role in reducing the probability of successful pregnancy and delivery at an older female age, including increased rates of miscarriage and increased risk for complications that result in pregnancy loss [35–37]. We accounted for this in our model by defining “success” as being able to conceive and carry pregnancy to term. While HIV providers are often not trained in reproductive health topics related to conception and pregnancy complications and may have a limited understanding of the role of age and fertility, increased emphasis should be placed on early discussion of childbearing intentions with HIV-infected patients. Discussions about safer conception strategies and the role of age in achieving safest conception are critically important in facilitating informed decisions.
Limitations
We used a simple probabilistic model using available data at the time the model was created. P-values obtained from the model must be interpreted with caution, as statistical significance does not equate with clinical significance, and we find small differences to be statistically significant due to the number of simulations performed. In the above discussion, we have presented our views on the clinically significant lessons learned from this model and believe that, ultimately, providers need to individualize decisions about safer conception utilizing this model alongside existing and evolving clinical data, patient preferences, and resource availability.
We made several assumptions, including that HIV-infected men would have semen analysis prior to attempts at conception (as per DHHS Guidelines) [16], HIV-infected male partners using ART would be adherent and fully suppressed, women would have a basic evaluation of fertility before attempts at conception, and when using PrEP, women would be adherent. Finally, we estimated the range of possible sex acts for both the optimal and suboptimal scenarios and sampled most frequently around a number we felt may represent average behavior in each scenario. Real-world behavior may be different than modeled ranges, resulting in different outcome probabilities.
Conclusions
Based on our model inputs, PrEP for an HIV-seronegative female partner attempting conception with an HIV-infected male partner provides little added benefit when the male is on ART, STIs are diagnosed and treated in both partners, and condomless sex is limited to the window of ovulation. Our model highlights that younger maternal age is associated with the desired outcome of successful conception and delivery, and raises the importance of proactive conversations with HIV-infected patients and partners about childbearing desires. Our data are reassuring in that, provided ART is available for the HIV-infected male partner, serodiscordant couples can have desired results without the addition of female PrEP for conception, as long as they are motivated to optimize modifiable risk factors.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge the assistance of Dr Sally Blower in the original development of the model. We thank Amber Smith for her assistance with data collection and Dr Mousa Shamonki for sharing his expertise on reproductive endocrinology. We additionally thank the University of California, Los Angeles (UCLA) Center for AIDS Research and UCLA Center for HIV Identification, Prevention, and Treatment Services for providing infrastructure and support that has made this research possible.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (grant number R01HD072633); the National Institute of Allergy and Infectious Diseases (grant numbers K24AI56933, 5P30AI028697); the National Institute of Mental Health via the UCLA Center for HIV Identification, Prevention, and Treatment (grant number MH58107); and the National Center for Advancing Translational Sciences through the UCLA Clinical and Translational Science Institute (CTSI) (grant number UL1TR000124).
Potential conflicts of interest. J. E. L. has served as a consultant to Glaxo Smith Kline and has received travel and honoraria for advisory board participation from Gilead Sciences; R. L. reports receiving drug-only support for a clinical research study, travel, and honoraria for advisory board participation from Gilead Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Stanwood NL, Cohn SE, Heiser JR, Pugliese M. Contraception and fertility plans in a cohort of HIV-positive women in care. Contraception 2007; 75:294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finocchario-Kessler S, Sweat MD, Dariotis JK et al. Understanding high fertility desires and intentions among a sample of urban women living with HIV in the United States. AIDS Behav 2010; 14:1106–14. [DOI] [PubMed] [Google Scholar]
- 3.Cooper D, Moodley J, Zweigenthal V, Bekker LG, Shah I, Myer L. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav 2009; 13(suppl 1):38–46. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz SR, Mehta SH, Taha TE, Rees HV, Venter F, Black V. High pregnancy intentions and missed opportunities for patient-provider communication about fertility in a South African cohort of HIV-positive women on antiretroviral therapy. AIDS Behav 2012; 16:69–78. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker SG, Bukusi EA, Odoyo J, Achando J, Okumu A, Cohen CR. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med 2011; 12:316–21. [DOI] [PubMed] [Google Scholar]
- 6.Weber S, Waldura JF, Cohan D. Safer conception options for HIV serodifferent couples in the United States: the experience of the National Perinatal HIV Hotline and Clinicians’ Network. J Acquir Immune Defic Syndr 2013; 63:e140–1. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick RJ, Mantell JE, Moodley J, Harries J, Zweigenthal V, Cooper D. Safer conception interventions for HIV-affected couples: implications for resource-constrained settings. Top Antivir Med 2011; 19:148–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews LT, Smit JA, Cu-Uvin S, Cohan D. Antiretrovirals and safer conception for HIV-serodiscordant couples. Curr Opin HIV AIDS 2012; 7:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker L-G, Black V, Myer L et al. Guideline on safer conception in fertile HIV-infected individuals and couples. South Afr J HIV Med 2011; 12:31–44. [Google Scholar]
- 10.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 13.Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011; 25:2005–8. [DOI] [PubMed] [Google Scholar]
- 14.Whetham J, Taylor S, Charlwood L et al. Pre-exposure prophylaxis for conception (PrEP-C) as a risk reduction strategy in HIV-positive men and HIV-negative women in the UK. AIDS Care 2014; 26:332–6. [DOI] [PubMed] [Google Scholar]
- 15.Matthews LT, Baeten JM, Celum C, Bangsberg DR. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS 2010; 24:1975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. http://aidsinfo.nih.gov/lvguidelines/PerinatalGL.pdf Accessed 27 August 2014.
- 17. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States - 2014 Clinical Practice Guideline, 2014. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf Accessed 2 September 2014.
- 18.Mugo NR, Hong T, Celum C et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. Jama 2014; 312:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. ISBN 3-900051-07-0. http://www.R-project.org. Accessed 27 August 2014. [Google Scholar]
- 20.Stanford JB, White GL, Hatasaka H. Timing intercourse to achieve pregnancy: current evidence. Obstet Gynecol 2002; 100:1333–41. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333:1517–21. [DOI] [PubMed] [Google Scholar]
- 22.Quinn TC, Wawer MJ, Sewankambo N et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 23.Gray RH, Wawer MJ. Probability of heterosexual HIV-1 transmission per coital act in sub–Saharan Africa. J Infect Dis 2012; 205:351–2. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JP, Baeten JM, Lingappa JR et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis 2008; 198:687–93. [DOI] [PubMed] [Google Scholar]
- 26.Wawer MJ, Gray RH, Sewankambo NK et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191:1403–9. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor EM, Sperling RS, Gelber R et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331:1173–80. [DOI] [PubMed] [Google Scholar]
- 29.De Cock KM, Fowler MG, Mercier E et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 2000; 283:1175–82. [DOI] [PubMed] [Google Scholar]
- 30.Cooper ER, Charurat M, Mofenson L et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 2002; 29:484–94. [DOI] [PubMed] [Google Scholar]
- 31.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLOS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roongpisuthipong A, Siriwasin W, Simonds RJ et al. HIV seroconversion during pregnancy and risk for mother-to-infant transmission. J Acquir Immune Defic Syndr 2001; 26:348–51. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro RL, Hughes MD, Ogwu A et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010; 362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS 2007; 21(suppl 4):S65–71. [DOI] [PubMed] [Google Scholar]
- 35.Cleary-Goldman J, Malone FD, Vidaver J et al. Impact of maternal age on obstetric outcome. Obstet Gynecol 2005; 105:983–90. [DOI] [PubMed] [Google Scholar]
- 36.Seoud MA, Nassar AH, Usta IM, Melhem Z, Kazma A, Khalil AM. Impact of advanced maternal age on pregnancy outcome. Am J Perinatol 2002; 19:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Newcomb WW, Rodriguez M, Johnson JW. Reproduction in the older gravida. A literature review. J Reprod Med 1991; 36:839–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.