Abstract
The current investigation examined stressors upon the coupling of the hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–gonadal (HPG) axes. Emphasis is placed on the moderating role of context and time. One hundred and eighteen adolescent males and females provided up to 32 diurnal saliva samples across a visit to a research lab. This visit constituted a day-long stress through which the impact on HPA–HPG axis coupling could be assessed. We tested four models of HPA–HPG axis coupling across the lab day. Sex and stress hormones operated synchronously (β = .404, p <.001), and the coupling of sex and stress hormones was moderated by the stress of the lab day (β = .010, p = .05). This pattern of co-elevation did not appear to be moderated by the distal experience of early life adversity. Findings suggest that the notion of “stress” must disentangle proximal and distal challenges, each of which appears to impact neurobiological processes.
Keywords: stress, coupling, HPA, HPG, adolescence, cortisol, testosterone, DHEA
INTRODUCTION
The human stress response system consists of a coordinated set of actions carried out by reactive physiological systems. These include the hypothalamic–pituitary–adrenal (HPA) axis, which culminates in the production of cortisol; the hypothalamic–pituitary–gonadal (HPG) axis, which results in the production of testosterone; and DHEA, which is an end product of both axes. Individual differences in stress-responsivity are apparent early in development, and disruptions to stress physiology impact many aspects of child development (Bergman, Sarkar, O’Connor, Modi, & Glover, 2007; Mulder et al., 2002). While often considered uniquely, these axes may operate in a more synergistic way than previously considered plausible. The current study tested whether the HPA and HPG axes may operate synergistically. To do so, we evaluated functioning of these systems in a particularly salient context: that of a day-long stress event, and examined a diurnal time-course. HPA and HPG axis activity can be considered as processes that allow the body to adapt to changing contexts. Here, we examine these systems in the context of a day-long visit to a novel environment, and examine the effects of this experience on the diurnal rhythm.
The HPA and HPG Axes
The HPA axis changes within minutes or across the day in response to social evaluative threat and uncontrollable contexts (Dickerson & Kemeny, 2004). The acute change of hormone systems in reactivity to extrinsic stressors has been thoroughly investigated (Bateup, Booth, Shirtcliff, & Granger, 2002; Dickerson & Kemeny, 2004; Salvadore, Simon, Suay, & Llorens, 1987). A small number of studies have found that individuals with early adversity also respond differently from other youth in acute stress challenges (Seltzer, Ziegler, Connolly, Prosoksi, & Pollak, 2014; Tarullo & Gunnar, 2006) raising the possibility that different time-courses of stress exposure (e.g., long-term vs. acute) may operate inter-dependently.
The impact of stress exposure could also be manifested through the HPG axis. Testosterone and DHEA—physiological end products of the HPG axis—advance pubertal maturation and are implicated in the timing and tempo of puberty. Indirectly, these sex hormones are linked with stress exposure through studies that find pubertal processes are influenced by stress and the particulars of the environment (Belsky et al., 2007; Ellis, 2004). For example, organisms experiencing extreme stress demonstrate later pubertal maturation compared to individuals within stable environments rich with resources (Gluckman & Hanson, 2006). Conversely, psychosocial stress such as exposure to maternal depression appears to accelerate puberty (Ellis & Garber, 2000), demonstrating an effect on HPG-mediated processes. The HPG also operates within shorter timeframes, acutely responding to contexts such as reward, competition, and challenge (Archer, 2006). For example, testosterone levels rise in response to competition (Bateup et al., 2002; Booth, Shelley, Mazur, Tharp, & Kittok, 1989), and continue to rise in the case of the winners (Eisenegger, Haushofer, & Fehr, 2011). Taken together, it is possible that the HPG axis is responsive to stressful contexts across short and long time frames, much like the HPA axis.
This relationship between HPA and HPG axis hormones can be extended to include several studies that look at activity of these axes simultaneously. Findings from these investigations are somewhat inconsistent at this point. Basal testosterone has been examined in conjunction with basal cortisol and there are modest correlations between the two (Popma et al., 2007; Vicennati et al., 2006). The relationship between basal levels and reactivity has been investigated in several contexts. Mehta, Jones, and Josephs (2008) have demonstrated that high testosterone individuals who lose a task experience elevations in cortisol but low testosterone people do not, and Glenn, Raine, Schug, Gao, and Granger (2011) have shown that an increased ratio of baseline cortisol to reactive testosterone is associated with psychopathy, highlighting that the association between cortisol and testosterone may be both environmentally responsive and behaviorally relevant.
Coupling of the HPA and HPG Axes: Context Matters
The HPA and HPG axes are a set of dynamic systems that may fluctuate in the service of adapting to contextual circumstances. A pattern of simultaneous activation of the HPA and HPG axes, or coupling, putatively represents the ability of these physiological processes to jointly calibrate to the specific needs of the individual. The current study combines the rigor of a laboratory setting with the ecological validity of a naturalistic challenge, and does so by examining the novel laboratory context as a social challenge for youth, in contrast to a more familiar “home” and “school” day. In sum, both the HPA and HPG axes are responsive physiological processes that may operate in a coordinated manner in response to some contexts. However, little is know about the range of settings or time-course for such actions.
In the present study, a novel day long experience was used to approximate adolescent’s responses to normative types of daily stressors. This is an innovative strategy, but not wholly without precedent. Specific to cortisol, same day reports of tension and anger have been associated with elevated evening cortisol levels, and as such, a flattened diurnal rhythm (Adam, Hawkley, Kudielka, & Caccioppo, 2006). Children in day care settings display elevated cortisol secretion patterns relative to non-day care settings, and it has been suggested that this is related to elevated stressful interactions in this context (Vermeer & van Ijzendoorn, 2006).
First, arriving at an unknown, laboratory-type setting is novel, which triggers hormone responsivity (Battaglia et al., 1997). Second, adolescents underwent magnetic resonance imaging (MRI), a challenge that impacts both HPA and HPG axis functioning (Eatough, Shirtcliff, Hanson, & Pollak, 2009; Muehlhan, Lueken, Wittchen, & Kirschbaum, 2011). Participants next underwent a physical exam and a puberty assessment, as well as several hours of interviews and assessments (described below). While normatively questionnaires are considered a resting phase for participants, the battery of instruments and semi-structured interviews of this study are more challenging than is common, specifically with regard to the Life Stress Interview. This interview asks participants to recall and discuss all of the adverse events that have occurred in the last year that they are willing to speak about, and it can be taxing to the participant to recall life stressors. All in all, this experience adds up to a day-long series of potentially challenging events which may cause engagement of the HPA and HPG axes. While none of these unique experiences represented an extreme event, they do represent a series of progressive events that sum into a challenging day for the participant.
The Diurnal Rhythm
The present study focused on the diurnal rhythm for several reasons. First, the diurnal rhythm represents wide-ranging hormone concentrations, capturing the highest physiological values early in the waking day, followed by extremely low concentrations anticipated in the evening hours. In this capacity, the diurnal rhythm operates as a measure of malleability and flexibility of these axes. Second, hormones of the HPA and HPG all display a diurnal rhythm. The slope of the diurnal rhythm is an important outcome of interest whose pattern is likely influenced by context. In addition, diurnal rhythms are linked to behavioral outcomes.
Cortisol and the Diurnal Rhythm
The HPA axis is particularly susceptible to environmental contexts (Doom, Cicchetti, & Rogosch, 2014). For example, Ockenfels et al. (1995) found steeper diurnal slopes for cortisol in the context of unemployment stress. This pattern is not ubiquitous; Grossi, Perski, Lundberg, and Soares (2001) found that financial hardship was related to elevated evening cortisol levels in women, and Ranjit, Young, and Kaplan (2005) focus on a blunted awakening response in relation to material hardship, each highlighting a different aspect of the diurnal rhythm. DeSantis et al. (2007) found racial discrimination was associated with flatter diurnal slopes. Higher levels of grief after a bereavement have also been associated with flattened cortisol rhythm (O’connor, Wellisch, Stanton, Olmstead, & Irwin, 2012). The diurnal rhythm is relevant for short-term stress exposure because this natural rhythm is also susceptible to acute stressors. Proximal effects of mood and experience interact and influence the trajectory of the cortisol rhythm over the course of a given day (Adam et al., 2006). In sum, the diurnal rhythm of cortisol varies in response to a wide variety of stressful environmental contexts.
Testosterone and the Diurnal Rhythm
Like cortisol, environmental context also influences testosterone’s diurnal rhythm, though there is less research on this hormone. Prolonged challenges alter testosterone’s diurnal rhythm, triggering elevated evening levels after a hunt in !Kung San men (Worthman & Konner, 1987). Furthermore, Peckins and Susman (2015) found that early life adversity is related to flatter diurnal slope of testosterone. Taken together, these studies demonstrate that the diurnal rhythm of testosterone is susceptible to environmental moderation.
DHEA and the Diurnal Rhythm
Similarly, DHEA’s diurnal rhythm appears altered within maltreated youth compared to other children (Cicchetti & Rogosch, 2007), as well as in people who exercise regularly (Heaney, Carroll, & Phillips, 2014). Like cortisol and testosterone, the diurnal rhythm of DHEA appears to be amenable to and moderated by context, although more work is needed on this neurosteroid.
In addition to being susceptible to environmental context, diurnal rhythms have behavioral and health implications. For example, a flattened cortisol rhythm has been linked to increased mortality in breast cancer patients (Sephton, Sapolsky, Kraemer, & Spiegel, 2000), as well as lung cancer (Sephton et al., 2013). Granger et al. (2003) found that a flatter testosterone diurnal rhythm in adolescents is associated with elevated levels of anxiety-depression and attention problems, whereas steeper patterns of testosterone rhythm were related to more externalizing problems in girls. Shirtcliff, Zahn-Waxler, Klimes-Dougan, and Slattery (2007) found that flatter diurnal rhythms of DHEA were associated with concurrent internalizing problems. All told, the diurnal rhythm is a complementary time-scale for measurement of the impact of a day-long stress event. Changes to this rhythm potentially best represent the summation of the impact of the lab day stressor.
The Importance of Adolescence
This is an adolescent sample, and adolescence is a critical window for examining the activity of these hormones. During adolescence, there are normative increases across DHEA, testosterone, and cortisol (Matchock, Dorn, & Susman, 2007). Furthermore, adolescence is a critical period for integration of environmental information; as adolescents transition into adulthood contextual information about the environment can be incorporated into re-calibrating physiological processes such as the HPA and HPG axes. As such, this period of environmental susceptibility and physiological reorganization marks adolescence as a critical window in which to observe the HPA and HPG axes.
Moderation by Lifetime Stress Exposure
Several authors in this issue have found coupling of the HPA and HPG axes and their end products occurring months, and even years, after stress exposure (see Dismukes et al., Bobadilla et al., Simmons et al., Ruttle et al., this issue). In concert, the present study includes lifetime adversity ranging from normative to extreme, including over 40 participants who had experienced substantiated child abuse. Child maltreatment has been shown to impact HPA axis functioning including the diurnal rhythm (Cicchetti & Rogosch, 2001; Tarullo & Gunnar, 2006) as well as impact HPG functioning (Booth, Johnson, Granger, Crouter, & McHale, 2003). We investigate whether child maltreatment likewise impacts HPA–HPG axis coupling.
Hypotheses
First, based on previous examinations of the reactivity of HPA and HPG axes to stress and challenge, we hypothesize that the HPA and HPG axes are coupled. We test this hypothesis by examining the overall coupling of cortisol, testosterone, and DHEA in the sample by predicting cortisol by testosterone and DHEA, respectively, and then trivariately following the analytic strategy outlined by Marceau and colleagues. Secondly, we hypothesize that context (the stressful lab day) will increase the degree of coupling, and this change in coupling will manifest across the diurnal rhythm of cortisol, testosterone, and DHEA. We test this hypothesis by examining an interaction between testosterone and time across the day and DHEA and time across the day, in the prediction of cortisol. Third, we hypothesize that lifetime stress exposure will moderate coupling of the HPA and HPG axes on the lab day in this analysis. This hypothesis is based upon previous reports of lifetime stress exposure influencing coupling distally. If confirmed, it would suggest that lifetime stress exposure impacts development across neuroendocrine systems.
METHODS
Participants
Participants included 118 adolescents (mean age = 11.33, SD = 1.72), 64 of whom were male (53.3%) and 56 were female (46.7%). Thirty three percent of participants were African-American, 49% were white, 12% were mixed, and 2% were Asian. Physically abused children (PA group N = 42) and their families were recruited by letters forwarded by the Department of Human Services in Dane County, WI, to families with substantiated cases of physical maltreatment. Children whose parents scored at least 20 on the physical abuse CTS subscale (described below) and/or had substantiated cases of physical abuse on record with the Dane County Department of Human Services were classified as abused. Non-abused children were recruited from the community by posting fliers (N = 78). To qualify as controls, children were required to have CTS scores below 10, and be free of any child protective services records. All procedures were approved by the Institutional Review Board, and parents and youth provided informed consent and assent, respectively
Procedures
Participants provided saliva samples across 5 days. On the first day, participants underwent a physical exam, an MRI, and a series of personal interviews and questionnaires; this visit lasted approximately 5–8 hr with the variability almost exclusively due to the length of the interview and not to the length of the “stressful” tasks. Eight hormone samples were collected via passive drool on the lab day: these are sampled (1) upon arrival, (2) after the puberty assessment, (3) before the MRI, (4) after the MRI, (5) after lunch, (6) after the interviews, (7) before dinner, and (8) at bedtime. This culminated in 944 total distinct sampling points for the 118 adolescents participating in this study on the lab day. We also collected samples from participants on an additional four days. On two home days and two school days, adolescents provided six daily saliva samples: (1) upon awakening, (2) mid-morning, at least an hour after breakfast, (3) prior to lunch, (4) mid-afternoon after school, (5) before dinner, and (6) before bedtime. It is worth noting that the school day may contain elements of stress, with samples one, two, and three occurring at school. These samples were self-collected using basal boxes with tubes featuring Medication Event Monitoring Caps that accurately captured sample times, and sample times were designed not to interfere with the normal school day. All together, this culminates in 5 days of sample collection at up to 32 time points per individual, with 2,525 total hormone samples collected for the 118 individuals. This allows for a data-rich mapping of the diurnal rhythm across multiple contexts.
Measures
Daily Diary
Participants completed a daily diary at the time of each sample collection on the home and school days, to record mood, time of awakening, time of collection, medication use, sleep, and daily hassles or uplifts.
Child Abuse Severity
Parents completed the 32 item Parent–Child Conflict Tactics Scale (CTS) (Straus, Hamby, Finkelhor, Moore, & Runyan, 1998) which measures the extent to which a parent has carried out specific acts of physical aggression toward the child. A Physical Abuse summary score was calculated by summing scores on three subscales: Minor Physical Assault, Physical Assault-Maltreatment, and Severe Physical Maltreatment.
Life Stress Interview
The Life Stress Interview (LSI) is a semi-structured interview administered one-on-one with the adolescent and then their caregiver to index stress in adolescent environments. Participants describe challenges and buffers across academic, behavioral, peer, family, romantic, marital, and cross-gender domains, with scores reported across each of these domains, as well as episodes of major stressors within the prior year to the interviewer. An independent rating team of three or more raters blinded to the child rates the stressfulness of the events later provided a consensual judgment of severity of stress within each domain. In addition, at the end of the study, LSI lifetime stressors were then aggregated and ranked on a 10-point scale by trained raters. The Life Stress Interview (LSI) has been found to be a valid and reliable measure of stress (Adrian & Hammen, 1993).
Hormone Assays
Saliva was collected via passive drool, frozen immediately and transferred to the lab on ice. On the day of assay, samples were thawed, centrifuges for 15 min, and the clear-top phase was pipetted into the appropriate wells. Assays were performed at Middleton Biodiagnostics (Middleton, WI) using Salimetrics assay kits. Intra-assay coefficients of variation (CV’s) were 7% for cortisol, 5.8% for DHEA, and 6.7% for testosterone; inter-assay CV’s were 11% for cortisol, 8.5% for DHEA, and 14.05% for testosterone. Hormone data were log transformed to normalize and out of range values were windsorized.
ANALYTIC STRATEGY
The present study design necessitated a 3-level hierarchical linear model to accurately assess hormonal coupling, as samples were collected within a day and across 5 days within each of the 118 individuals, giving rise to three hierarchical levels of analysis. Level 1 focused on samples varying from moment to moment within a day. As described below, cross-axis coupling was modeled at level 1. The “day” level (level 2) was modeled to be systematically different across days (i.e., school, lab, home). Third, given that the relevant measure of stress was specific to the entire rhythm of cortisol on the lab day and it is diurnal stress indices that were of interest, the day-level captured the impact of the lab day stress on the entire diurnal rhythm. In these models, Age, SES, race, gender, body mass index, and medication usage were all statistically controlled at level 3—the level of the individual.
Following the advice of Marceau (this issue), a series of progressive models that evaluate specific hypotheses were run. These attempted to capture the questions raised by each of our three main hypotheses. First, a baseline model with no predictors partialled out the amount of variance attributable to each level; in this case, 78% of the total variance was attributable to level 1, level 2 differences accounted for 9% (x2 = 544.64, p <.001), and level 3 accounted for 13% (x2 = 360.75, p <.001). The first model predicted cortisol by DHEA and testosterone, respectively, and then DHEA and testosterone simultaneously, in order to establish levels of bivariate and trivariate coupling from moment to moment across each sample, and tested hypothesis 1.
Next, models included DHEA and testosterone in the prediction of cortisol, which added unique time variables to level 1 (individual level), and added level 2 (day level) variables to distinguish the labday from the home and school day. These time variables included time since waking, which controlled for linear changes in time predicting cortisol, and cubic and quadratic time variables that allowed cortisol to change nonlinearly with time as expected with the diurnal rhythm, which drops faster in the morning than evening hours (Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012). Furthermore, the effects of the lab day were brought in on level 2, allowing us to differentiate coupling patterns across basal days and the stressful lab day, and began to address our second hypothesis. In effect, these models answer the question, are these hormones coupled above and beyond the effects of time on cortisol, and after controlling for time, is there an influence of the lab day?
A subsequent analysis built upon the first and second series by adding a time by hormone interaction term, which indexed the diurnal rhythm, or the degree to which the coupling of cortisol with DHEA or testosterone changes over the course of a day. This model tested hypothesis 2. The school and home variables allowed for the curve to be different on these days, and the interaction term allowed that cross-axis coupling is not fixed or invariant, but can change in magnitude over the course of a day. The presence of the lab day on level 2 examined differences in those samples collected on the day in which the participant is in the lab from those assessed on home and school days. In this model, with no level three predictors added, this established a diurnal rhythm stress coupling parameter.
Last, to address hypothesis 3, a follow-up set of analyses indexed diurnal rhythm coupling in the context of moderating variables of life history that could potentially change the coupling pattern across the stress day, with these predictors added at level 3.
RESULTS
Hypothesis 1: Are the HPA and HPG Axes Coupled in This Sample?
In the first coupling model, testosterone was added as a single predictor of cortisol, and positively (β = .404, p <.001) predicted cortisol. The same pattern was true for DHEA (β = .335, p <.001). When DHEA and testosterone were modeled simultaneously in the prediction of cortisol, a trivariate model, both testosterone and DHEA were significant predictors of cortisol (β = .200, p <.001, and β = .240, p<.001, respectively). This initial model indicated, on average, that positive coupling of cortisol with testosterone and DHEA was occurring (see Tabs. 1 and 2 for results from bivariate coupling of cortisol, testosterone, and DHEA across models).
Hypothesis 2: Does the Lab Day Context Moderate the Degree of Coupling Between the HPA and HPG Axes, and Does This Manifest on the Diurnal Rhythm?
Inclusion of Time
After discrete time, predictors (time since waking, time since waking squared, and time since waking cubed) were added as predictors of cortisol, testosterone remained a significant, positive (β = .286, p <.001) predictor of cortisol. This demonstrated that coupling with cortisol occurred over and above the influence of cortisol’s diurnal rhythm. The same pattern held true for DHEA (β = .243, p <.001) as well as the trivariate model where DHEA (β = .170, p <.001) and testosterone (β = .152, p <.001) both predicted cortisol in the same model above and beyond the influence of cortisol’s diurnal rhythm.
Inclusion of the Lab Day
Next, we were interested in whether coupling occurred beyond the influence of the challenge of a lab day or, put another way, the influence of day-to-day events on cortisol. Initially, to capture time (across days), we included lab day as a level 2 predictor of cortisol and the other hormones. The lab day effect on the coupling of cortisol with DHEA (β = .013) and testosterone (β = −.022) seemed to indicate decoupling, but this must be thought of as a correction when all the other predictors were held to zero and because morning samples were collected at different times across the lab and basal days. For this reason, we predicted coupling to manifest across the diurnal rhythm, and not on waking samples.
Lab Day Effects on the Diurnal Rhythm
Time and hormone levels both change at every point in which a hormone is collected, and are, therefore, both level 1 variables. For this reason, hormone by time interaction terms were created and added as level 1 variables to index the way that DHEA and testosterone changed in the prediction of cortisol across the diurnal rhythm, which addressed hypothesis 2.
For testosterone, there was a significant, positive effect (β = .417, p <.05) predicting cortisol, which indicated that cortisol and testosterone were coupled on waking, such that those participants who had higher testosterone had significantly higher cortisol as well.
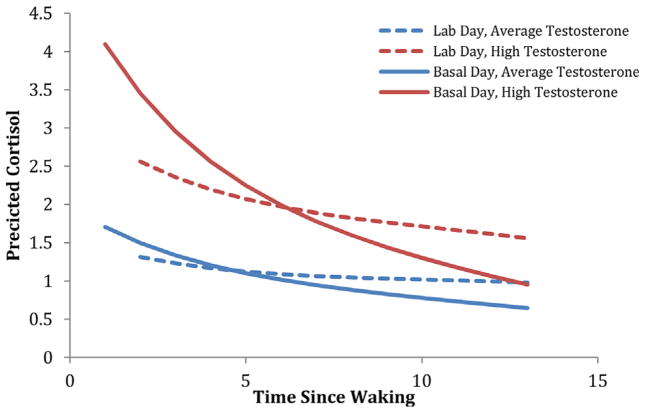
In this model, the lab day effect was brought in on level 2, allowing variability between days in which the participant was in the lab, and alternative home and school days for comparison. The effect on the slope was significant for lab day (β = .021, p <.001), such that cortisol did not decline as quickly on the lab day as it did on other days. The interaction term was negative on basal days (β = −.019, p <.05) indicating that, on average, as the day goes by, cortisol and testosterone appeared to de-couple. However, the time by hormone interaction term was significant and positive on the lab day (β = .010, p = .05), which suggested that testosterone remained coupled with cortisol across challenges unfolding on the lab day, whereas it did not in the context of the basal day (See Fig. 1). The interaction between DHEA and time in the prediction of cortisol on the lab day was not significant.
FIGURE 1.
Coupling of testosterone and cortisol across the day in the context of a short-term stressor. Participants on the lab day have a relatively flat coupling pattern across the day, such that cortisol and testosterone remain coupled as the day progresses, whereas on the basal day participants de-couple.
In the trivariate model, with testosterone, DHEA, and cortisol all loaded onto the same model, testosterone and DHEA did not significantly predict cortisol on the lab day. This indicated that neither testosterone nor DHEA were recruited preferentially to account for the challenges associated with a stressful lab day, but instead both DHEA and testosterone may be coupled with cortisol on the lab day through overlapping mechanisms; it is unclear whether it is DHEA or testosterone is driving coupling observed in model 3, though the bivariate effects are strong.
Hypothesis 3: Does Elevated Life Adversity Moderate the Lab Day Experience Effect on Coupling?
The impact of lifetime stress exposure mostly evidenced on the unique hormone terms, and did not exhibit a pattern of significantly moderating the interaction term in the four-way interaction between lifetime stress exposure, day, hormone, and time. The influence of life stress exposure is subtle, and manifested only in the three-way stress exposure by hormone by context interaction in the prediction of DHEA and testosterone on cortisol, as opposed to the four-way life adversity by hormone by time by context interaction that was a priori postulated as the meaningful coupling parameter. Initial analyses of lifetime stress exposure included the CTS, life stress interview domains, and the effect of the high abuse group. Specifically, on the lab day, higher levels of marital stress in the past year increased the coupling of cortisol and testosterone (β = .023, p <.05) on average, but did not evidence significant changes on the way cortisol and testosterone change together across time. The same was true of family stress (β = .023, p <.10) at a trend level. The effect of group (physically abused vs. control) did not exhibit a fully significant effect on the hormone × time interaction term across the lab day for testosterone or DHEA in the prediction of cortisol.
DISCUSSION
This article examined the coupling of cortisol, testosterone, and DHEA over the course of a stressful day. This was accomplished by creating time by hormone interaction terms for testosterone and DHEA to predict cortisol as the outcome of interest. We found that cortisol and testosterone decoupled over the course of a day, but that the context of a stressful lab day attenuated this, such that these hormones decoupled less, and maintained a more synchronous or “coupled” relationship.
In our analysis, hypothesis 1 was borne out: the HPA and HPG axes were positively coupled, consistent with several other studies in this special issue. These hormones decoupled over the course of a given day as each hormone generally declined across its own diurnal rhythm. However, de-coupling was diminished when the day was stressful and challenging, as on the lab day (hypothesis 2). We emphasize the importance of context in driving this dynamic interplay of hormones in an attempt to establish predictions for when positive coupling was anticipated. As an additional context, we hypothesized (hypothesis 3) that the impact of early life adversity would moderate the degree of coupling across the lab day, but this did not occur.
We speculate, consistent with known literature and commensurate with our lab day observation of persistent positive HPA–HPG axis coupling, that the first and most important factor in establishing whether HPA and HPG axis coupling is expected is an environment that promotes co-activation. Firstly, we suspect that there is an element that promotes activity of the HPA axis—a stress element. A robust literature suggests that the HPA axis responds to a number of lab based and ecological stressors with changes in cortisol levels. We contend that a stress-inducing element that stimulates the HPA axis is likely to be observed prior to or concurrent with coupling.
Second, we speculate that there must be an element of challenge present to elicit an HPG axis response (Archer, 2006). Challenge and stress can share many characteristics. If the event is performance-based or if better performance allows one to have better control over the outcome, then the HPG axis may be implicated. Testosterone would be predicted to rise to motivate the individual through the competition, and cortisol would be expected to rise as this is an instance of social judgment by others (e.g., lab techs). This idea has received empirical support; for example, testosterone and cortisol have both been seen to rise in response to competition, both before and after the event (Bateup et al., 2002). There are times when stress and challenge are unique events that would not induce coupling, but it is our argument that many situations involve the mechanistic overlap of stress and challenge, and as such, the body is adapted to mobilize all the resources at its disposal to meet the needs of that situation, utilizating both the HPA and HPG axes.
It is worthwhile to consider how this emphasis on positive coupling fits with the conventional wisdom that the HPG axis is not mobilized in response to threat more broadly. Much of this evidence is largely borne out of research using animal models with precise experimental manipulations. For example, a number of different stress induction paradigms (restraint stress, foot shock, cold and forced swimming, sleep deprivation) reduce circulating testosterone levels in rats (Andersen, Bignotto, Machado, & Tufik, 2004), but this is to be expected as none of these delineated circumstances contain the necessary components that would induce coupling to occur (i.e., competition, challenge, or reward). However, in studies where HPG axis activation is context-appropriate, inhibition of the HPG axis by the HPA axis is not observed even within animal studies. Rats experiencing social stress relevant to sexual behavior (rotation into different cages and exposure to females) failed to find concomitant inhibition of the HPG axis coupled to stimulation of the HPA axis (Lemaire, Taylor, & Morméde, 1997), and the importance of context in moderating the relationship between adrenal and gonadal end products has been highlighted in several animal studies (Retana-Marquez, Socorro, & Velazquez-Moctezuma, 1996; Taylor, Weiss, & Rupich, 1987). Thus, when there is overlap between stress and challenge, even within temporally proximal settings, mutual activation of the HPA and HPG axes may be expected.
The effect of life stress exposure on the lab day was largely non-significant. There are several reasons that could account for this discrepancy in results and a priori hypotheses. Firstly, the lab day experience is a highly stressful one for most individuals who undergo this experience, regardless of whether they have experienced lifetime stress or not. If so, then the proximal challenge of the lab day may overwhelm the distal influence of lifetime stress, leading to a pattern of life stress moderating the coupling parameter, but not influencing the way the coupling parameter changes across the day (the time interaction). The effects of life stress exposure may be constrained to those time periods on the lab day when the participant is experiencing the most or least amounts of proximal stress, which is a level of nuance beyond the scope of the present investigation. Alternatively, the experience of the lab day stress might not have been fundamentally different for those who experienced elevated lifetime stress compared to youth with less lifetime stress. In support of this interpretation, lifetime stress impacted coupling but did not further diverge on the lab day, suggesting the elements of stress and challenge were similarly appraised by these youth and early life adversity was not context relevant for the lab day.
The present investigation is novel in that it focuses on the contextually responsive nature of diurnal coupling across the HPA and HPG axes. Some limitations apply, however. The protocol we have used for induction of day long stress is not an established paradigm, but instead leverages an extensive, day long data collection protocol to proxy a day-long stress event. This strategy is novel, but is not empirically validated in the way many laboratory-based stress induction protocols have been. The use of a day long laboratory visit with an MRI, puberty assessment, and questionnaire period has not been characterized or reported on previously as a stress inducing environment, although we do see changes across the stress response system in this context, adding validity to this vantage point. We argue that the summative influence of this day of unique stress events, occurring one after another, does aggregate into a stressful day for participants. It must be noted, also, that there may be elements of stress in the school day as well, which were not characterized and are beyond the scope of the current investigation to address. Furthermore, we anticipated that individuals with a history of early stress exposure would demonstrate altered HPA and HPG axis reactivity to diurnal rhythms (Ellis & Boyce, 2008). Our most involved models employ a unique time by hormone interaction term, allowing for sample to sample differences, to index the way hormone levels change with time, with further interaction terms brought in at the level of the day and the level of the individual, which results in a cumulative four-way interaction model. While we have extensive hormone data on these participants, such extensive interactions with life stress may emerge with more participants.
Several future directions are suggested by these data as well. Further examinations of coupling across diurnal rhythms, across alternative age ranges, would be an important next step to validate the current information and provide additional context about when, in development, coupling may or may not play more meaningful roles. Secondly, coupling has thus far been investigated as an outcome of environmental context. It would be important to extend investigations of coupling to include prediction of relevant outcomes; as both the HPA and HPG axes have uniquely been associated with a number of behavioral and health relevant outcomes it is likely that the interaction between the two may be an important factor to consider as contributing to risk or resilience as well.
In sum, multiple levels of analyses across proximal and distal influences of time, with regard to whether or not a context is regarded as stressful, challenging, or some combination of the two, likely informs the pattern of coupling across the diurnal rhythm for the HPA and HPG axes. Both the HPA axis and the HPG axis have been characterized as responsive elements to context, but, to the authors’ knowledge, have not been modeled simultaneously in this fashion before. Coupling can be viewed a physiological resource utilized to meet environmental needs of situations characterized as both stressful and challenging. It is our contention that the HPA and HPG axes exhibit nuanced responses to environmental stimuli that orient the individual to best navigate the circumstances surrounding him or her, and that context and time course are vital components of experience—driven patterns of activity across the HPA and HPG axes. This is a novel vantage point that clarifies the role of these rarely co-modeled systems.
Table 1.
Beta Weights for Bivariate Coupling of Testosterone and Cortisol Across Models
| Model | Level 1 | β | Level 2 | β | Level 3 | β |
|---|---|---|---|---|---|---|
| Model 1 | Intercept | .389*** | ||||
| Testosterone | .404*** | |||||
| Model 2 | Intercept | −.181* | Labday | −.154* | ||
| Testosterone | .286*** | Labday | −.022 | |||
| Time | −.107*** | Labday | .021*** | |||
| Model 3 | Intercept | −.634*** | Labday | .071 | ||
| Testosterone | .417*** | Labday | −.09* | |||
| Time | −.085*** | Labday | .021*** | |||
| Testoxtime | −.019*** | Labday | .01* | |||
| Model 4 | Intercept | −.519*** | Labday | −.012 | Abused | −.241 |
| Testosterone | .392*** | Labday | −.081 | Abused | .058 | |
| Time | −.094*** | Labday | .035** | Abused | .012* | |
| Testoxtime | −.018*** | Labday | .010 | Abused | .004 |
Intercept terms represent waking levels (when time is 0). Testosterone represents the value of testo in the prediction of cortisol. Testoxtime represents the interaction term between testosterone and time in the prediction of cortisol. Labday represents the effect of the labday on testosterone, time, waking level, and the interaction between time and testosterone. Abused denotes the effect of the high abuse group
p <.001,
p <.01,
p <.05
Table 2.
Beta Weights for Bivariate Coupling of DHEA and Cortisol Across Models
| Model | Level 1 | β | Level 2 | β | Level 3 | β |
|---|---|---|---|---|---|---|
| Model 1 | Intercept | .409*** | ||||
| DHEA | .335*** | |||||
| Model 2 | Intercept | −.222** | Labday | −.228*** | ||
| DHEA | .243*** | Labday | .013 | |||
| Time | −.101*** | Labday | .015*** | |||
| Model 3 | Intercept | −.167 | Labday | −.116 | ||
| DHEA | .231*** | Labday | −.027 | |||
| Time | −.092*** | Labday | .008 | |||
| DHEAxtime | −.004 | Labday | .002 | |||
| Model 4 | Intercept | −.501*** | Labday | −.202 | Abused | .121 |
| DHEA | .314*** | Labday | .012 | Abused | −.011 | |
| Time | −.050** | Labday | .009 | Abused | −.020 | |
| DHEAxtime | −.011*** | Labday | .001 | Abused | .001 |
Intercept terms represent waking levels (when time is 0). DHEA represents the value of DHEA in the prediction of cortisol. DHEAxtime represents the interaction term between DHEA and time in the prediction of cortisol. Labday represents the effect of the labday on DHEA, time, waking level, and the interaction between time and DHEA. Abused denotes the effect of the high abuse group.
p <.001,
p <.01,
p <.05
Acknowledgments
Contract grant sponsor: National Institute of Mental Health
Contract grant number: R01-MH061285
Contract grant sponsor: National Institute of Child Health and Human Development
Contract grant number: P30HD03352
Contract grant sponsor: k01 Career Development
Contract grant numbers: k01 mh077687, pi shirtcliff
Funding was provided by National Institute of Mental Health grant R01-MH061285 (Pollak); with additional support from the National Institute of Child Health and Human Development, P30HD03352 and a k01 career development grant (k01 mh077687, pi shirtcliff).
Footnotes
The authors declare no conflicts-of-interest.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Brazilian Journal of Medical and Biological Research. 2004;37(6):791–797. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology. 1993;61(2):354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women’s competition. Evolution and Human Behavior. 2002;23(3):181–192. [Google Scholar]
- Battaglia M, Bajo S, Strambi LF, Brambilla F, Castronovo C, Vanni G, Bellodi L. Physiological and behavioral responses to minor stressors in offspring of patients with panic disorder. Journal of Psychiatric Research. 1997;31(3):365–376. doi: 10.1016/s0022-3956(97)00003-4. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, Susman E. Family rearing antecedents of pubertal timing. Child Development. 2007;78(4):1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(11):1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Bobadilla L, Asberg K, Johnson M, Shirtcliff EA. Experiences in the military may impact dual-axis neuroendocrine processes in veterans. Dev Psychobiol. 2014 doi: 10.1002/dev.21259. [DOI] [PubMed] [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Hormones and Behavior. 1989;23(4):556–571. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Booth A, Johnson DR, Granger DA, Crouter AC, McHale S. Testosterone and child and adolescent adjustment: The moderating role of parent-child relationships. Developmental Psychology. 2003;39(1):85–98. doi: 10.1037//0012-1649.39.1.85. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007;19(3):787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dismukes Andrew R, et al. Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Developmental psychobiology. 2014 doi: 10.1002/dev.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA. Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(11):1206–1215. doi: 10.1016/j.jaac.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34(8):1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in Cognitive Sciences. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17(3):183–187. [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls’ pubertal timing: Maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71(2):485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130(6):920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Gao Y, Granger DA. Increased testosterone-to-cortisol ratio in psychopathy. Journal of Abnormal Psychology. 2011;120(2):389–399. doi: 10.1037/a0021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends in Endocrinology & Metabolism. 2006;17(1):7–12. doi: 10.1016/j.tem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Grossi G, Perski A, Lundberg U, Soares J. Associations between financial strain and the diurnal salivary cortisol secretion of long-term unemployed individuals. Integrative Physiological & Behavioral Science. 2001;36(3):205–219. doi: 10.1007/BF02734094. [DOI] [PubMed] [Google Scholar]
- Heaney JL, Carroll D, Phillips AC. Physical activity, life events stress, cortisol, and DHEA: Preliminary findings that physical activity may buffer against the negative effects of stress. Journal of Aging and Physical Activity. 2014;22(4):465–473. doi: 10.1123/japa.2012-0082. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Taylor GT, Morméde P. Adrenal axis activation by chronic social stress fails to inhibit gonadal function in male rats. Psychoneuroendocrinology. 1997;22(8):563–573. doi: 10.1016/s0306-4530(97)00051-6. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C. Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents. Dev Psychobiol. 2013 doi: 10.1002/dev.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24(5):969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology. 2008;94(6):1078–1093. doi: 10.1037/0022-3514.94.6.1078. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C. The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. International Journal of Psychophysiology. 2011;79(2):118–126. doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, Robles de Medina PG, Huizink AC, Van den Bergh BRH, Buitelaar JK, Visser GHA. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Human Development. 2002;70(1):3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Olmstead R, Irwin MR. Diurnal cortisol in complicated and non-complicated grief: Slope differences across the day. Psychoneuroendocrinology. 2012;37(5):725–728. doi: 10.1016/j.psyneuen.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: Overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57(5):460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Peckins MK, Susman EJ. Variability in diurnal testosterone, exposure to violence, and antisocial behavior in young adolescents. Development and Psychopathology. doi: 10.1017/S095457941400145X. available on CJO2015. [DOI] [PubMed] [Google Scholar]
- Popma A, Doreleijers TAH, Jansen LMC, Van Goozen SHM, Van Engeland H, Vermeiren R. The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology. 2007;32(7):1622–1628. doi: 10.1038/sj.npp.1301289. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology. 2005;34(5):1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Retana-Marquez S, Salazar ED, Velazquez-Moctezuma J. Effect of acute and chronic stress on masculine sexual behavior in the rat. Psychoneuroendocrinology. 1996;21(1):39–50. doi: 10.1016/0306-4530(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol. 2013 doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore A, Simon V, Suay F, Llorens L. Testosterone and cortisol responses to competitive fighting in human males. Aggressive Behavior. 1987;13:9–13. [Google Scholar]
- Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, Pollak SD. Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Development. 2014;85(2):501–512. doi: 10.1111/cdev.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior, and Immunity. 2013;30:S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48(6):580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Simmons Julian G, et al. Dual-axis hormonal covariation in adolescence and the moderating influence of prior trauma and aversive maternal parenting. Developmental psychobiology. 2015 doi: 10.1002/dev.21275. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the parent-child conflict tactics scales: Development and psychometric data for a national sample of American parents. Child Abuse and Neglect. 1998;22(4):249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Rupich R. Male rat behavior, endocrinology and reproductive physiology in a mixed-sex, socially stressful colony. Physiology & Behavior. 1987;39(4):429–433. doi: 10.1016/0031-9384(87)90368-4. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior. 2012;62(1):36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer HJ, van Ijzendoorn MH. Children’s elevated cortisol levels at daycare: A review and meta-analysis. Early Childhood Research Quarterly. 2006;21(3):390–401. [Google Scholar]
- Vicennati V, Ceroni L, Genghini S, Patton L, Pagotto U, Pasquali R. Sex difference in the relationship between the hypothalamic-pituitary-adrenal axis and sex hormones in obesity. Obesity. 2006;14(2):235–243. doi: 10.1038/oby.2006.30. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Konner MJ. Testosterone levels change with subsistence hunting effort in !Kung San men. Psychoneuroendocrinology. 1987;12(6):449–458. doi: 10.1016/0306-4530(87)90079-5. [DOI] [PubMed] [Google Scholar]