Abstract
Background
It remains unclear whether or not a positive family history of affective disorders predicts the effectiveness of antidepressant treatment of depression.
Aims
Assess the relationship of a family history of affective disorders to the efficacy of duloxetine in the treatment of depressive disorder.
Methods
Seventy-seven patients with depressive disorder (as defined by the 10th edition of the International Classification of Diseases, ICD-10) were enrolled in the study and treated with standard doses of duloxetine for 12 weeks. Among these patients 37 had a family history of affective disorder in first-degree relatives and 40 did not. The Hamilton Depression rating scale (HAMD-17), Hamilton Anxiety rating scale (HAMA), Side Effects Rating Scale (SERS), Snaith-Hamilton Pleasure Scale (SHAPS), and Beck Depression Inventory (BDI) were assessed at baseline and at the end of the 2nd, 4th, 6th, 8th, and 12th week after enrollment. Repeated measures analysis of variance and logistic regression were used to analyze the association between a family history of affective disorders and the efficacy of duloxetine.
Results
Patients with a positive family history of affective disorders had an earlier age of onset, a longer duration of illness, a higher level of psychic anxiety, and more prominent anhedonia. Repeated measures analysis of variance showed a significant improvement in the severity of depression over the 12 weeks but no differences in the magnitude or speed of improvement between the two groups. Treatment was considered effective (i.e., drop in baseline HAMD-17 total score of ≥50%) in 75.7% of those with a family history of affective disorders and in 77.5% of those without a family history (X2=0.04, p=0.850).
Conclusions
Family history of affective disorders is not associated with the effectiveness of duloxetine in the acute treatment of depressive disorder.
Keywords: family history, depressive disorders, duloxetine, China
Abstract
背景
阳性情感障碍家族史能否预测抗抑郁药疗效尚不清楚。
目的
评估情感障碍家族史与度洛西汀治疗抑郁症的疗效之间的相关性。
方法
研究纳入符合国际疾病分类第10版中抑郁症定义的77例患者,采用标准剂量的度洛西汀治疗12周。其中37例患者有情感障碍家族史(一级亲属中),另外40例家族史阴性。采用汉密尔顿抑郁量表 (Hamilton Depression rating scale, HAMD-17)、汉密尔顿焦虑量表 (Hamilton Anxiety rating scale, HAMA)、抗抑郁药副反应量表 (Side Effects Rating Scale, SERS)、斯奈思-汉密尔顿快感量表 (Snaith-Hamilton Pleasure Scale, SHAPS) 和贝克抑郁自评量表 (Beck Depression Inventory, BDI) 在基线与纳入研究后第2、4、6、8、12周末对患者进行评估。采用重复测量方差分析和logistic回归分析情感障碍家族史与度洛西汀疗效之间的相关性。
结果
情感障碍阳性家族史的患者发病较早,病程较长,精神性焦虑水平更高,而且快感缺乏更加突出。重复测量方差分析显示经过12周治疗抑郁症严重程度显著改善,但两组之间的改善幅度或速度没有显著差异。情感障碍家族史阳性的患者中有75.7%治疗有效(即 HAMD-17 总分较基线下降 ≥50%),而家族史阴性的患者有77.5%有效 (X2=0.04, p=0.850)。
结论
情感障碍家族史与度洛西汀对抑郁症的急性期治疗的疗效不相关。
中文全文
本文全文中文版从2015年10月26日起在http://dx.doi.org/10.11919/j.issn.1002-0829.215080可供免费阅览下载
1. Introduction
Previous studies have shown that heritability of depression ranges from 40 to 70%.[1,2,3] A positive family history of depression may reflect both genetic heritability and common environmental factors.[4] The genetic profile and clinical course of depressed patients with a positive family history of affective disorders may be different from that of depressed patients without a positive family history.[5] Given these differences, it is reasonable to hypothesize that a family history of affective disorders may be one of the factors that predicts the effectiveness of different antidepressant medications, but previous research about this issue has been inconclusive.[6,7,8] To address this issue, the current study compares the treatment response to duloxetine of depressed patients with and without a positive family history of affective disorders.
2. Methods
2.1. Subjects
The enrollment process is shown in Figure 1. All participants were inpatients or outpatients with depression at the Huzhou Third People’s Hospital or at the Sir Run Run Shaw Hospital (both in Zhejiang Province, China) admitted from November 1, 2013 to August 1, 2014 who were treated by seven participating clinicians (SW, HZ, GS, ML, RF, LZ, WC). When comparing the treatment effect in patients with and without a positive family history of affective disorders, it was necessary to remove the confounding effect of different types of treatment, so we only included patients treated with duloxetine, currently the most commonly used antidepressant at our two centers. Of the 108 depressed patients treated with duloxetine in the correct age range (18-65), 93 agreed to participate and 77 (68 inpatients and 9 outpatients) met the inclusion and exclusion criteria (below).
Figure 1. Flowchart of the study.
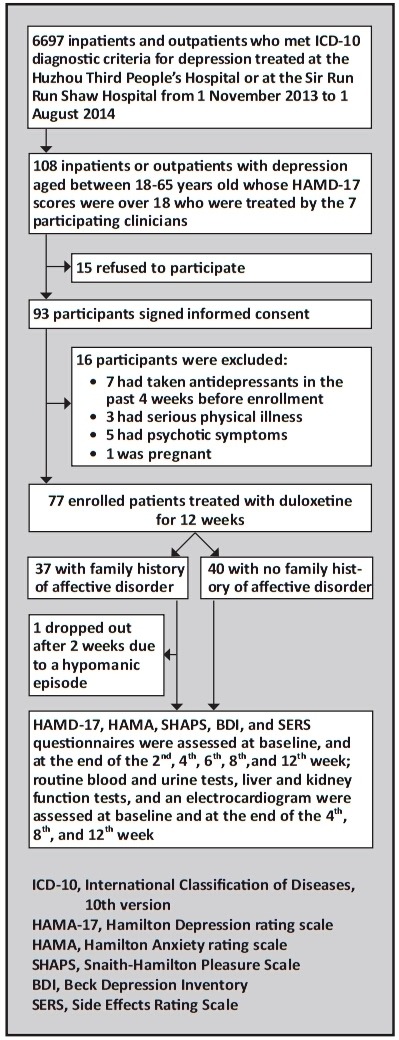
All enrolled participants met the following inclusion criteria: a) met the diagnostic criteria for depression according to International Classification of Diseases, 10th version (ICD-10);[9] b) 18 to 65 years of age; c) had a score of 18 or over on the 17-item Hamilton Depression rating scale (HAMD-17);[10] d) were able to provide sufficient information about their first-degree relatives (i.e. parents, children, and siblings); and e) signed the informed consent form for this study. The exclusion criteria were: a) pregnant or lactating; b) use of any antidepressant within four weeks of enrollment; c) a history of serious heart, liver or kidney disorder, cancer, hematonosis, or epilepsy; d) any other comorbid mental or substance abuse disorder; or f) had psychotic symptoms as part of the depressive episode.
2.2. Classification of probands’ family history of affective disorder
In our study, a positive family history of affective disorders was defined as ‘any first-degree relative who had bipolar disorder, depression, or other affective disorders’. By interviewing the patients and their guardians, all first-degree relatives of the proband (i.e., parents, sibs, and adult children) were enumerated. A total of 297 first-degree relatives of the 77 probands with depression were considered: 11 patients had two first-degree relatives, 26 patients had three first-degree relatives, 18 patients had four first-degree relatives, and 22 patients had five or more first-degree relatives. The 37 patients in the group with a positive family history had 154 first-degree relatives and the 40 patients in the group without a positive family history had 143 first-degree relatives. Among all of these first-degree relatvies 41 (13.8%) had previously been diagnosed with an affective disorder (n=33) or were suspected of having suffered from an affective disorder based on the history (n=8). Most of these individuals (n=30) were interviewed and the presence or absence of a current or prior affective disorder was determined based on ICD-10 criteria; for the 11 first-degree relatives who had passed away or could not be interviewed due to other reasons, a retrospective diagnosis was made based on the description of the informants. Based on this method, 37 of the 77 participating patients (48%) had a family history of affective disorders and 40 (52%) did not.
2.3. Treatment
All the enrolled patients were prescribed only duloxetine (trade mark name: Cymbalte, produced by Eli Lilly, 60 mg per capsule). The starting dosage for each patient was 60 mg/d (after breakfast) and the dosage was gradually increased to 120 mg/d over two weeks. Patients with sleeping disturbance were prescribed benzodiazepines at bedtime if necessary for a maximum of three weeks. All participating patients were followed up for 12 weeks.
Participants who met any of the following criteria were terminated from this study: a) emergence of a serious physical illness; b) emergence of mania or hypomania; c) the patient or guardian withdrew consent; or d) the researcher advised the patient to drop out of this study due to their condition (e.g., suicidal or could not use the medication as directed).
2.4. Assessments
Five questionnaires were administered to all participants at baseline and at the end of the 2nd, 4th, 6th, 8th, and 12th week after enrollment. a) Two questionnaires were completed by research clinicians who were blind to the family history status of the patients: Chinese versions of the HAMD-17 and the Hamilton anxiety scale (HAMA).[10] Previous studies have confirmed the reliability and validity of these scales.[10] In the current study, the inter-rater reliability of the total scores for these measures between the two raters (based on simultaneous evaluation of five patients) was excellent (kappa>0.80). b) Side effects were assessed by the seven treating clinicians (who were not blind to the family history status of the patients) using the Rating Scale for Side Effects (SERS).[10] c) Two self-completion instruments were also administered to participants: the Beck Depression Rating Scale (BDI)[10] and the Snaith-Hamilton Pleasure Scale (SHAPS).[11] The BDI[10] includes 13 items rated on four-level (0-3) Likert scales with a total score of 0 to 39; a total score of ‘0-4’ indicates ‘almost no depressive symptoms’; ‘5-7’ indicates ‘mild depressive symptoms’, ‘8-15’ indicates ‘moderate depressive symptoms’, and ’16 or over’ indicates ‘serious depressive symptoms’. The test-retest reliability of the BDI total score is excellent (rs=0.92) and its correlation with the HAMD total score is good (r=0.57). SHAPS[11] includes 14 items rated on 4-point Likert scales (1=strongly agree, 2=agree, 3=disagree, 4=strongly disagree); higher total scores (range 14-56) reflect greater levels of anhedonia. The internal consistency of SHAPS is excellent (Cronbach’s alpha=0.93) and the test-retest reliability is fair (kappa=0.64).
Routine blood and urine tests, liver and kidney function tests, and electrocardiography (EKG) were conducted at the baseline and at the end of 4th, 8th, and 12th week of treatment.
2.5. Statistical analysis
The data was analyzed using SPSS 16.0 software. Repeated measures analysis of variance (ANOVA) was used to compare the changes of HAMD-17, HAMA, SHAPS, and BDI scores over time between depressed patients who did and did not have a family history of affective disorders. Comparisons of scores between the two groups of patients at each specific time were made using t-tests. Comparison of baseline characterisitics used t-tests or Mann-Whitney tests for continuous and ranked variables and Chi-square tests of Fisher’s exact tests for categorical data. This was an intent-to-treat (ITT) analysis: only one patient dropped out during the 12-week course of treatment (due to development of a hypomanic episode); the last observed values of the evaluation scales (at two weeks) for this patient were carried forward (LOCF). For the purpose of this study, ‘effective treatment’ was defined as a ≥50% drop in the total HAMD-17 score from baseline at the end of the trial (i.e., 12 weeks);[12] and ‘remission’ was defined as a final HAMD-17 score of less than 7.[10] Logistic regression was used to identify factors associated with the effectiveness and remission rate of duloxetine. The statistical significance level was set at p<0.05 (two sided).
The study was approved by the Ethics Committee of Huzhou Third People’s Hospital.
3. Results
3.1. Pre-treatment comparison of the two groups of depressed patients
Among the 37 patients with a family history of affective disorder 23 (62.2%) were being treated for their first episode of illness while among the 40 patients without a positive family history 29 (72.5%) were being treated for their first episode of illness (X2=0.94, p=0.333). As shown in Table 1, depressed patients with a family history of affective disorders had an earlier age of onset, a longer total duration of illnesses and a longer duration of the current episode. As shown in Table 2, at the time of the baseline assessment (prior to starting treatment), patients with a family history of affective disorders had significantly higher levels of clinician-assessed total anxiety and psychic anxiety, and significantly more severe self-reported anhedonia.
Table 1.
Comparison of baseline demographic and clinical characteristics of patients with depression with or without a history of affective disorders in first-degree relatives
| characteristics | with family
history (n=37) |
without family
history (n=40) |
statistic | p |
|---|---|---|---|---|
| inpatient at time of enrollment, n (%) | 33 (89.2%) | 35 (87.5%) | X2=0.05 | 0.818 |
| weeks as outpatient during 12-weeks of treatment, median (IQR) | 5 (4-8) | 4 (4-8) | Z=0.04 | 0.969 |
| female gender, n (%) | 21 (56.8%) | 23 (57.5%) | X2=0.03 | 0.869 |
| age, mean (sd) | 41.9 (9.6) | 39.0 (11.3) | t=1.18 | 0.240 |
| unmarried, n (%) | 5 (13.5%) | 9 (22.5%) | X2=2.35 | 0.503 |
| age of onset, mean (sd) | 30.9 (12.3) | 36.7 (11.0) | t=2.20 | 0.031 |
| total duration of illness in months, median (IQR) | 12 (4-318) | 6 (2-33) | Z=2.03 | 0.043 |
| duration of the current episode in months, median (IQR) | 6 (2-12) | 2.5 (1-6) | Z=1.99 | 0.047 |
| number of episodes, median (IQR) | 1 (1-2) | 1(1-2) | Z=0.70 | 0.486 |
IQR, interquartile range
3.2. Treatment efficacy
With the exception of the patient who dropped out after 2 weeks of treatment due to a hypomanic episode, all enrolled patients took 60 mg/d of duloxetine during the first two weeks of treatment and 120 mg/d during the subsequent 10 weeks of treatment, so the mean dosage over the 12 weeks was 110 mg/d.
The results of the treatment with duloxetine in the two groups of patients are shown in Figure 2 and Table 3. With the sole exceptions of significant differences at baseline in the HAMA and SHAPS total scores (shown in Table 2), there were no significant differences between the two groups in any of the four outcome scales at any of the six assessment times. The repeated measures analysis of variance for the total scores of the four measures shows a dramatic improvement over time but no differences by group and no significant time by-group interaction effects. That is, a family history of affective disorders is not related to the change in scores of any of these measures during 12 weeks of treatment with duloxetine.
Figure 2. Comparison of outcome measures over the 12-week duloxetine treatment period in patients with depression with (n=37) or without (n=40) a history of affective disorders in first-degree relatives.
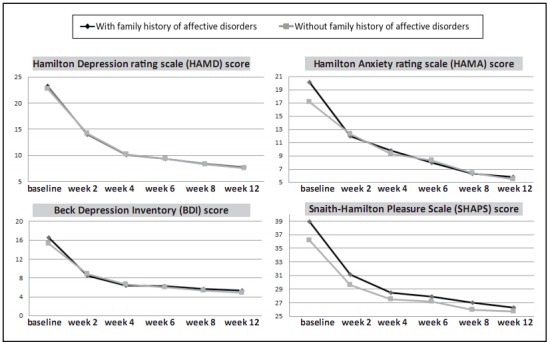
Table 2.
Comparison of baseline mean (sd) clinical assessment scores in patients with depression with or without a history of affective disorders in first degree relatives
| Scale (theoretical range in scores) |
with family
history (n=37) |
without family
history (n=40) |
statistic | p |
|---|---|---|---|---|
| HAMD-17 total score (0-51) | 23.22 (3.74) | 22.75 (3.43) | t=0.57 | 0.570 |
| anxiety/somatic symptoms (0-17) | 6.92 (2.07) | 6.15 (1.78) | t=1.75 | 0.084 |
| weight lossa (0-2) | 0 (0-1)a | 1 (0-1)a | Z=-0.95a | 0.343 |
| cognitive symptoms (0-12) | 3.30 (1.33) | 3.00 (1.55) | t=0.90 | 0.372 |
| retardation (0-14) | 7.68 (1.55) | 7.73 (1.88) | t=-0.13 | 0.901 |
| sleep problems (0-6) | 4.73 (1.45) | 5.13 (1.28) | t=-1.27 | 0.208 |
| HAMA total score (0-56) | 20.16 (5.13) | 17.18 (4.99) | t=2.59 | 0.012 |
| somatic anxiety (0-28) | 7.59 (2.30) | 6.50 (2.98) | t=1.62 | 0.110 |
| psychic anxiety (0-28) | 12.57 (2.93) | 10.68 (2.59) | t=3.01 | 0.004 |
| SHAPS (14-56) | 38.92 (4.46) | 36.15 (5.04) | t=2.55 | 0.013 |
| BDI (0-39) | 16.70 (5.24) | 15.25 (4.35) | t=1.33 | 0.188 |
HAMD-17, 17-item version of the Hamilton Depression rating scale
HAMA, Hamilton Anxiety rating scale
SHAPS, Snaith-Hamilton Pleasure Scale
BDI, Beck Depression Inventory
a medians (interquartile range) were listed for each group and Mann-Whitney U test was used for comparison.
Table 3.
Comparison of mean (sd) outcome measures before and after duloxetine treatment in patients with depression with (n=37) or without (n=40) a history of affective disorders in first-degree relativesa
| measure | group | baseline | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 12 weeks | Ftime (p) | Fgroup (p) | Ftime*group (p) |
|---|---|---|---|---|---|---|---|---|---|---|
| HAMD-17 | with family history | 23.2 (3.7) | 14.0 (4.5) | 10.1 (3.6) | 9.3 (3.4) | 8.4 (3.3) | 7.8 (3.8) | 443.97 (<0.001) | 0.01 (0.912) | 0.01 (0.938) |
| without family history | 22.8 (3.4) | 14.2 (5.0) | 10.2 (4.1) | 9.4 (3.6) | 8.3 (3.5) | 7.6 (4.2) | ||||
| HAMA | with family history | 20.2 (5.1)a | 12.0 (4.1) | 9.8 (3.7) | 8.0 (3.9) | 6.3 (3.0) | 5.8 (2.8) | 247.04 (<0.001) | 0.74 (0.392) | 3.01 (0.087) |
| without family history | 17.2 (5.0)a | 12.3 (4.4) | 9.3 (2.9) | 8.3 (2.1) | 6.4 (2.5) | 5.5 (2.4) | ||||
| SHAPS | with family history | 38.9 (4.5)a | 31.2 (4.2) | 28.5 (3.1) | 27.9 (3.1) | 27.0 (3.1) | 26.3 (3.6) | 251.13 (<0.001) | 3.95 (0.051) | 3.31 (0.073) |
| without family history | 36.2 (5.0)a | 29.6 (3.9) | 27.5 (3.0) | 27.2 (2.8) | 26.0 (2.9) | 25.7 (3.1) | ||||
| BDI | with family history | 16.7 (5.2) | 8.5 (3.9) | 6.4 (2.3) | 6.3 (2.3) | 5.7 (2.2) | 5.4 (2.6) | 260.18 (<0.001) | 0.40 (0.530) | 0.07 (0.791) |
| without family history | 15.3 (4.4) | 8.9 (3.7) | 6.6 (2.5) | 6.0 (2.3) | 5.3 (2.1) | 4.9 (2.5) |
HAMD-17, 17-item version of the Hamilton Depression rating scale
HAMA, Hamilton Anxiety rating scale
SHAPS, Snaith-Hamilton Pleasure Scale
BDI, Beck Depression Inventory
a except for the baseline scores of HAMA (t=2.59, p=0.012) and SHAPS (t=2.55, p=0.013), there were no statistically significant differences in any of the measures at any of the 6 time periods.
Using a ≥50% decrease in baseline HAMD-17 scores as the cutoff for effective treatment, at the end of week 12, 28 of the 37 patients (75.7%) with a family history of affective disorders were effectively treated and 31 of the 40 patients (77.5%) without a family history of affective disorders were effectively treated (X2=0.04, p=0.850). Using a HAMD-17 score ≤7 as the threshold for remission, the remission rates in patients with and without a family history of affective disorders were 54.1% (20/37) and 57.5% (23/40), respectively (X2=0.09, p=0.761).
As shown in Table 4, after adjusting for other factors in logistic regression, a family history of affective disorders was not significantly associated with the effectiveness or the rate of remission of 12 weeks of treatment with duloxetine. With the exception of a borderline significant association between a lower baseline HAMD-17 total score and remission (OR=0.87, 95%CI=0.75-1.00), none of the other factors considered (e.g., age, gender, marital status, age of onset, duration of illness, and number of episodes) were significantly associated with the effectiveness or remission rate after 12 weeks of treatment with duloxetine.
Table 4.
Logistic regression of association between demographic and clinical factors of 77 patients with depression and the effectiveness (≥50% reduction in baseline HAMD-17 total score) and remission (final HAMD-17 under 7) of 12 weeks of treatment with duloxetinea
| variables | effectively treated | remission | |
|---|---|---|---|
| odds ratio (95% confidence interval) |
odds ratio (95% confidence interval) |
||
| Family history of affective disorders | 1.03 (0.31-3.42) | 1.28 (0.44-3.71) | |
| baseline HAMD-17 value | 1.03 (0.88-1.20) | 0.87 (0.75-1.00) | |
| age | 3.86 (0.29-51.00) | 1.74 (0.32-9.56) | |
| female gender | 1.67 (0.54-5.21) | 0.97 (0.35-2.65) | |
| unmarried | 1.05 (0.18-6.13) | 1.42 (0.31-6.52) | |
| age of onset | 0.26 (0.20-3.44) | 0.59 (0.11-3.27) | |
| total duration of illness | 0.89 (0.72-1.11) | 0.95 (0.83-1.10) | |
| number of episodes | 1.03 (0.54-2.00) | 1.22 (0.69-2.15) |
HAMD-17, 17-item version of the Hamilton Depression rating scale
a during the 12 weeks of treatment, 77% (59/77) of the patients were effectively treated and 55.8% (43/77) remitted
3.3. Adverse effects and use of adjunctive medication
As shown in Table 5, about half of the participants in both groups reported one or more adverse effects during the 12 weeks of treatment. Most of the adverse effects occurred during the early stage of the treatment and were mild to moderate; none of them were severe enough to require removal from the trial. Among the reported adverse effects, only the incidence of sexual dysfunction was significantly different between the two groups; it was more common in the group without a positive family history. None of the blood tests, urine tests, liver and kidney function tests, or EKG examinations conducted at the end of the 4th, 8th, and 12th week for treatment were abnormal.
Table 5.
Comparison of incidence (n, %) of adverse effects in patients with depression with and without history of affective disorders in first-degree relatives
| adverse effect | with family
history (n=37) |
without
family
history (n=40) |
statistic | p |
|---|---|---|---|---|
| dizziness | 8 (21.6%) | 7 (17.5%) | X2=0.21 | 0.648 |
| palpitation | 4 (10.8%) | 3 (7.5%) | Fisher’s exact | 0.705 |
| tremor | 3 (8.1%) | 2 (5.0%) | Fisher’s exact | 0.667 |
| dry mouth | 6 (16.2%) | 6 (15.0%) | X2=0.02 | 0.883 |
| constipation | 5 (13.5%) | 4 (10.0%) | X2=0.23 | 0.731 |
| sexual dysfunction | 5 (13.5%) | 14 (35.0%) | X2=4.74 | 0.029 |
| nausea | 5 (13.5%) | 6 (15.0%) | X2=0.04 | 0.850 |
| ANY adverse effect | 19 (51.4%) | 22 (55.0%) | X2=0.10 | 0.749 |
Adjunctive treatment with benzodiazepines for sleep (only used in the first 3 weeks of treatment) was provided to 81.1% (30/37) of the patients with a positive family history of affective disorders but to only 52.5% (21/40) of the patients without a positive family history (X2=5.80, p=0.016). Among the 30 patients in the positive family history group given benzodiazepines 13 used alprazolam, 8 used clonazepam, 5 used lorazepam, and 4 used oxazepam. Among the 21 patients without a family history of affective disorders given benzodiazepines 9 used alprazolam, 7 used clonazepam, 2 used lorazepam, and 3 used oxazepam. Among the patients who used benzodiazepines, the mean duration of use in patients with a positive family history of affective disorders was 16.7 (3.7) days, while that in patients without a positive family history was 17.2 (2.4) days (t=0.55, p=0.583).
4. Discussion
4.1. Main findings
Similar to the STAR*D study,[13] we found that depressed patients with a family history of affective disorders had an age of onset that is, on average, about 6 years earlier than depressed patients without a family history. We also found that depressed patients with a positive family history had a longer duration of illness, more prominent psychic anxiety, and more severe anhedonia. Other reports have suggested that depressed patients with a positive family history for affective disorders also have more severe social dysfunction and a greater risk of suicide than depressed patients without a family history,[14] a finding that highlights the clinical importance of identifying patients with a positive family history. The differences may be more evident in treatment-resistant patients: a multicenter study from Europe[15] reported that among 538 patients who were not responsive to antidepressant treatment, those with a positive family history of major depression had more severe levels of the core symptoms of depression.
The association between a family history of affective disorders and treatment efficacy among patients with depressive disorders is controversial. We found no difference in the effectiveness of 12 weeks of treatment with duloxetine between patients with and without a family history, both when assessing the result as a continuous measure (i.e., change in HAMD-17 total score) and as a dichotomous variable (i.e., whether or not treatment was ‘effective’). Retrospective studies from Japan also found no association between a family history of affective disorders and the effectiveness of treatment for depression with sertraline[6] or fluvoxamine.[16] One report from the STAR*D study[17] found that patients with a positive family history respond to antidepressants more quickly than those without a family history, but in our study both the magnitude and the rate of response was almost identical in both groups. However, a 12-week trial comparing depressed pediatric patients with and without a family history of affective disorders found that those with a family history were more responsive to antidepressant treatment, even after adjusting for age, race, number of episodes, comorbidity, and so forth.[18]
In our study, significantly more patients with a family history of affective disorders were prescribed benzodiazepines by their treating clinicians during the early phases of treatment (81% v. 53%). The primary goal of using benzodiazepines was to help with sleep, but their more frequent use in those with a positive family history may be related to the higher levels of anxiety at the outset of the study among patients with a positive family history.
We also found significantly more frequent reports of sexual dysfunction during treatment in patients without a family history of affective disorders (35% v. 14%). Further studies with larger samples will be need to determine whether or not this was a statistical artifact or a real difference that has a biological etiology.
4.2. Limitations
In some cases the determination of whether or not a patient had a positive family history of affective disorders depended on the patient’s report of family members’ prior behavior, so there may have been some misclassification. The lack of significant differences in the various measures of effectiveness and side effects between groups may have been related to the small sample size (i.e., a ‘type II’ error) but the very small magnitude of the differences suggests that there was, in fact, no difference in the treatment outcomes for the two groups. However, given the very large confidence intervals for the reported odds ratios in the logistic regression models of factors associated with effective treatment and remission, the failure to find any significant correlates of the effectiveness and remission rate of treatment with duloxetine may be the result of the small sample size. Further studies with larger samples and more rigorous methods of assessing family history of affective disorders are needed to confirm these results.
4.3. Significance
Despite the relatively small sample and the inability to be certain about the classification of the family history status, the very similar clinical results between the two groups throughout the 12 weeks of treatment provides reasonably robust support for the contention that a positive family history of affective disorders does not influence the effectiveness of acute treatment with duloxetine. Further studies are need to determine whether or not this is generally true for all antidepressants, whether or not it is true for children as well as adults, and whether or not it remains true for chronic depression.
Biography

Shiliang Wang obtained his bachelor’s degree in medicine from Hubei Yunyang Medical College (now renamed as Hubei College of Medicine and Pharmacology) in 2006 and began work in the psychiatry department of Huzhou Third People’s Hospital. He is currently an attending doctor in his department and is enrolled as a master’s degree candidate in Zhejiang University. His main research interest is depressive disorders.
Funding Statement
The study was funded by the General Program of the National Natural Science Foundation (81371490), the Open Fund of the Zhejiang Key Research Laboratory for Cognitive Disorder Evaluation Technology (PD11001005002008), and the Program of Health and Family Planning Commission of Zhejiang Province Foundation (2011KYA029).
Conflict of interest: The authors declared no conflict of interest related to this manuscript.
Ethics approval: The study was approved by the Ethics Committee of Huzhou Third People’s Hospital.
Informed consent: All participants and their legal guardians provided signed informed consent to participate this study.
References
- 1.Liu XH, Xu YF, Jiang KD, Zhao LY, Jiang SD. [A preliminary study on the genetic effects and genetic mode of first episode depression] Zhong Hua Jing Shen Ke Za Zhi. 2005;38(1):7–10. Chinese. [Google Scholar]
- 2.Qi SG, Dong XH, Zhang YB, An BF. [Gender differences in the genetic effect of unipolar depression] Zhong Hua JingShen Ke Za Zhi. 2007;33(9):562–564. doi: 10.3969/j.issn.1002-0152.2007.09.016. Chinese. [DOI] [Google Scholar]
- 3.Jiang KD. [Depression Prevention Guide] Beijing: Peking University Medical Press; 2007. Chinese. [Google Scholar]
- 4.Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 5.Talati A, Weissman MM, Hamilton SP. Using the high-risk family design to identify biomarkers for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368(1615):20120129. doi: 10.1098/rstb.2012.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara H, Sakamoto K, Harada T, Ishigooka J. Predictors of efficacy in lithium augmentation for treatment-resistant depression. J Affect Disord. 2010;125(1-3):165–168. doi: 10.1016/j.jad.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Morishita S, Kinoshita T. Predictors of response to sertralinein patients with major depression. Hum Psychopharmacol. 2008;23:647–651. doi: 10.1002/hup.969. [DOI] [PubMed] [Google Scholar]
- 8.Duggan C, Sham P, Minne C, Lee A, Murray R. Family history as a predictor of poor long-term outcome in depression. Br J Psychiatry. 1998;173:527–530. doi: 10.1192/bjp.173.6.527. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. International Statistical Classification of Disease and Related Health Problems, 10th Revision. Geneva: World Health Organization; 1992. [Google Scholar]
- 10.Zhang MY. [Psychiatric Rating Scale Manual] Changsha: Hunan Science and Technology Press; 1998. Chinese. [Google Scholar]
- 11.Liu WH, Wang LZ, Zhu YH, Li MH, Chan RC. Clinical utility of the Snaith- Hamilton- Pleasure scale in the Chinese settings. BMC Psychiatry. 2012;12:184. doi: 10.1186/1471-244X-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Xu XF, Wang G, Gao CG, Zhao JP, Tang MN, et al. [Exploratory study of optimal treatment plan for treatment resistant depression] Zhong Hua Jing Shen Ke Za Zhi. 2009;42(1):17–20. doi: 10.3760/cma.j.issn.1006-7884.2009.01.006. Chinese. [DOI] [Google Scholar]
- 13.Nierenberg AA, Trivedi MH, Fava M, Biggs MM, Shores-Wilson K, Wisniewski SR, et al. Family history of mood disorder and characteristics of major depressive disorder:a STAR*D (sequenced treatment alternatives to relieve depression) study. J Psychiatr Res. 2007;41(3-4):214–221. doi: 10.1016/j.jpsychires.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holma KM, Melartin TK, Holma IA, Paunio T, Isometsä ET. Family history of psychiatric disorders and the outcome of psychiatric patients with DSM-IV major depressive disorder. J Affect Disord. 2011;131(1-3):251–259. doi: 10.1016/j.jad.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Serretti A, Chiesa A, Calati R, Sentissi O, Akimova E, Kasper S, et al. Family history of major depression and residual symptoms in responder and non-responder depressed patients. Compr Psychiatry. 2014;55(1):51–55. doi: 10.1016/j.comppsych.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Morishita S, Arita S. Possible predictors of response tofluvoxamine for depression. Hum Psychopharmacol. 2003;18(3):197–200. doi: 10.1002/hup.469. [DOI] [PubMed] [Google Scholar]
- 17.Husain MM, Rush AJ, Wisniewski SR, McClintock SM, Fava M, Nierenberg AA, et al. Family history of depression and therapeutic outcome: findings from STAR*D. J ClinPsychiatry. 2009;70(2):185–195. doi: 10.4088/jcp.07m03571. [DOI] [PubMed] [Google Scholar]
- 18.Tao R, Emslie G, Mayes T, Nakonezny P, Kennard B, Hughes C, et al. Early prediction of acute antidepressant treatment response and remission in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(1):71–78. doi: 10.1097/CHI.0b013e318190043e. [DOI] [PMC free article] [PubMed] [Google Scholar]


