Abstract
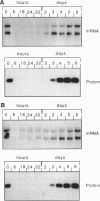
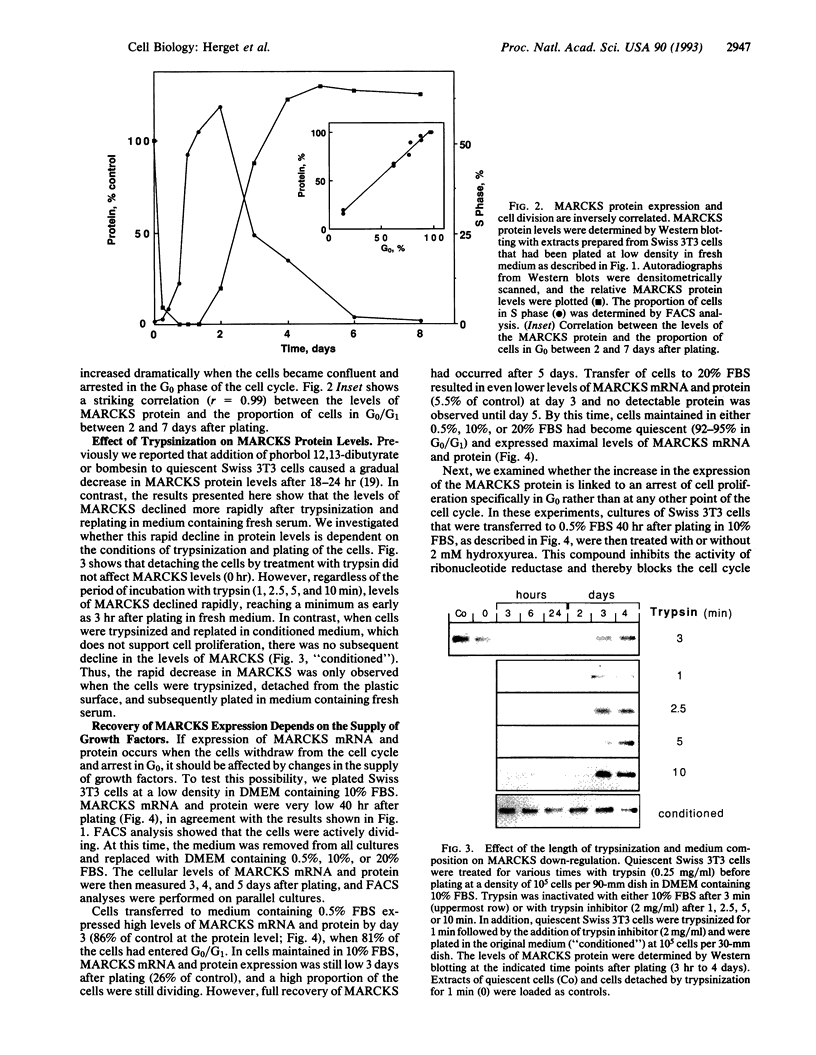
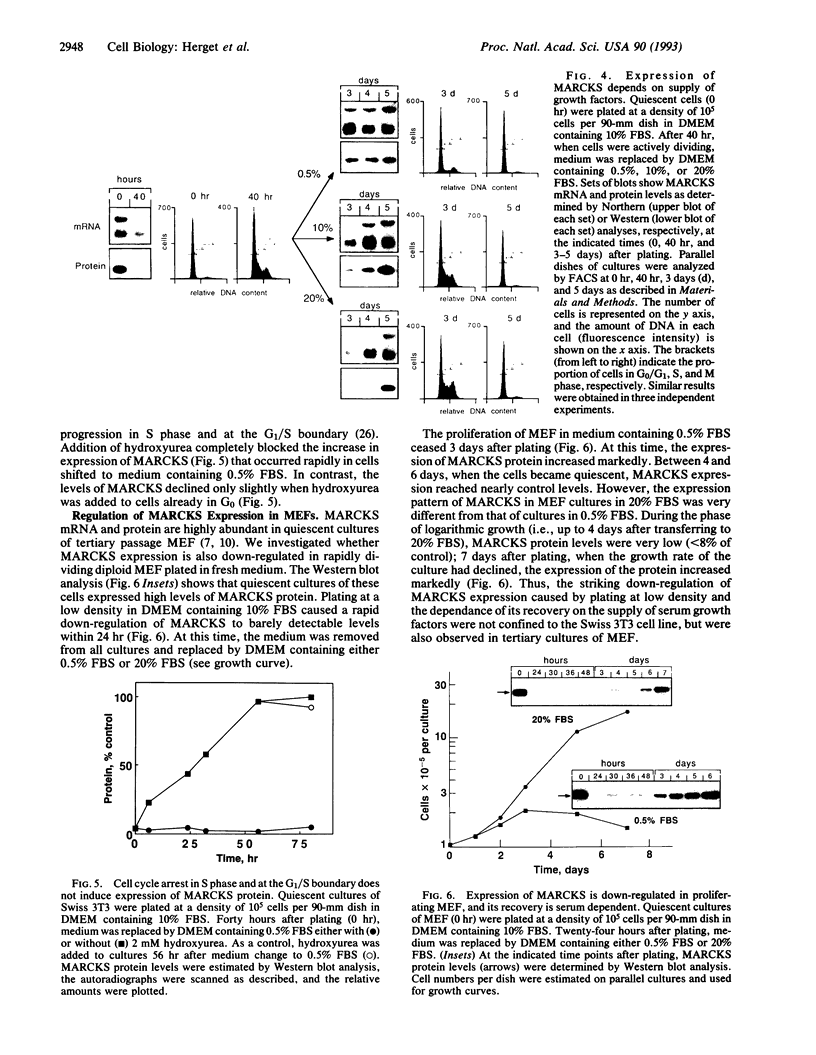
The expression of the major protein kinase C (PKC) substrate, originally called "80K" for acidic SDS/PAGE-observed 80-kDa PKC substrate and now called "MARCKS" for myristoylated alanine-rich C kinase substrate, in Swiss 3T3 fibroblasts changes strikingly (15- to 22-fold) during transitions of cell growth. Quiescent cells in G0 express high levels of MARCKS mRNA and protein. However, plating these cells in fresh medium at low density to stimulate multiple rounds of cell division caused a striking down-regulation of MARCKS expression. The mRNA level declined to a minimum of 4.5% compared with quiescent control cells 6 hr after plating, and protein levels declined during the same period to 6.5% of the control value. This rapid down-regulation was independent of PKC activation and length of exposure to trypsin (1-10 min) but required plating in medium containing fresh serum. MARCKS mRNA and protein levels remained down-regulated for 3 days, during which time the cells were actively progressing through the cell cycle as judged by fluorescence-activated cell sorting analysis. However, on reaching quiescence, the expression of MARCKS mRNA and protein increased markedly. Furthermore, the rate of recovery of MARCKS mRNA and protein levels was shown to be dependent on the supply of serum-derived growth factors in the medium. Addition of hydroxyurea to arrest the cells in S phase or at the G1/S boundary rather than G0 completely prevented the recovery of MARCKS protein. The down-regulation of MARCKS following plating and its serum-dependent recovery was also demonstrated in tertiary cultures of mouse embryo fibroblasts. The results suggest that MARCKS may play a role in the regulation of entry and exit of cells from G0.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks S. F., Herget T., Broad S., Rozengurt E. The expression of 80K/MARCKS, a major substrate of protein kinase C (PKC), is down-regulated through both PKC-dependent and -independent pathways. Effects of bombesin, platelet-derived growth factor, and cAMP. J Biol Chem. 1992 Jul 15;267(20):14212–14218. [PubMed] [Google Scholar]
- Brooks S. F., Herget T., Erusalimsky J. D., Rozengurt E. Protein kinase C activation potently down-regulates the expression of its major substrate, 80K, in Swiss 3T3 cells. EMBO J. 1991 Sep;10(9):2497–2505. doi: 10.1002/j.1460-2075.1991.tb07789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Erusalimsky J. D., Brooks S. F., Herget T., Morris C., Rozengurt E. Molecular cloning and characterization of the acidic 80-kDa protein kinase C substrate from rat brain. Identification as a glycoprotein. J Biol Chem. 1991 Apr 15;266(11):7073–7080. [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Harlan D. M., Graff J. M., Stumpo D. J., Eddy R. L., Jr, Shows T. B., Boyle J. M., Blackshear P. J. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem. 1991 Aug 5;266(22):14399–14405. [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Herget T., Brooks S. F., Broad S., Rozengurt E. Relationship between the major protein kinase C substrates acidic 80-kDa protein-kinase-C substrate (80K) and myristoylated alanine-rich C-kinase substrate (MARCKS). Members of a gene family or equivalent genes in different species. Eur J Biochem. 1992 Oct 1;209(1):7–14. doi: 10.1111/j.1432-1033.1992.tb17255.x. [DOI] [PubMed] [Google Scholar]
- Joseph C. K., Qureshi S. A., Wallace D. J., Foster D. A. MARCKS protein is transcriptionally down-regulated in v-Src-transformed BALB/c 3T3 cells. J Biol Chem. 1992 Jan 15;267(2):1327–1330. [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Blackshear P. J., Johnson J. D. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991 Mar 15;266(8):4959–4964. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Riddle P., Rozengurt E. Stimulating the proliferation of quiescent 3T3 fibroblasts by peptide growth factors or by agents which elevate cellular cyclic AMP level has opposite effects on motility. Exp Cell Res. 1985 Jan;156(1):65–78. doi: 10.1016/0014-4827(85)90262-9. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Identification of multiple PKC isoforms in Swiss 3T3 cells: differential down-regulation by phorbol ester. J Cell Physiol. 1992 Aug;152(2):240–244. doi: 10.1002/jcp.1041520204. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yang H. C. Decreased expression of the myristoylated alanine-rich C kinase substrate in transformed BALB/C 3T3 mouse fibroblasts. Biochem Biophys Res Commun. 1991 Jul 31;178(2):494–500. doi: 10.1016/0006-291x(91)90134-s. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Rapp U., Cuddy M. P. Transformed 3T3 cells have reduced levels and altered subcellular distribution of the major PKC substrate protein MARCKS. Cell Signal. 1991;3(6):569–576. doi: 10.1016/0898-6568(91)90033-q. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Keenan K. F., Thelen M., Nairn A. C., Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990 Oct 1;172(4):1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Neuropeptides as cellular growth factors: role of multiple signalling pathways. Eur J Clin Invest. 1991 Apr;21(2):123–134. doi: 10.1111/j.1365-2362.1991.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Seykora J. T., Ravetch J. V., Aderem A. Cloning and molecular characterization of the murine macrophage "68-kDa" protein kinase C substrate and its regulation by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2505–2509. doi: 10.1073/pnas.88.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S. L., Kligman D., Patel J., Colburn N. H. Differential expression of an 80-kDa protein kinase C substrate in preneoplastic and neoplastic mouse JB6 cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7410–7414. doi: 10.1073/pnas.86.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Pardee A. B. Cell cycle and growth factor-dependent phosphoprotein of 78kD differently regulated in normal and transformed mouse fibroblasts. J Cell Physiol. 1987 Nov;133(2):377–382. doi: 10.1002/jcp.1041330224. [DOI] [PubMed] [Google Scholar]