Abstract
Few studies have investigated Seomae mugwort (a Korean native mugwort variety of Artemisia argyi H. Lév. & Vaniot), exclusively cultivated in the southern Korean peninsula, and the possibility of its use as a food resource. In the present study, we compared the nutritional and chemical properties as well as sensory attributes of Seomae mugwort and the commonly consumed species Artemisia princeps Pamp. In comparison with A. princeps, Seomae mugwort had higher contents of polyunsaturated fatty acids, total phenolic compounds, vitamin C, and essential amino acids. In addition, Seomae mugwort had better radical scavenging activity and more diverse volatile compounds than A. princeps as well as favorable sensory attributes when consumed as tea. Given that scant information is available regarding the Seomae mugwort and its biological, chemical, and sensory characteristics, the results herein may provide important characterization data for further industrial and research applications of this mugwort variety.
1. Introduction
Mugworts (the genus Artemisia) have been widely used as tea, spices, and food ingredients in East Asia. Much attention has been recently paid to their multiple health benefits including anti-tumor-promoting effects [1], induction of apoptosis in various types of cancer cells [2, 3], antidiabetic effects [4], anti-inflammatory effects [5], and anticoagulant/antiplatelet activities [6]. Amongst a plethora of Artemisia species, Artemisia princeps Pamp., which is widely consumed in Korea, and its bioactive compounds (e.g., eupatilin and jaceosidin) have been most extensively studied in various experimental models [5, 6], yet little information is available regarding the Korean native mugwort variety (also known as Seomae mugwort) of Artemisia argyi H. Lév. & Vaniot, cultivated in the southern Korean peninsula.
Considering that (1) environmental factors play a significant role in growth as well as the content of active compounds of Artemisia species [7], (2) diverse Artemisia species have been demonstrated to have varying biological effects [4], and (3) scant information is available regarding the native variety of A. argyi (exclusively cultivated in Namhae County, Republic of Korea) and its biological, chemical, and sensory characteristics, it would be important and timely to report the chemical composition and functionality of this variety and the possibility of its use as a food ingredient. Specifically, in the present study both fatty acids and amino acids profiles were analyzed in order to compare contents of essential fatty acids (e.g., linoleic acid) and essential amino acids. Further, antioxidative capacity, vitamin C contents (i.e., a major vitamin of mugworts), and total phenolic compounds were assessed to address potential health promoting effects thereof. In addition, mugwort teas were prepared using Seomae mugwort and A. princeps and their sensory attributes were analyzed to test potential for the practical use of mugwort tea. All parameters of Seomae mugwort analyzed in the study were compared with those of A. princeps.
2. Materials and Methods
2.1. Materials
The Seomae mugwort (a Korean native variety of A. argyi) was kindly provided by the Namhae Agricultural Association Corporation (Namhae, Republic of Korea) where all Seomae mugworts harvested in the entire Namhae County are collected. This variety was specifically cultivated in Namhae County, Republic of Korea. A. princeps was purchased from a local store (Jinju, Republic of Korea). After being obtained, both mugworts were identified and specimen vouchers were issued by the Department of Agriculture and Herbal Resources of the Gyeongnam National University of Science and Technology (GFA-006 and GFA-007 for A. princeps and Seomae mugwort, resp.). Leaf samples were completely dried at room temperature, ground, and then stored at −80°C until being analyzed. Heptadecanoic acid (98% purity) and a lipid standard mixture (37 fatty acid methyl esters (FAME)) were from Sigma-Aldrich Co. (St. Louis, MO, USA). Other chemicals were of analytical grade.
2.2. Analysis of Free Amino Acids
Briefly, 1 g of each sample was added to 20 mL of pure ethanol and agitated for 10 min. After agitation, samples were centrifuged at 3000 ×g for 20 min and the supernatants were evaporated using a rotary evaporator (R-III; BÜCHI, Postfach, Switzerland). The residues were dissolved in 25 mL of Lithium Loading Buffer (Biochrom Ltd., Cambridge, UK) and incubated for 1 h at 4°C after addition of 20 mg of sulfosalicylic acid. Subsequently, samples were centrifuged at 3000 ×g for 20 min and filtered through a 0.2 μm membrane filter. Samples were analyzed using an amino acid analyzer (L-8900; Hitachi High Tech, Tokyo, Japan) equipped with an ion exchange column (2622PF, 4.6 mm × 60 mm; Hitachi High Tech). The column temperature ranged between 30°C and 70°C and the detection wavelengths were 570 nm and 440 nm.
2.3. Fatty Acid Composition
2.3.1. Lipid Extraction
Total lipids were extracted as reported elsewhere with slight modifications [8]. Ten grams of ground samples was suspended in 20 mL of deionized water, 50 mL of methanol, and 25 mL of chloroform, and ~10 mg of hydroquinone was subsequently added. The contents were agitated on an orbital shaker for 2 min at 3000 ×g and the resulting slurry was filtered using a filter paper (Whatman No. 1; GE Healthcare, Little Chalfont, UK). One gram of sodium chloride was added to the filtrate to facilitate phase separation and the filtrate was placed at room temperature overnight. The resulting chloroform phase was then evaporated and samples were stored under a nitrogen headspace at −80°C until being further analyzed.
2.3.2. Fatty Acid Methylation
To analyze fatty acid profiles, FAME were prepared as described previously [9]. In brief, extracted lipids (25 mg) were transferred into a Reacti-Vial (Thermo Fisher Scientific, Rockford, IL, USA) and their mass was accurately measured. The internal standard (heptadecanoic acid in hexane, 1 mg/mL) was added and samples were mixed with 0.5 N sodium hydroxide in methanol followed by flushing with nitrogen gas. The mixtures were then placed in a heating block set at 100°C for 5 min. After cooling, 2 mL of 14% boron trifluoride solution (in methanol) was added to each vial equipped with a Reacti-Vial magnetic stirrer. The vials were vortexed and placed in the Reacti-Block B-1 aluminum block within a Reacti-Therm III Heating/Stirring Module (Thermo Fisher Scientific) at 100°C for 30 min. After derivatization, each sample was extracted with 1.5 mL of hexane.
2.3.3. GC Analysis
An Agilent Technologies (Santa Clara, CA, USA) 7890A Network GC system equipped with a flame ionization detector (FID) was used to quantify fatty acids. Chromatography was performed on an SP-2560 capillary column (100 m × 0.25 mm i.d., 0.25 μm film thickness; Sigma-Aldrich Co.). The analyses were performed in the constant flow mode. A split liner with glass wool was installed in the injector and the injector temperature was set at 220°C for injection. The FID temperature was set at 240°C, and ultrahigh purity hydrogen (flow rate: 40 mL/min) and scientific-grade air (flow rate: 450 mL/min) were used as the FID fuel gases. The temperature of the oven was initially held at 140°C for 5 min and then was ramped up at 4°C/min to 230°C and maintained at 230°C for an additional 35 min. Triplicate readings were taken.
2.3.4. Fatty Acid Identification
Using the internal standard (heptadecanoic acid), the relative response factor for each FAME was calculated by using the following equation:
| (1) |
where R i is the relative response factor for fatty acid i, Ps i is the peak area of individual FAME i in the FAME standard solution, Ws C17:0 is the mass (mg) of heptadecanoic acid FAME in the injected FAME standard solution, Ps C17:0 is the peak area of heptadecanoic acid FAME in the FAME standard solution, and Ws is is the mass (mg) of individual FAME i in the injected FAME standard solution.
2.3.5. Method Validation for Fatty Acid Analysis
The relative repeatability standard deviation and % relative standard deviation were determined to validate the method for fatty acid analysis in lipid extracts of Seomae mugwort and A. princeps by assaying Standard Reference Material (SRM) 1849a (Infant/Adult Nutritional Formula) purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA).
2.4. Determination of Total Phenolic Compounds
The total phenolic contents of Seomae mugwort and A. princeps were compared using a previously described spectrophotometric method with slight modifications [10]. The dried samples were prepared at a concentration of 1 mg/mL of water and then a 40 μL aliquot of each sample was diluted with 200 μL of distilled water. Folin-Ciocalteu's reagent (200 μL; Sigma-Aldrich Co.) was added to the mixture, followed by the addition of 600 μL of sodium carbonate solution (30%, w/v) and 160 μL of distilled water. The mixture was thoroughly mixed and kept in the dark for 2 h at 25°C, after which the absorbance was read at 750 nm. The total phenolic compounds in each sample were determined from interpolation of the calibration curve constructed by using gallic acid solution (0–500 μg/mL).
2.5. Analysis of Vitamin C Contents
One gram of ground mugwort sample was added to 1 mL of 10% formic acid and then diluted with 19 mL of 5% formic acid. Samples were thoroughly vortexed and placed at room temperature for 20 min followed by centrifugation (1,000 ×g; 10 min). Resulting supernatants were filtered through a HPLC membrane filter (Sigma-Aldrich Co., Nylon 66 Filter Membranes, 0.45 μm) and injected to a HPLC system (10 μL injection; Shimadzu, Kyoto, Japan). Isocratic method (0.05 M of phosphate buffer and acetonitrile, 60 : 40) was used for the separation of vitamin C using the Bondapak C18 column (Waters, Milford, MA, USA) which was utilized for separation and analytes were monitored at 245 nm wavelength. The standard curve was constructed using the authentic standard for quantification of vitamin C. The r-squared value of the standard curve was greater than 0.99.
2.6. Antioxidative Capacity Measurement
The antioxidant capacity of Seomae mugwort and A. princeps was compared using a typical 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as described elsewhere [11]. In brief, serial dilutions of samples were prepared (200, 400, 600, and 800 μg/mL) and then 80 μL of each sample was added to 320 μL of DPPH solution (0.2 mM, dissolved in pure ethanol). The reactions were performed in an incubator at 37°C and the absorbance was measured at 517 nm. The IC50 of each sample was calculated by using the following equation:
| (2) |
2.7. Analysis of Volatile Compound Composition
A Likens and Nickerson–type simultaneous steam distillation and extraction apparatus (SDE) was used for the extraction of volatile compounds according to the method reported elsewhere [12]. Ground samples (100 g) were mixed with distilled water (1 L) followed by the addition of internal standard (1 mL of n-pentadecane, 1 mg/mL, Sigma-Aldrich Co.). Atmospheric steam distillation was performed to collect sample volatiles in a 100 mL mixture of n-pentane and diethyl ether (1 : 1, v/v) over 3 h at 110°C. Anhydrous sodium sulfate (10 g) was added to the extracts, which were then placed at 4°C overnight. Samples were then filtered and reduced to a volume of 1 mL using a nitrogen evaporator. Concentrated samples were analyzed using a GC fitted with a mass spectrometer (Agilent 7890A and 5975C, resp.), which was operated in electron impact ionization mode (70 eV), scanning a mass range (m/z) from 30 to 550 amu. An HP-5MS column (30 m × 0.25 mm, i.d. × 0.25 μm film thickness, Agilent Technologies) was used for the analysis. The temperature of the column was maintained at 4°C for the first 5 min and then increased to 200°C at a rate of 5°C/min. The analysis was carried out in the splitless mode, using helium as the carrier gas (1 mL/min flow rate). The injector temperature was 220°C. Separated peaks in the total ionization chromatogram were identified using a database (The NIST 12 Mass Spectrum Library; Gaithersburg, MD, USA) and then confirmed by matching the retention indices (RI) with data from published literature. RI were calculated according to the following formula [13] and based on a series of n-alkanes (C8–C20):
| (3) |
where RIx is RI of the unknown compound, t Rx is retention time of the unknown compound, t Rn is retention time of the n-alkane, and t Rn+1 is retention time of the next n-alkane. Each t Rx is between t Rn and t Rn+1 (n = number of carbon atoms).
2.8. Olfactometry Analysis
Separated volatile compounds were further analyzed through an olfactory detection port with a heated mixing chamber (ODP 3; Gerstel, Linthicum, MD, USA). In advance of performing experiments, panels were trained with the instrument operation and data collection. Specifically, they were asked to respond to their perceived intensity of odor through the detection port using a signal generator. The intensity scales of the signal generator ranged from 0 (no perception) to 5 (the strongest perception). To take into account individual variations, 3 trained panels performed an identical experiment and recorded the intensity of each volatile compound isolated from the samples.
2.9. Evaluation of Sensory Attributes of Seomae Mugwort and A. princeps
2.9.1. Study Participants
A total of 15 participants evaluated the sensory attributes of the two Artemisia species. All subjects were recruited from the Gyeongnam National University of Science and Technology through fliers and received a gift card incentive for participation. People who discovered themselves having allergy to either Seomae mugwort or A. princeps were screened prior to the sensory evaluation. The study was approved by the University Institutional Review Board and consent forms were provided to participants. The demographic characteristics are summarized in Table 1.
Table 1.
Demographic information of study participants and frequency of tea consumption(a).
| Percentage (n) | |
|---|---|
| Gender | |
| Male | 33 (5) |
| Female | 67 (10) |
|
| |
| Age | |
| 19–29 | 87 (13) |
| 30–40 | 13 (2) |
| ≤40 | 0 |
|
| |
| Frequency of tea consumption (per month) | |
| Never | 13 (2) |
| ≥5 times | 60 (9) |
| ≥10 times | 27 (4) |
| ≥20 times | 0 (0) |
| Daily | 0 (0) |
(a)A total of 15 participants were recruited from the Gyeongnam National University of Science and Technology through fliers. The protocol was approved by the University Institutional Review Board and written consent forms were obtained from the participants in advance ofcollecting data.
2.9.2. Tea Preparation and Sensory Evaluation
To prepare mugwort tea, 5 g of a dried sample was added to 1 L of boiling water and brewed for 5 min. All preparation steps were performed by a professional cook and samples were prepared about 10 min before sensory evaluation. Teas prepared from both species (100 mL each) were provided to each subject. Participants evaluated the teas for perceived color acceptability, flavor acceptability, saltiness, bitterness, sourness, astringency, sweetness, and overall preference using labeled affective magnitude (LAM) scales; the scales were labeled with the phrases “greatest imaginable like,” “like extremely,” “like very much,” “like moderately,” “like,” “neither like nor dislike,” “dislike moderately,” “dislike very much,” “dislike extremely,” and “greatest imaginable dislike.” The scales ranged from 0 (greatest imaginable dislike) to 15 (greatest imaginable like) [14].
2.10. Statistical Analysis
All results were expressed as the mean ± standard deviation (SD). The statistical significance between groups (i.e., Seomae mugwort versus A. princeps) was tested via Student's t-test, using the Statistical Analysis System (SAS; Cary, NC, USA). A P value less than 0.05 was considered to be statistically significant.
3. Results and Discussion
To compare general nutritional compositions of Seomae mugwort and A. princeps, we analyzed the content of free amino acids, fatty acids, vitamin C, and total phenolic compounds. First, we found that the content of free amino acids of A. princeps was significantly different from that of Seomae mugwort (Table 2). Specifically, the content of the essential amino acids valine and phenylalanine was significantly higher in Seomae mugwort (by approximately 63% and 41%, resp.) than in A. princeps. The content of total essential amino acids was approximately 57% in A. princeps and 61% in Seomae mugwort. Notably, it has been reported that γ-aminobutyric acid (GABA), a nonprotein amino acid, is beneficial for treatment of general anxiety and anxiety disorders [15, 16]. We found that Seomae mugwort had approximately 3.8-fold higher content of GABA than A. princeps, indicating potential benefits of this variety in medicinal psychopharmacology, which warrants further investigations.
Table 2.
Free amino acids profile of Artemisia princeps Pamp. and Seomae mugwort(a).
| A. princeps | Seomae mugwort | |
|---|---|---|
| Essential amino acid (mg/100 g of dried material) | ||
| Histidine | 7.18 ± 0.16a | 2.54 ± 0.06b |
| Phenylalanine | 66.05 ± 0.26b | 93.78 ± 0.74a |
| Valine | 102.71 ± 1.97b | 167.07 ± 0.85a |
| Leucine | 59.26 ± 0.65a | 44.51 ± 0.60b |
| Isoleucine | 61.56 ± 0.89a | 47.62 ± 00.57b |
| Threonine | 22.20 ± 1.03a | 15.12 ± 0.29b |
|
| ||
| Nonessential amino acid (mg/100 g of dried material) | ||
| Arginine | 29.47 ± 0.68a | 20.55 ± 0.32b |
| γ-Aminobutyric acid | 12.60 ± 0.18b | 48.52 ± 0.87a |
| Alanine | 86.90 ± 0.88a | 34.29 ± 0.60b |
| Cysteine | 4.42 ± 0.30 | 4.52 ± 0.25 |
| Glutamic acid | 23.59 ± 0.68b | 33.45 ± 0.36a |
| Tyrosine | 7.62 ± 0.13b | 10.77 ± 0.38a |
| Glycine | 4.57 ± 0.25b | 11.39 ± 0.35a |
| β-Alanine | 16.18 ± 0.78 | 15.96 ± 0.09 |
| α-Aminobutyric acid | 2.53 ± 0.44b | 6.54 ± 0.37a |
| Aspartic acid | 7.01 ± 0.51b | 8.68 ± 0.29a |
| Serine | 47.52 ± 0.61 | 47.48 ± 0.65 |
|
| ||
| Total essential amino acid | 318.93 ± 1.22b | 370.64 ± 0.27a |
| Total nonessential amino acid | 242.35 ± 2.24 | 242.15 ± 2.80 |
| Total free amino acid | 561.28 ± 3.30b | 612.79 ± 2.97a |
(a)Data represents the mean ± SD (n = 3). Different superscript letters indicate statistical significance of the differences between Seomae mugwort and A. princeps groups, tested by Student's t-test using the SAS. P values less than 0.05 were considered statistically significant.
The fatty acid analysis method was validated before determination of the fatty acid composition of Seomae mugwort and A. princeps (Table 3). The accuracy of the method was calculated based on the percentage of the certified fatty acid content in SRM 1849a and expressed as the percentage of the accepted value. The accuracy ranged from 94.12 to 108.33%, while the reproducibility of the method, indicated by the relative standard deviation (RSD), was higher than 90% for all fatty acids. The complete fatty acid profiles of Seomae mugwort and A. princeps are shown in Table 4. In total, nine fatty acids, ranging from C16 to C24, were detected based on retention mapping with external standards. These fatty acids were quantified relative to the internal standard (heptadecanoic acid). In A. princeps, C18:1 and C18:2 were the most prevalent fatty acids (34.91% and 27.56%, resp.), followed by C18:3 ω-6 (9.83%), C16:0 (8.73%), and other fatty acids. Interestingly, the content of C18:3 ω-6 was much higher in Seomae mugwort (36.36%, Table 4). Artemisia princeps had a lower total content of saturated fatty acids than Seomae mugwort (27.47% versus 40.79%), while the content of polyunsaturated fatty acids was higher in Seomae mugwort, likely due to C18:3 ω-6 (Table 4).
Table 3.
Method validation of fatty acids analysis: % accepted values and % relative standard deviations (RSD) determined using SRM 1849a.
| Fatty acids | % weight | % of accepted value(d) | % RSD(e) | ||
|---|---|---|---|---|---|
| Accepted value(a) | Analytical value(b) | Bias(c) | |||
| C14:0 | 4.76 ± 0.14 | 4.79 ± 0.13 | −0.03 | 100.63 | 2.71 |
| C16:0 | 9.89 ± 1.10 | 9.81 ± 0.21 | 0.08 | 99.19 | 2.14 |
| C16:1 ω-7 | 0.12 ± 0.01 | 0.13 ± 0.01 | −0.01 | 108.33 | 7.69 |
| C18:0 | 4.21 ± 0.10 | 4.25 ± 0.05 | −0.04 | 100.95 | 1.18 |
| C18:1 ω-9 | 50.37 ± 5.51 | 50.45 ± 2.72 | −0.08 | 100.16 | 5.39 |
| C18:1 ω-7 | 1.02 ± 0.03 | 1.03 ± 0.05 | −0.01 | 100.98 | 4.85 |
| C18:2 ω-6 | 25.95 ± 2.11 | 25.82 ± 1.10 | 0.13 | 99.50 | 4.26 |
| C18:3 ω-3 | 0.42 ± 0.01 | 0.46 ± 0.02 | −0.04 | 109.52 | 4.35 |
| C20:0 | 0.24 ± 0.03 | 0.26 ± 0.01 | −0.02 | 108.33 | 3.85 |
| C20:1 ω-9 | 2.51 ± 0.26 | 2.52 ± 0.05 | −0.01 | 100.40 | 1.98 |
| C22:0 | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.02 | 94.12 | 3.13 |
| C24:0 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.01 | 94.12 | 6.25 |
(a)The accepted value was calculated using the certified fatty acids content of SRM 1849a based on % weight.
(b)Data represents the mean ± SD (n = 3). (c)Bias = accepted value − analytical value. (d)The ratio of the analytical value to accepted value expressed as a percentage. (e)RSD indicates interday relative standard deviation (SD × 100/mean) of analytical values.
Table 4.
Comparison of fatty acid profiles between Artemisia princeps Pamp. and Seomae mugwort(a).
| Fatty acids | A. princeps | Seomae mugwort |
|---|---|---|
| C16:0 | 8.73 ± 0.06b | 18.82 ± 0.15a |
| C16:1 | 0.23 ± 0.01b | 2.04 ± 0.05a |
| C18:0 | 3.54 ± 0.04a | 1.66 ± 0.07b |
| C18:1 | 34.91 ± 0.06a | 5.09 ± 0.09b |
| C18:2 | 27.56 ± 0.07a | 15.73 ± 0.12b |
| C20:0 | 2.53 ± 0.04b | 3.63 ± 0.13a |
| C18:3 ω-6 | 9.83 ± 0.06b | 36.36 ± 0.20a |
| C22:0 | 8.58 ± 0.14b | 10.91 ± 0.09a |
| C24:0 | 4.08 ± 0.14b | 5.76 ± 0.07a |
|
| ||
| SFA(b) | 27.47 ± 0.08b | 40.79 ± 0.10a |
| MUFA(c) | 35.14 ± 0.03a | 7.12 ± 0.07b |
| PUFA(d) | 37.39 ± 0.06b | 52.09 ± 0.16a |
(a)Data represents the mean ± SD (n = 3). Different superscript letters indicate statistical significance of the differences between Seomae mugwort and A. princeps groups, tested by Student's t-test using the SAS. P values less than 0.05 were considered statistically significant. (b)SFA: saturated fatty acids. (c)MUFA: monounsaturated fatty acids. (d)PUFA: polyunsaturated fatty acids.
The amount of phenolic compounds in A. princeps was 49.12 ± 1.23 mg per 100 g of dried material whilst it was much higher (by approximately 50%) in Seomae mugwort (74.53 ± 2.08 mg per 100 g, Table 5). Further, the vitamin C content of Seomae mugwort was 2-fold higher than that in A. princeps. Specifically, it was found that Seomae mugwort contains 209.1 ± 3.2 mg of vitamin C per 100 g of dried sample materials (Table 5). We compared the antioxidative capacities of the two mugwort species using the DPPH radical scavenging assay and found that the IC50 value of Seomae mugwort was 0.55 ± 0.09 mg, whereas A. princeps extract required a higher concentration, 0.82 ± 0.12 mg, which is expected given the significantly higher amounts of vitamin C/total phenolic compounds in the Seomae mugwort. Generally, the antioxidant activity is closely correlated with the amount of phenolic compounds [17, 18]; this trend was also observed in the present study (Table 5). However, due to the inherent limitations of the method (e.g., nonspecific oxidation by Folin-Ciocalteu's reagent), identification of specific phenolic constituents was not possible in our experiments. Their identification in the future might further elucidate the health benefits of these mugwort species.
Table 5.
Total phenolic contents, vitamin C contents, and antioxidative capacities of Artemisia princeps Pamp. and Seomae mugwort(a).
| A. princeps | Seomae mugwort | |
|---|---|---|
| Total phenolic content (mg/100 g of dried sample)(b) | 49.12 ± 1.23b | 74.53 ± 2.08a |
| IC50 in DPPH radical scavenging (mg)(c) | 0.82 ± 0.12a | 0.55 ± 0.09b |
| Vitamin C content (mg/100 g of dried sample)(d) | 100.6 ± 2.2b | 209.1 ± 3.2a |
(a)Data represents the mean ± SD (n = 3). Different superscript letters indicate statistical significance of the differences between Seomae mugwort and A. princeps groups, tested by Student's t-test using the SAS. P values less than 0.05 were considered statistically significant. (b)The total phenolic contents of samples were measured using Folin-Ciocalteu's reagent as described in the Materials and Methods. (c)The IC50 values of A. princeps and Seomae mugwort were calculated and compared using a typical 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. (d)The vitamin C was analyzed using the HPLC as described in the Materials and Methods.
Using SDE, 43 volatile compounds were identified in A. princeps and 50 in Seomae mugwort (Table 6). Representative chromatograms of both mugwort species are shown in Supplemental Figure 1 in the Supplementary Material available online at http://dx.doi.org/10.1155/2015/916346. Intuitively, it is clear that Seomae mugwort should have more diverse profiles of volatiles given the numbers of compounds listed in the table and identified in chromatograms, as well as their peak areas. This was further supported by olfactometry analysis by three trained panels. Strong intensities of Seomae mugwort were recorded mostly between 12 min and 21 min of the aromagram (Supplemental Figure 1(C)). Notably, within this range of retention times, a few volatile chemicals present in Seomae mugwort had significantly higher peak areas. For instance, terpenic compounds (e.g., α-terpinolene and α-terpinene) were significantly more abundant in Seomae mugwort than in A. princeps; most of these compounds were not detected in A. princeps (e.g., α-terpinene, 1,8-cineole, camphor, and 4-terpineol; Table 6). It has been reported that these terpenic compounds possess characteristic woody, citrus, floral, and herbal flavors [19], which possibly confer more favorable sensory characteristics when consumed in the present study. Importantly, the sensory attributes of volatile compounds are difficult to predict due to potential associations between aromas of different compounds (e.g., synergistic or masking effects) [19]. In the olfactometry analysis, we only recorded the aroma intensities but were unable to assess their flavor descriptions and acceptability. Hence, comparative sensory evaluation of Seomae mugwort and A. princeps was performed.
Table 6.
Volatile compounds present in Artemisia princeps Pamp. and Seomae mugwort(a).
| Peak number(b) | Compounds(c) | Retention time (min) | Peak area ×103 | |
|---|---|---|---|---|
| A. princeps | Seomae mugwort | |||
| 1 | Propanoic acid methyl ester | 3.32 | 4,655.5 ± 502.1b | 43,759.7 ± 1,202.3a |
| 2 | Acetic acid ethyl ester | 3.91 | 873.6 ± 90.2b | 8,365.1 ± 902.1a |
| 3 | 2,3-Dimethyl pentane | 4.79 | 89.1 ± 84.3b | 984.5 ± 42.5a |
| 4 | Butyl ethyl ether | 5.39 | 478.3 ± 63.1b | 4,321.9 ± 472.5a |
| 5 | Diethyl sulfide | 5.51 | 61.5 ± 33.2b | 1,687.2 ± 202.9a |
| 6 | Acetal | 6.29 | 1,122.9 ± 172.6b | 13,265.2 ± 1,502.5a |
| 7 | 2-Methyl-2-hexanol | 6.98 | Not detectedb | 1,178.4 ± 227.3a |
| 8 | Valeric acid methylbutyl ester | 7.07 | 1,646.2 ± 216.2b | 19,573.6 ± 1,312.4a |
| 9 | Methylbenzene | 7.32 | 372.7 ± 39.3b | 3,455.6 ± 482.1a |
| 10 | 2-Furancarboxaldehyde | 9.45 | 53.3 ± 32.1b | 953.3 ± 113.2a |
| 11 | Chlorobenzene | 9.87 | 59.5 ± 29.6b | 757.16 ± 221.5a |
| 12 | 2-Hexenal | 10.13 | Not detectedb | 1,323.3 ± 160.5a |
| 13 | Ethyl benzene | 10.40 | 1,253.9 ± 264.3b | 15,135.1 ± 1,302.1a |
| 14 | m-Xylol | 10.67 | 42.9 ± 40.7b | 624.01 ± 129.4a |
| 15 | o-Xylol | 11.47 | 89.3 ± 66.2b | 1,628.5 ± 278.4a |
| 16 | α-Terpinolene | 12.62 | Not detectedb | 60,468.9 ± 2,532.8a |
| 17 | α-Pinene | 12.85 | 252.9 ± 102.5b | 5,512.4 ± 762.0a |
| 18 | Camphene | 13.35 | Not detectedb | 2,842.3 ± 388.7a |
| 19 | Sabinene | 14.18 | 49.8 ± 36.1b | 357.3 ± 94.4a |
| 20 | β-Pinene | 14.29 | 220.3 ± 100.5b | 1,473.5 ± 233.5a |
| 21 | 1-Octen-3-ol | 14.34 | Not detected | 3,433.3 ± 582.3a |
| 22 | β-Myrcene | 14.72 | 119.4 ± 84.5a | Not detectedb |
| 23 | Yomogi alcohol | 15.08 | Not detectedb | 288,651.3 ± 1,321.1a |
| 24 | α-Terpinene | 15.57 | Not detectedb | 1,863.8 ± 282.4a |
| 25 | o-Cymene | 15.83 | Not detectedb | 1,483.3 ± 248.5a |
| 26 | D-Limonene | 15.96 | 75.9 ± 63.1b | 750.3 ± 121.5a |
| 27 | 1,8-Cineole | 16.05 | Not detectedb | 32,351.2 ± 1,321.8a |
| 28 | 2,4-Hexadiene | 16.17 | Not detectedb | 5,933.3 ± 567.3a |
| 29 | Phenyloxirane | 16.42 | 695.1 ± 111.8a | Not detectedb |
| 30 | Benzeneacetaldehyde | 16.43 | Not detectedb | 5,493.2 ± 484.3a |
| 31 | γ-Terpinene | 16.90 | Not detectedb | 1,384.6 ± 233.2a |
| 32 | cis-β-Terpineol | 17.21 | Not detectedb | 617.9 ± 171.3a |
| 33 | Artemisia alcohol | 17.78 | Not detectedb | 533,734.3 ± 8,242.0a |
| 34 | β-Linalool | 18.15 | 136.7 ± 70.3b | 17,562.3 ± 1,382.4a |
| 35 | Nonanal | 18.25 | 80.7 ± 29.5a | Not detectedb |
| 36 | Camphor | 19.57 | Not detectedb | 4,463.87 ± 529.4a |
| 37 | 4-Terpineol | 20.49 | Not detectedb | 4,215.52 ± 498.5a |
| 38 | β-Fenchyl alcohol | 20.88 | Not detectedb | 2,583.98 ± 200.4a |
| 39 | Indole | 23.69 | 144.0 ± 101.1b | 1,073.78 ± 218.3a |
| 40 | δ-Elemene | 24.87 | 75.4 ± 43.1a | Not detectedb |
| 41 | Eugenol | 25.34 | Not detectedb | 13,037.30 ± 1,009.3a |
| 42 | α-Copaene | 25.90 | 325.0 ± 112.2b | 2,643.10 ± 183.6a |
| 43 | β-Bourbone | 26.16 | 65.25 ± 45.6b | 2,933.01 ± 438.3a |
| 44 | β-Elemene | 26.27 | 845.4 ± 205.1a | Not detectedb |
| 45 | Caryophyllene | 27.06 | 13,728.3 ± 1,225.3b | 85,473.18 ± 5,384.5a |
| 46 | β-Copaene | 27.26 | Not detectedb | 1,417.52 ± 135.8a |
| 47 | α-Amorphene | 27.65 | 60.5 ± 60.9a | Not detectedb |
| 48 | cis-β-Farnesene | 27.75 | 1,890.6 ± 210.9b | 2,483.2 ± 499.3a |
| 49 | α-Humulene | 27.88 | 3,921.7 ± 673.3b | 9,065.3 ± 886.1a |
| 50 | γ-Muurolene | 28.37 | Not detectedb | 1,646.7 ± 245.3a |
| 51 | γ-Curcumene | 28.38 | 998.1 ± 89.0a | Not detectedb |
| 52 | β-Cubebene | 28.54 | 16,826.3 ± 1,533.2b | 29,434.57 ± 5,553.7a |
| 53 | β-Selinene | 28.68 | Not detectedb | 8,386.7 ± 1,334.3a |
| 54 | Zingiberene | 28.75 | 6,225.4 ± 562.1a | Not detectedb |
| 55 | Germacrene B | 28.91 | 1,347.8 ± 113.2a | Not detectedb |
| 56 | α-Farnesene | 28.99 | 889.5 ± 82.0a | Not detectedb |
| 57 | β-Bisabolene | 29.07 | 200.2 ± 121.2a | Not detectedb |
| 58 | γ-Cadinene | 29.29 | 353.5 ± 178.1b | 3,976.39 ± 529.9a |
| 59 | δ-Cadinene | 29.47 | 1,345.4 ± 203.1b | 4,073.7 ± 587.9a |
| 60 | α-Cadinene | 29.82 | 211.4 ± 52.2a | Not detectedb |
| 61 | trans-β-Farnesene | 30.33 | 158.2 ± 78.0a | Not detectedb |
| 62 | Nerolidol | 30.34 | Not detectedb | 3,122.5 ± 443.9a |
| 63 | Caryophyllene oxide | 30.95 | 315.3 ± 192.1a | Not detectedb |
| 64 | Diethyl phthalate | 31.02 | 233.6 ± 54.2a | Not detectedb |
| 65 | α-Guaiene | 31.30 | Not detectedb | 1,347.3 ± 309.4a |
| 66 | tau-Muurolol | 32.20 | 349.1 ± 120.2a | Not detectedb |
(a)Data represents the mean ± SD (n = 3). Different superscript letters indicate statistical significance of the differences between Seomae mugwort and A. princeps groups, tested by Student's t-test using the SAS. P values less than 0.05 were considered statistically significant. (b)Peak numbering was determined by the order of elution. (c)The gas chromatographic retention data and mass spectral data were compared to those of authentic samples and library compounds, respectively.
To examine the potential for the practical use of mugwort tea as a nutritious drink, we prepared tea samples from both mugwort species. As mentioned above, the participants were asked to evaluate perceived preference for each mugwort tea. Subjects evaluated color acceptability prior to consuming the samples. Then, other perceived qualities (flavor acceptability, saltiness, bitterness, sourness, astringency, sweetness, and overall preference) were evaluated after sample consumption by using the LAM scales of 0–15 points. We did not find any differences in sweetness (6.8 ± 1.8 and 7.3 ± 1.2 for A. princeps and Seomae mugwort), bitterness (7.7 ± 1.5 and 6.9 ± 2.1 for A. princeps and Seomae mugwort), sourness (6.1 ± 2.2 and 7.4 ± 2.4 for A. princeps and Seomae mugwort), astringency (7.7 ± 2.1 and 7.2 ± 2.2 for A. princeps and Seomae mugwort), and saltiness (6.1 ± 2.4 and 6.5 ± 1.8 for A. princeps and Seomae mugwort) between samples. There were, however, significant differences in overall preference (5.8 ± 0.9 and 8.9 ± 1.1 for A. princeps and Seomae mugwort; P < 0.05) and flavor acceptability (8.9 ± 1.1 and 10.6 ± 1.0 for A. princeps and Seomae mugwort; P < 0.05, Figure 1), which may be due to the differences in the profiles of volatile compounds between Seomae mugwort and A. princeps, in particular the difference in terpenic compounds (Table 6). Of many properties, we were specifically interested in “bitter taste” and “astringency,” which may impact consumers' preference and palatability. In the analysis of free amino acids, we found that the content of branched-chain amino acids was slightly higher in Seomae mugwort than in A. princeps (259.20 mg/100 g versus 223.53 mg/100 g; Table 2). Branched-chain amino acids (leucine, isoleucine, and valine) are known to confer bitter taste [20]. However, our results indicate that there was no difference in such unfavorable tastes between these mugwort species. No significant correlation was found between tested sensory attributes and the frequency of tea consumption as well as participants' sex (data not shown). Considering the small number of participants, further investigations may be warranted to clarify and confirm the observed trends. Furthermore, it would be interesting to include another type of tea (e.g., green tea) in sensory evaluation as a control for a direct comparison with its sensory attributes. Lastly, given the nature of sensory evaluation, it is also possible that perceived attributes relatively vary with individuals; thus, descriptive sensory evaluation with trained panelists might be warranted in the future.
Figure 1.
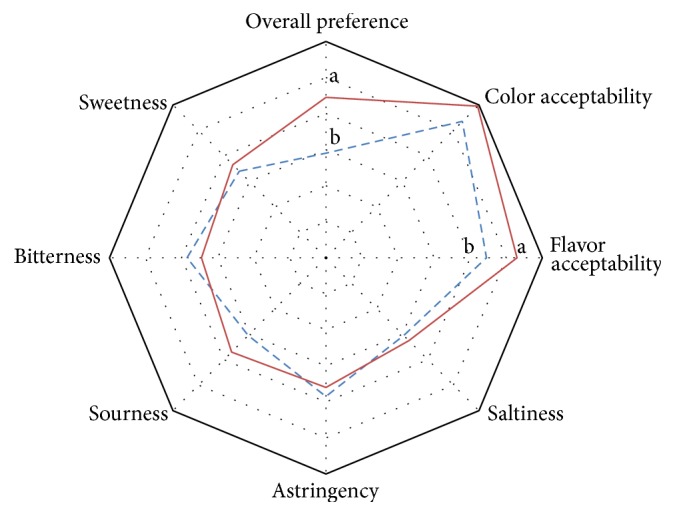
Comparison of the sensory profiles of mugwort tea prepared with either Artemisia princeps Pamp. or Seomae mugwort (a Korean native variety of Artemisia argyi H. Lév. & Vaniot). A total of 15 participants used LAM scales for perceived color acceptability, flavor acceptability, saltiness, bitterness, sourness, astringency, sweetness, and overall preference. Dashed line and solid line indicate A. princeps and Seomae mugwort, respectively. Preference scales ranged from 0 (greatest imaginable dislike) to 15 (greatest imaginable like). Different superscript letters indicate statistical significance of the differences between Seomae mugwort and A. princeps groups, tested by Student's t-test using the SAS. P values less than 0.05 were considered statistically significant.
4. Conclusions
In the present study, we compared the nutritional characteristics and sensory attributes of Seomae mugwort, a native mugwort variety of A. argyi cultivated in Namhae County in South Korea, and those of A. princeps. The native variety showed (1) higher contents of essential amino acids without compromising flavor, (2) higher amount of polyunsaturated fatty acids, likely due to an increased content of C18:3 ω-3, (3) better radical scavenging activity against DPPH and higher vitamin C/total phenolic compound contents, and (4) more diverse volatile compounds with more favorable sensory attributes when consumed as tea. Given that scant information is available regarding the Seomae mugwort and its biological, chemical, and sensory characteristics, the results of this study may provide important preliminary data for further industrial and research applications of this mugwort variety.
Supplementary Material
Representative total ion chromatograms of volatile compounds and aromagram of Seomae mugwort.
Acknowledgment
This research was supported by High Value-Added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Jae Kyeom Kim and Eui-Cheol Shin equally contributed to this work.
References
- 1.Seo H. J., Park K. K., Han S. S., et al. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. International Journal of Cancer. 2002;100(4):456–462. doi: 10.1002/ijc.10489. [DOI] [PubMed] [Google Scholar]
- 2.Cho J.-H., Lee J.-G., Yang Y.-I., et al. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food and Chemical Toxicology. 2011;49(8):1737–1744. doi: 10.1016/j.fct.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Sarath V. J., So C.-S., Young D. W., Gollapudi S. Artemisia princeps var orientalis induces apoptosis in human breast cancer MCF-7 cells. Anticancer Research. 2007;27(6B):3891–3898. [PubMed] [Google Scholar]
- 4.Jung U. J., Baek N.-I., Chung H.-G., et al. The anti-diabetic effects of ethanol extract from two variants of Artemisia princeps Pampanini in C57BL/KsJ-db/db mice. Food and Chemical Toxicology. 2007;45(10):2022–2029. doi: 10.1016/j.fct.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Kim M. J., Han J. M., Jin Y. Y., et al. In vitro antioxidant and anti-inflammatory activities of jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Archives of Pharmacal Research. 2008;31(4):429–437. doi: 10.1007/s12272-001-1175-8. [DOI] [PubMed] [Google Scholar]
- 6.Ryu R., Jung U. J., Kim H.-J., et al. Anticoagulant and antiplatelet activities of artemisia princes pampanini and its bioactive components. Preventive Nutrition and Food Science. 2013;18(3):181–187. doi: 10.3746/pnf.2013.18.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thu B. T. T., van Minh T., Lim B. P., Keng C. L. Effects of environmental factors on growth and artemisinin content of Artemisia annua L. Tropical Life Sciences Research. 2011;22(2):37–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 9.Ngeh-Ngwainbi J., Lin J., Chandler A., et al. Determination of total, saturated, unsaturated, and monounsaturated fats in cereal products by acid hydrolysis and capillary gas chromatography: collaborative study. Journal of AOAC International. 1997;80(2):359–372. [PubMed] [Google Scholar]
- 10.Kim D.-O., Lee K. W., Lee H. J., Lee C. Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. Journal of Agricultural and Food Chemistry. 2002;50(13):3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 11.Su B.-L., Zeng R., Chen J.-Y., Chen C.-Y., Guo J.-H., Huang C.-G. Antioxidant and antimicrobial properties of various solvent extracts from Impatiens balsamina L. stem. Journal of Food Science. 2012;77(6):C614–C619. doi: 10.1111/j.1750-3841.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- 12.Schultz T. H., Flath R. A., Mon T. R., Eggling S. B., Teranishi R. Isolation of volatile components from a model system. Journal of Agricultural and Food Chemistry. 1977;25(3):446–449. doi: 10.1021/jf60211a038. [DOI] [Google Scholar]
- 13.Vandendool H., Kratz P. D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. Journal of Chromatography. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 14.Schutz H. G., Cardello A. V. A labeled affective magnitude (LAM) scale for assessing food liking/disliking. Journal of Sensory Studies. 2001;16(2):117–159. doi: 10.1111/j.1745-459X.2001.tb00293.x. [DOI] [Google Scholar]
- 15.Sarris J., McIntyre E., Camfield D. A. Plant-based medicines for anxiety disorders, part 1: a review of preclinical studies. CNS Drugs. 2013;27(3):207–219. doi: 10.1007/s40263-013-0044-3. [DOI] [PubMed] [Google Scholar]
- 16.Sarris J., McIntyre E., Camfield D. A. Plant-based medicines for anxiety disorders, part 2: a review of clinical studies with supporting preclinical evidence. CNS Drugs. 2013;27(4):301–319. doi: 10.1007/s40263-013-0059-9. [DOI] [PubMed] [Google Scholar]
- 17.Zheleva-Dimitrova D. Z. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacognosy Magazine. 2013;9(34):109–113. doi: 10.4103/0973-1296.111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Nasr S., Aazza S., Mnif W., Miguel M. G. Antioxidant and anti-lipoxygenase activities of extracts from different parts of Lavatera cretica L. grown in Algarve (Portugal) Pharmacognosy Magazine. 2015;11(41):48–54. doi: 10.4103/0973-1296.149743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson A. J., Heymann H., Ebeler S. E. Volatile and sensory profiling of cocktail bitters. Food Chemistry. 2015;179:343–354. doi: 10.1016/j.foodchem.2015.01.114. [DOI] [PubMed] [Google Scholar]
- 20.Mukai J., Tokuyama E., Ishizaka T., Okada S., Uchida T. Inhibitory effect of aroma on the bitterness of branched-chain amino acid solutions. Chemical & Pharmaceutical Bulletin. 2007;55(11):1581–1584. doi: 10.1248/cpb.55.1581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative total ion chromatograms of volatile compounds and aromagram of Seomae mugwort.


