Abstract
Aim
Evaluation of effects of intravitreal aflibercept therapy on choroidal thickness (CT) in neovascular age-related macular degeneration.
Methods
Retrospective cohort study evaluating the change in CT following a loading dose of three intravitreal aflibercept injections at 4 weeks interval. Pretreated and treatment-naive eyes as well as untreated fellow eyes were evaluated at five retinal locations (subfoveal, 300 and 2500 µm nasal and temporal to the fovea) using spectral domain optical coherence tomography prior to and 4 weeks after a loading dose of three intravitreal aflibercept injections.
Results
A total of 84 treated eyes (61 pretreated, 23 treatment naive) and 48 fellow eyes were enrolled into the study. Treatment-naive and pretreated eyes showed a significant reduction in CT at all retinal locations. The effect was more pronounced in treatment-naive eyes. In the pretreated group, the mean reduction in CT was greatest at 2500 µm temporal to the fovea at 10.7 µm compared with 22.4 at 300 µm nasal to the fovea in the treatment-naive group. Only the fellow eyes in the treatment-naive group showed a significant CT reduction 12 weeks after initiation of therapy to the partner eye.
Conclusions
Aflibercept induces a reduction in CT in treatment-naive and pretreated eyes with neovascular age-related macular degeneration. There is some evidence of a systemic effect of aflibercept reflected by CT reduction in untreated fellow eyes.
Keywords: Angiogenesis, Choroid, Degeneration, Macula, Treatment Medical
Introduction
For almost a decade, intravitreal antivascular endothelial growth factor (VEGF) injections have been the first-line therapy for neovascular age-related macular degeneration (AMD).1 2 Large, independent randomised clinical trials comparing ranibizumab and bevacizumab have found overall similar functional and anatomic results, with a trend towards a greater reduction of retinal thickness for ranibizumab.3 4 Aflibercept is the latest approved VEGF-inhibitor and, when given every 4 or 8 weeks, has shown functional and anatomic outcomes comparable with 4-weekly ranibizumab.5 However, there is evidence that aflibercept induces a further reduction in retinal thickness and pigment epithelial detachments in patients insufficiently responding to anti-VEGF therapy with either ranibizumab or bevacizumab.6 Furthermore, aflibercept appears to be most effective in diseases associated with a thicker choroid such as idiopathic polypoidal choroidal vasculopathy (IPCV), choroidal vascular hyperpermeability and central serous chorioretinopathy.7–9 One potential mechanism that could explain these findings is the effect of aflibercept on choroidal vasculature, leading to thinning of this structure.
To assess this hypothesis, choroidal thickness (CT) was measured by spectral domain optical coherence tomography retrospectively in eyes pretreated with ranibizumab or bevacizumab as well as untreated (treatment naive) eyes, before and after aflibercept therapy. A potential systemic effect of intravitreal aflibercept injection was evaluated by measuring CT also in fellow eyes of aflibercept-treated eyes.
Methods
This retrospective cohort study was approved by the local ethics committee in Zurich, Switzerland, and was conducted in adherence with the tenets of the Declaration of Helsinki. All included patients signed research consent forms on initial presentation allowing their data to be used for retrospective analysis.
The study consists of two distinct groups of patients with neovascular AMD: those who commenced intravitreal aflibercept therapy after previous treatment with ranibizumab or bevacizumab for neovascular AMD (ie, pretreated) and those who received intravitreal aflibercept as initial treatment at the onset of neovascular AMD (ie, treatment naive). The first group consisted of consecutive patients with wet AMD whose treatment was changed from intravitreal injection of 0.5 mg ranibizumab (Lucentis; Novartis Pharma Schweiz AG, Basel, Rotkreuz) or 1.25 mg bevacizumab (Avastin; Roche Pharma SChweiz AG, Reinach, Switzerland) to intravitreal injection of 2 mg aflibercept (Eylea; Bayer Schweiz AG, Zurich, Switzerland). All patients in this group had received at least three intravitreal injections of bevacizumab or ranibizumab within 4 months prior to their first aflibercept injection. The second group consisted of consecutive treatment-naive patients. All patients (pretreated and treatment naive) were given a standard loading dose regimen of three intravitreal 2 mg aflibercept injections with a frequency of one injection every 4 weeks. All consecutive patients had received their first aflibercept injection between November 2012 and March 2013. In addition, untreated fellow eyes were evaluated at each time point in both groups.
OCT measurements of CT were performed at all time points using the spectral domain Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany). A standard set of 19 B-scans (512 A-scans; 20°×15°) was used. All OCT scans were performed in ‘follow-up’ mode, allowing follow-up scans in the same location because of the system′s eye tracking. For inclusion into the study, all enrolled eyes had to have OCT scans of sufficient quality to allow CT measurement through the fovea and all other measurement points. CT was defined as the distance from the sclera to Bruch`s membrane. In each image, measurements were made in five different areas: subfoveal, 300 µm nasal, 2500 µm nasal, 300 µm temporal and 2500 µm temporal (figure 1). All CT measurements were independently performed by two ophthalmologists (KM and HF-R).
Figure 1.
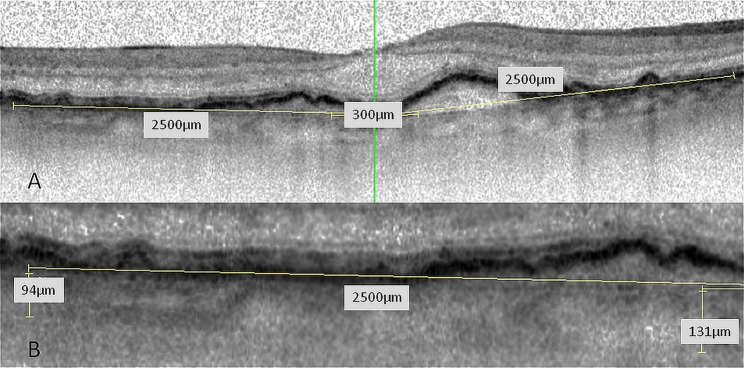
(A) Optical coherence tomography (OCT) scan through the fovea; five locations (subfoveal, 300 µm and 2500 µm nasal and temporal to the fovea) were measured. (B) CT-measurements were performed by two independent readers. The imaging software of Spectralis OCT includes contrast enhancement, copying of overlays, side by side comparison of different OCT scans and flickering of two scans allowing for precise measurements.
In addition to the OCT examination, all patients underwent a complete ophthalmic examination at each visit including best corrected visual acuity, slit-lamp examination and funduscopy. Prior to any anti-VEGF treatment, colour fundus photography and fluorescein angiography using a confocal scanning laser ophthalmoscope (HRA2; Heidelberg Engineering GmbH, Heidelberg, Germany) were performed.
All data were checked for normal distribution using histograms and the Shapiro–Wilks test. Normally distributed datasets were compared using paired and unpaired t tests and all pair-wise group comparisons in non-normally distributed datasets were performed using the Wilcoxon–Mann–Whitney test. As this was a pilot study, no posthoc analysis for multiple pair-wise comparisons was performed. All statistical calculations were performed using R V.3.1.0 and the graphics package ggplot2 (R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/). All calculations and graphics were generated with the RStudio Integrated Development Environment V.0.98.507 (RStudio (2014). RStudio: Integrated development environment for R, Boston, Massachusetts, USA).
Results
Eighty-four treated eyes (61 pretreated, 23 treatment naive) and 48 fellow eyes were included in this study. The mean age was 79.3 years in the pretreated group and 78.0 years in the treatment-naive group. The majority of patients were women (59% and 57% respectively). In the pretreated group the mean number of prior anti-VEGF injections was 28.2 over a mean follow-up time of 37.7 months.
In the pretreated group there was a statistically significant reduction in mean CT in treated eyes from prior to the first aflibercept injection to 4 weeks after the third aflibercept injection at all tested areas (table 1).
Table 1.
Mean choroidal thickness in pretreated and treatment-naive patients prior to and after a loading dose of three 2 mg aflibercept injections at 4 weeks interval
| Retinal area | Pretreated patients mean choroidal thickness | Treatment-naive patients mean choroidal thickness | ||||
|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |
| 2500NCT | 112.4±49.7 | 104.8±49.4 | <0.001 | 125.3±52.1 | 110.0±41.2 | <0.001 |
| 300NCT | 173.7±58.2 | 164.9±56.8 | <0.001 | 218.7±69.2 | 196.3±71.2 | <0.001 |
| SFCT | 182.8±57.4 | 172± 57.7 | <0.001 | 226.4±68.5 | 208.0±66.6 | <0.001 |
| 300TCT | 173.6±57.5 | 163.7±56.2 | <0.001 | 225.7±71.1 | 209.3±68.8 | <0.001 |
| 2500TCT | 176.2±55.1 | 165.5±54.6 | <0.001 | 213.9±71.3 | 195.7±78.5 | <0.001 |
2500NCT=choroidal thickness 2500 µm nasal from the central fovea, 300NCT=choroidal thickness 300 µm nasal from the central fovea, SFCT=subfoveal choroidal thickness, 300TCT=choroidal thickness 300 µm temporal from the central fovea, 2500TCT=choroidal thickness 2500 µm temporal from the central fovea.
Data presented as mean±SD.
The same was found for the treatment-naive group with all tested areas demonstrating a statistically significant reduction in mean CT after three aflibercept injections at 4-week intervals (table 1).
In the pretreated group, the mean reduction in CT was greatest at 2500 µm temporal to the fovea at 10.7 µm, while in the treatment-naive group the greatest reduction in mean CT was seen at 300 µm nasal to the fovea at 22.4 µm (table 2).
Table 2.
Comparison of mean change in choroidal thickness after a loading dose of three 2 mg aflibercept injections at 4 weeks interval between pretreated and treatment naive patients
| Retinal area | Pretreated patients mean change in CT | Treatment-naive patients mean change in CT | p Value |
|---|---|---|---|
| 2500NCT | 7.7±8.4 | 15.3±19.2 | 0.077 |
| 300NCT | 8.8±9.4 | 22.4±21.9 | 0.008 |
| SFCT | 10.6±14.5 | 18.3±22.0 | 0.047 |
| 300TCT | 9.9±12.8 | 16.4±12.7 | 0.041 |
| 2500TCT | 10.7±12.3 | 18.3±22.0 | 0.128 |
2500NCT=choroidal thickness 2500 µm nasal from the central fovea, 300NCT=choroidal thickness 300 µm nasal from the central fovea, SFCT=subfoveal choroidal thickness, 300TCT=choroidal thickness 300 µm temporal from the central fovea, 2500TCT=choroidal thickness 2500 µm temporal from the central fovea.
Data presented as mean±SD.
The treatment-naive group showed a greater decrease in mean CT at all areas compared with the pretreated group but the difference reached statistical significance only within 300 µm from the fovea (table 2).
For untreated fellow eyes in the pretreated group no statistically significant difference was observed in CT measurements from prior to the first aflibercept injection to four weeks after the third aflibercept injection at any retinal location measured (table 3).
Table 3.
Mean choroidal thickness in untreated fellow eyes of pretreated and treatment-naive patients prior to and after a loading dose of three 2 mg aflibercept injections at 4 weeks interval
| Retinal area | Fellow eye—pretreated choroidal thickness (n=31) | Fellow eye—treatment-naive choroidal thickness (n=17) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Change | p Value | Before | After | Change | p Value | |
| 2500NCT | 131.5±49.2 | 130.0±49.8 | 1.5±4.6 | 0.082 | 123.3±63.7 | 117.5±62.7 | 5.8±7.6 | 0.007 |
| 300NCT | 195.5±47 | 193.7±48.0 | 1.7±4.7 | 0.051 | 216.6±81.8 | 209.3±83.7 | 7.3±9.2 | 0.002 |
| SFCT | 198.4±45.8 | 198.7±45.7 | −0.3±3.9 | 0.645 | 222.5±80.2 | 218.5±82.7 | 4.0±11.9 | 0.067 |
| 300TCT | 197.7±43.7 | 196.5±45.1 | 1.2±4.3 | 0.126 | 224.6±81.0 | 219.6±81.6 | 5.1±10.1 | 0.006 |
| 2500TCT | 200.5±42.3 | 200.0±42.3 | 0.5±3.2 | 0.404 | 222.0±73.6 | 219.3±74.4 | 2.7±2.6 | 0.002 |
2500NCT=choroidal thickness 2500 µm nasal from the central fovea, 300NCT=choroidal thickness 300 µm nasal from the central fovea, SFCT=subfoveal choroidal thickness, 300TCT=choroidal thickness 300 µm temporal from the central fovea, 2500TCT=choroidal thickness 2500 µm temporal from the central fovea.
Data presented as mean±SD.
For fellow eyes in the treatment-naive group, except for the subfoveal measurement, all other areas showed a significant reduction in CT after treatment (table 3).
Discussion
Our findings indicate that three monthly intravitreal injections of aflibercept significantly reduce CT in eyes with previously treated neovascular AMD (ie, treated with ranibizumab or bevacizumab) as well as in treatment-naive eyes. This finding is in keeping with the findings of Branchini et al10 who reported a decrease in CT after treatment with ranibizumab or bevacizumab and Hikichi et al11 who reported a significant decrease in CT after ranibizumab treatment in eyes with polypoidal choroidal vasculopathy. Possible explanations for a decrease in CT could be that VEGF inhibitors, by decreasing levels of nitric oxide, induce choroidal vasoconstriction or that VEGF inhibitors reduce choroidal fenestrations.12
Our results show that, after aflibercept therapy, the reduction in CT was greater in treatment-naive exudative AMD eyes than in eyes which had received previous bevacizumab or ranibiumab therapy. This finding may be a result of the significantly thinner baseline CT in the pretreated group compared with that in the treatment-naive group; the baseline subfoveal CT in the pretreated group was 182.8 μm (±57.3) compared with 226.4 μm (±68.5) in the treatment naïve group. A further plausible explanation is that the choroid has been previously exposed to other anti-VEGF agents. However, it is surprising that in extensively pretreated eyes (mean of 28.2 anti-VEGF injections over a mean of 37.7 months), aflibercept is still able to induce a further significant decrease in CT.
For all intravitreally applied pan-VEGF inhibitors, an effect on systemic VEGF levels has been shown.4 13 However, it is interesting that except for the subfoveal area, fellow eyes of treatment-naive eyes treated with aflibercept showed a statistically significant decrease in CT in all tested retinal areas, indicating a clinically relevant systemic effect. No significant difference was seen in fellow eyes of pretreated eyes after aflibercept therapy. The reason this effect was only seen in the treatment-naive group may be a decreased choroidal sensitivity in a choroid previously exposed to anti-VEGF agents as described above.
Klettner et al14 showed, in tissue culture, that aflibercept has a prolonged duration of VEGF inhibition compared with either bevacizumab or ranibizumab; this may partially explain the further choroidal thinning seen with aflibercept following pretreatment with other anti-VEGF agents. Our study indicates a significant reduction in CT with aflibercept in treatment-naive and pretreated eyes. These findings could account for superior efficacy of aflibercept in the treatment of IPCV and pigment epithelial detachments (PEDs) in AMD. While a reduction in CT in AMD may lead to a further reduction of exudative changes, especially PEDs, choroidal thinning has been associated with retinal pigment epithelium (RPE) atrophy. Therefore, the long-term implications of a pharmacologically induced thinned choroid are to be determined.15 In addition, Julien et al16 recently showed in monkeys that a 2 mg intravitreal injection of aflibercept induced more haemolysis in the choriocapillaris, resulting in more RPE cell death compared with 0.5 mg of ranibizumab.
It is not yet established that anti-VEGF agents indeed cause choroidal thinning or if this is part of the disease process of neovascular AMD. A direct comparison between untreated and treated eyes with neovascular AMD is impossible for ethical reasons. There is however some rationale that this effect can be attributed to anti-VEGF treatment. One would expect that upregulated VEGF in untreated neovascular AMD would rather lead to increased CT as a result of vasodilatation and increased blood vessel fenestration. This difference is also seen in our baseline findings, indicating a thicker choroid for eyes with neovascular AMD in comparison with fellow eyes with non-neovascular AMD. In addition, it is plausible that anti-VEGF agents could influence CT and vascular permeability. Our data suggest that aflibercept influences CT; if these findings are confirmed in prospective studies, CT may become a relevant parameter for drug selection and evaluation in follow-up.
Footnotes
Twitter: Follow Kyriaki Mazaraki at @kyriaki Mazaraki
Correction notice: This article has been corrected since it was published Online First. The provenance and peer review statement has been corrected.
Acknowledgements: Special thanks to the study nurses Sarah Eisenstein, Manuela Noe and Ute Hornberger for continuous support.
Contributors: All authors have contributed to the planning, conduct and reporting of the work.
Funding: The study was supported by the “Stiftung wissenschaftliche Forschung, Fonds Ophthalmologie, City Hospital Triemli” and the Werner H. Spross Foundation. Both funding organisations had no role in the design or conduct of this research.
Competing interests: The “Stiftung wissenschaftliche Forschung, Fonds Ophthalmologie, City Hospital Triemli” received research grants from Novartis Schweiz AG and Bayer Schweiz AG and payments for invited talks or advisory board participations for MB and SM from Allergan, Novartis, Alimera, Bayer, Roche and Clanotech.
Ethics approval: Local ethics committee Zurich.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 2.Gragoudas ES, Adamis AP, Cunningham ET Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805–16. 10.1056/NEJMoa042760 [DOI] [PubMed] [Google Scholar]
- 3.Martin DF, Maguire MG, Fine SL, et al. , Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–98. 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–67. 10.1016/S0140-6736(13)61501-9 [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–48. 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht-Riederle H, Becker M, Graf N, et al. Effect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMD. Graefes Arch Clin Exp Ophthalmol 2014;252:1705–9. 10.1007/s00417-014-2589-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CM, Mohla A, Wong TY. Resolution of persistent pigment epithelial detachment secondary to polypoidal choroidal vasculopathy in response to Aflibercept. Eye 2014;28:1148–9. 10.1038/eye.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata M, Oishi A, Tsujikawa A, et al. Efficacy of intravitreal injection of aflibercept in neovascular age-related macular degeneration with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci 2014;55:7874–80. 10.1167/iovs.14-14610 [DOI] [PubMed] [Google Scholar]
- 9.Pitcher JD III, Witkin AJ, DeCroos FC, et al. A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study. Br J Ophthalmol 2015;99:848–52.. 10.1136/bjophthalmol-2014-306018 [DOI] [PubMed] [Google Scholar]
- 10.Branchini L, Regatieri C, Adhi M, et al. Effect of intravitreous anti-vascular endothelial growth factor therapy on choroidal thickness in neovascular age-related macular degeneration using spectral-domain optical coherence tomography. JAMA Ophthalmol 2013;131:693–4. 10.1001/jamaophthalmol.2013.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hikichi T, Kitamei H, Shioya S, et al. Relation between changes in foveal choroidal thickness and 1-year results of ranibizumab therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol 2014;98:1201–4. 10.1136/bjophthalmol-2013-304555 [DOI] [PubMed] [Google Scholar]
- 12.Peters S, Heiduschka P, Julien S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol 2007;143:995–1002. 10.1016/j.ajo.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 13.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636–41. 10.1136/bjophthalmol-2014-305252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klettner A, Recber M, Roider J. Comparison of the efficacy of aflibercept, ranibizumab, and bevacizumab in an RPE/choroid organ culture. Graefes Arch Clin Exp Ophthalmol 2014;252:1593–8. 10.1007/s00417-014-2719-y [DOI] [PubMed] [Google Scholar]
- 15.Sohn EH, Khanna A, Tucker BA, et al. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci 2014;55:1352–60. 10.1167/iovs.13-13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julien S, Biesemeier A, Taubitz T, et al. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol 2014;98:813–25. 10.1136/bjophthalmol-2013-304019 [DOI] [PubMed] [Google Scholar]


