Abstract
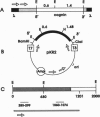
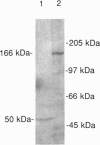
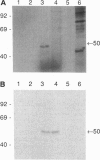
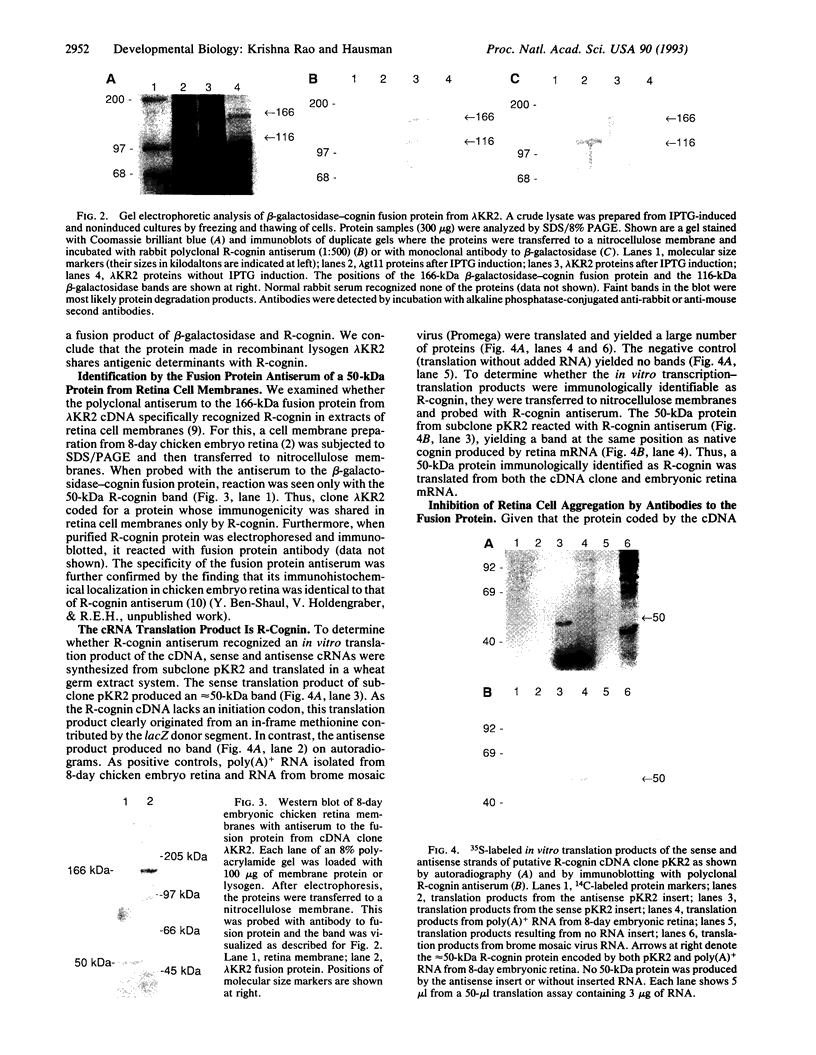
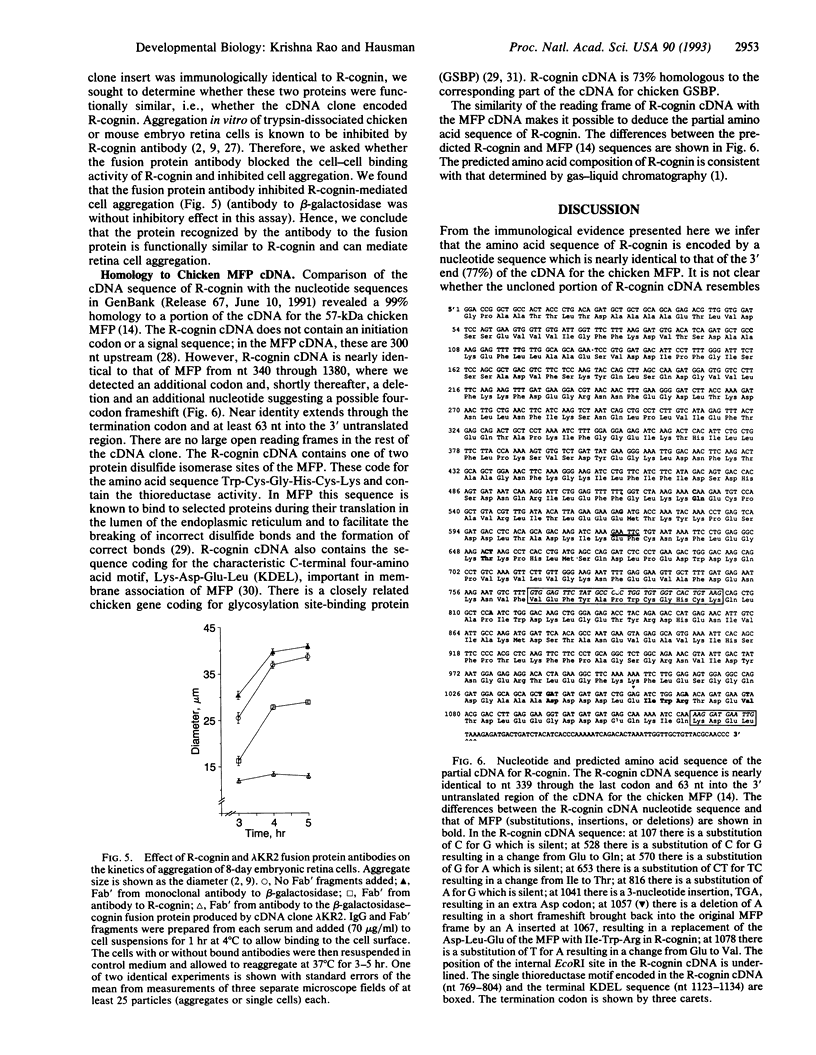
Retina cognin (R-cognin) is a developmentally regulated 50-kDa protein that was isolated from chicken embryo retina cell membranes. It mediates the adhesion and reaggregation in vitro of retina cells from chicken and mouse embryos, but not of cells from other tissues, and may be involved in neuronal differentiation. We report here the cloning of a cDNA for R-cognin. A chicken embryo retina cDNA library was constructed in lambda gt11 vector and was screened with polyclonal R-cognin antiserum, yielding several immunoreactive clones. Antiserum prepared to the R-cognin-beta-galactosidase fusion protein produced by one recombinant lysogen recognized the 50-kDa R-cognin protein derived from retina cell membranes. This antiserum inhibited the reaggregation of dissociated retina cells and immunostained chicken embryo retina tissue in a pattern similar to that obtained with R-cognin antiserum. In vitro translation of RNA from a cDNA subclone yielded a 50-kDa protein that was recognized by R-cognin antiserum on a Western blot. By these criteria we identify the cDNA clone as representative of the gene encoding R-cognin. This cDNA is nearly identical to a major portion of the cDNA for the multifunctional protein that is the beta subunit of prolyl 4-hydroxylase and has both protein disulfide isomerase activity and thyroid hormone-binding activity. These findings demonstrate that R-cognin differs from other cell adhesion molecules and suggest possible mechanisms for its action in cell adhesion and neuronal differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi S., Yamamoto A., Yoshimori T., Masaki R., Ogawa R., Tashiro Y. Localization of protein disulfide isomerase on plasma membranes of rat exocrine pancreatic cells. J Histochem Cytochem. 1988 Aug;36(8):1069–1074. doi: 10.1177/36.8.3292644. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaul Y., Hausman R. E., Moscona A. A. Visualization of a cell surface glycoprotein, the retina cognin, on embryonic cells by immuno-latex labeling and scanning electron microscopy. Dev Biol. 1979 Sep;72(1):89–101. doi: 10.1016/0012-1606(79)90100-3. [DOI] [PubMed] [Google Scholar]
- Dobi E. T., Naya F. J., Hausman R. E. Distribution of R-cognin and choline acetyltransferase in the ganglion cell layer of developing chick neural retina. Cell Differ. 1988 Jan;22(2):115–123. doi: 10.1016/0045-6039(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Dobi E. T., Troccoli N. M., Hausman R. E. Distribution of R-cognin in late embryonic and post-hatching chicken retina. Invest Ophthalmol Vis Sci. 1986 Mar;27(3):323–329. [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Immunologic detection of retina cognin on the surface of embryonic cells. Exp Cell Res. 1979 Mar 15;119(2):191–204. doi: 10.1016/0014-4827(79)90348-3. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Isolation of retina-specific cell-aggregating factor from membranes of embryonic neural retina tissue. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3594–3598. doi: 10.1073/pnas.73.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Purification and characterization of the retina-specific cell-aggregating factor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):916–920. doi: 10.1073/pnas.72.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman R. E., Sagar G. D., Shah B. H. Initial cholinergic differentiation in embryonic chick retina is responsive to insulin and cell-cell interactions. Brain Res Dev Brain Res. 1991 Mar 18;59(1):31–37. doi: 10.1016/0165-3806(91)90026-f. [DOI] [PubMed] [Google Scholar]
- Helaakoski T., Vuori K., Myllylä R., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Rao A. S., Bolten B. M., Balakrishnan R., Goldberg E. B. Cloning and identification of bacteriophage T4 gene 2 product gp2 and action of gp2 on infecting DNA in vivo. J Bacteriol. 1989 Jan;171(1):488–497. doi: 10.1128/jb.171.1.488-497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay D. R., Moscona A. A. Purification of the specific cell-aggregating factor from embryonic neural retina cells. Exp Cell Res. 1974 Aug;87(2):438–443. doi: 10.1016/0014-4827(74)90514-x. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nakazawa M., Aida T., Everson W. V., Gonda M. A., Hughes S. H., Kao W. W. Structure of the gene encoding the beta-subunit of chicken prolyl 4-hydroxylase. Gene. 1988 Nov 30;71(2):451–460. doi: 10.1016/0378-1119(88)90062-5. [DOI] [PubMed] [Google Scholar]
- Noiva R., Lennarz W. J. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992 Feb 25;267(6):3553–3556. [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar G. D., Rao A. S., Ren Y., Hausman R. E. The cell recognition molecule, cognin, mediates choline acetyltransferase activity in embryonic chick retina. Brain Res. 1992 Jul 10;585(1-2):63–70. doi: 10.1016/0006-8993(92)91191-g. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B. H., Rao A. S., Hausman R. E. Role of the cell recognition molecule, cognin, in GABAergic differentiation in chick retina. Brain Res. 1992 Sep 4;589(2):268–274. doi: 10.1016/0006-8993(92)91286-n. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tasanen K., Parkkonen T., Chow L. T., Kivirikko K. I., Pihlajaniemi T. Characterization of the human gene for a polypeptide that acts both as the beta subunit of prolyl 4-hydroxylase and as protein disulfide isomerase. J Biol Chem. 1988 Nov 5;263(31):16218–16224. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troccoli N. M., Hausman R. E. Retina cognin does not bind to itself during membrane interaction in vitro. Cell Differ. 1988 Feb;22(3):225–231. doi: 10.1016/0045-6039(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Troccoli N. M., Hausman R. E. Vesicle interactions as a model for the retinal cell-cell recognition mediated by R-cognin. Cell Differ. 1985 Feb;16(1):43–49. doi: 10.1016/0045-6039(85)90606-2. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Semba T., Takemoto H., Akagi S., Yamamoto A., Tashiro Y. Protein disulfide-isomerase in rat exocrine pancreatic cells is exported from the endoplasmic reticulum despite possessing the retention signal. J Biol Chem. 1990 Sep 15;265(26):15984–15990. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]