Best practices for integrating HIV testing and antiretroviral interventions for prevention and treatment are suggested based on research evidence and existing normative guidance. The goal is to provide high impact prevention services during periods of substantial risk.
Keywords: early treatment, HIV, postexposure prophylaxis, pre-exposure prophylaxis, prevention
Abstract
Best practices for integrating human immunodeficiency virus (HIV) testing and antiretroviral interventions for prevention and treatment are suggested based on research evidence and existing normative guidance. The goal is to provide high-impact prevention services during periods of substantial risk. Antiretroviral medications are recommended for postexposure prophylaxis (PEP), pre-exposure prophylaxis (PrEP), and treatment of HIV infection. We reviewed research evidence and current normative guidelines to identify best practices for integrating these high-impact prevention strategies. More sensitive HIV tests used for screening enable earlier diagnosis and treatment of HIV infection, more appropriate counseling, and help limit drug resistance. A fully suppressive PEP regimen should be initiated based on exposure history or physical findings when sensitive diagnostic testing is delayed or not available and antibody tests are negative. Transitions from PEP to PrEP are often warranted because HIV exposure events may continue to occur. This algorithmic approach to integrating PEP, PrEP, and early treatment decisions may increase the uptake of these interventions by a greater number and diversity of knowledgeable healthcare providers.
There is growing consensus that antiretroviral medications have an important role to play in preventing the transmission and acquisition of human immunodeficiency virus (HIV) infection. More than 2 million new HIV infections occur every year worldwide, including an estimated 50 000 per year in the United States [1]. Prevention uses of antiretroviral medications include postexposure prophylaxis (PEP) after an isolated, significant exposure to fluids that may contain HIV, or pre-exposure prophylaxis (PrEP) if exposure is frequent, or early treatment after infection has occurred [2]. The management of transitions from PEP to PrEP and from prophylaxis to early treatment remain challenging, partly because these concepts are new and best practices are still evolving. Although more information would be beneficial, research to optimize antiretroviral service integration will be complex, largely observational, partly based on animal models, and not definitive [3]. In this narrative review, we suggest approaches for healthcare providers and potential users, referring to available evidence and normative guidance and drawing on published clinical experience. Providing approaches to integrating high-impact prevention interventions is expected to mitigate common barriers to their use and leverage potential synergies.
ANTIRETROVIRAL STRATEGIES FOR PREVENTION
Postexposure Prophylaxis
Postexposure prophylaxis is recommended by the Centers for Disease Control and Prevention (CDC) and the World Health Organization after a substantial exposure to body fluids likely to contain virus from a person who is HIV infected, or possibly infected [4–6]. Postexposure prophylaxis with zidovudine alone was shown to be approximately 80% effective if started within 72 hours after exposure based on case-control studies after needlestick injury among healthcare workers [7]. The safety of PEP after nonoccupational exposure to HIV has been confirmed [8, 9]. There have been no studies comparing the effectiveness of 1, 2, or 3 drug PEP regimens. In 2013, the US Public Health Service recommended that occupational PEP regimens include at least 3 drugs [10], typically including 2 nucleoside or nucleotide reverse-transcriptase inhibitors and an integrase inhibitor, which was also recommended in 2013 for nonoccupational PEP in New York State [11]. Nonoccupational PEP use has been low in most settings, with barriers including a lack of awareness among potential users, difficulty identifying exposures or denial of their risk, the requirement that action be taken within hours up to 2–3 days of exposure, and a lack of nonoccupational PEP awareness among healthcare providers [9, 12].
Pre-Exposure Prophylaxis
Pre-exposure prophylaxis using daily oral emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) (brand name Truvada) is safe and effective for substantially reducing the acquisition of HIV among sexually active adults, including gay, bisexual, and other men who have sex with men [13–15], transgender women [13], and heterosexual men and women [16, 17]. Daily oral TDF alone was also effective among HIV-discordant heterosexual couples and among injection drug users [16, 18]. The US Food and Drug Administration (FDA) approved daily oral FTC/TDF for PrEP in 2012, and recommendations for its use have been published by the CDC [19] and the World Health Organization [20]. Consumer demand for PrEP increased 332% in the United States in 2014 [21, 22]. By the end of 2014, approximately 10% of men who have sex with men surveyed in San Francisco had taken PrEP [23]. Pre-exposure prophylaxis uptake is high when offered by experienced providers [24–26]. The barriers to more rapid uptake of PrEP include lack of information among healthcare providers and potential users [27], indecision about whether PrEP should be provided by HIV treatment specialists or general practitioners [28], fear of medication side effects [25], and stigma associated with antiretroviral medications. Most public and private insurance covers PrEP (http://myprepexperience.blogspot.com/p/truvada-track.html), and the drug manufacturer has a medication assistance program for uninsured people with low income and a copayment assistance program that applies regardless of income (http://start.truvada.com/individual/truvadaprep-copay). The Patient Assistance Network Foundation also provides assistance for purchasing PrEP medications (https://www.panfoundation.org/fundingapplication/patientEnrollment.php).
Early Treatment
Antiretroviral treatment of HIV infection prolongs life, restores health, and significantly reduces onward HIV transmission [29]. Treatment of HIV infection has continually improved over the past 18 years, including the development of combinations of medications that are more active, have more convenient dosing [30], and are safer. Initiating therapy very early after infection has been associated with reductions in viral reservoirs in tissues [31] and decreased clinical outcomes [32]. Although US Department of Health and Human Services (DHHS) guidelines have been revised to recommend antiretroviral therapy regardless of CD4+ T cell count, rates of treatment-induced viral suppression are relatively low in the United States [33]. The challenges regarding treatment are how best to engage people in HIV testing services, how to foster uptake of treatment and retention in care during phases of infection when people feel well, and how best to inform people and their partners of the prevention benefits of early therapy. Side effects, fear of medications, and stigma linked to HIV infection are substantial barriers to timely initiation of, and adherence to, antiretroviral treatment [34].
A Common Entry Point: Human Immunodeficiency Virus Testing
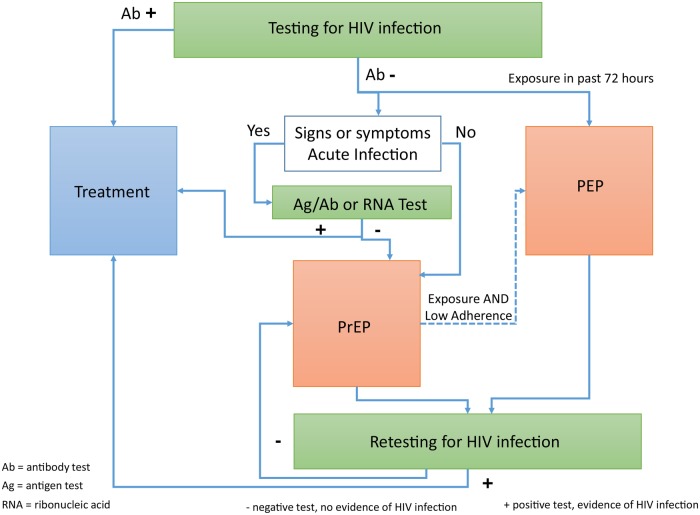
Initiating PEP, PrEP, or HIV treatment each require first determining the presence or absence of HIV infection (Figure 1). The CDC recommends HIV testing be performed for all people 13–64 years of age at least once, with more frequent testing among people with identifiable risk factors for HIV acquisition [35]. Barriers to HIV testing include fear of HIV, fear of HIV-related stigma, fear of HIV medications, uncertain access to medical care, and low perception of personal risk. Making early HIV treatment available provides a motivation for people to test who suspect that HIV infection is already present. Likewise, making PrEP available provides a motivation for people who still believe, or hope, that they are HIV uninfected, and offers an opportunity for ongoing discussions of ways to maintain sexual health. In addition, offering PEP can create a sense of urgency after a potential HIV exposure, which can motivate people's learning about HIV and HIV prevention opportunities, including the critically important role of HIV testing.
Figure 1.
An integrated postexposure prophylaxis (PEP), pre-exposure prophylaxis (PrEP), and treatment transition algorithm. In people reporting mucosal exposure to fluids likely to be infected with human immunodeficiency virus (HIV) in the past 72 hours, start PEP with a 3-drug regimen while awaiting the results of HIV testing. In people with repeated HIV exposures, a negative HIV antibody test, and no signs or symptoms consistent with acute HIV infection, start PrEP with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). A negative test for HIV nucleic acids or antigen is preferred, especially if there are signs or symptoms of an acute viral syndrome. If any HIV test is positive, initiate antiretroviral therapy without delay and send specimens for HIV-confirmatory and drug-resistance testing as soon as possible.
The Challenge of Detecting Acute Human Immunodeficiency Virus Infection
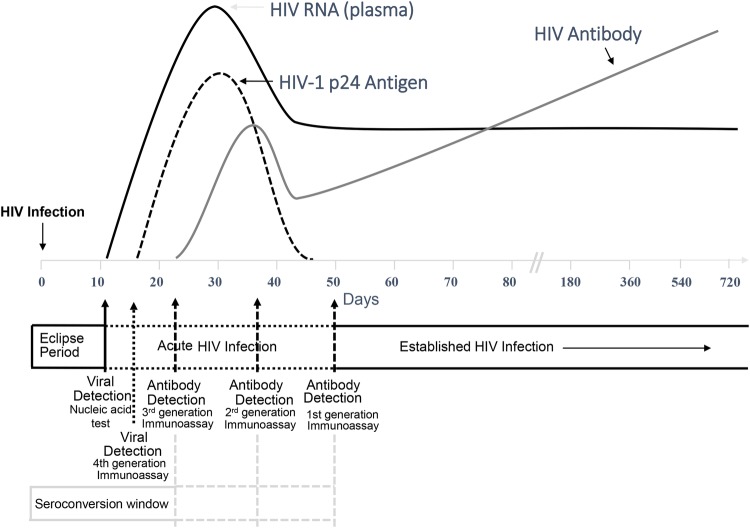
Human immunodeficiency virus antibody testing will guide the decision to start antiretroviral treatment vs PEP or PrEP in the majority of situations. However, the detection of acute HIV infection can be challenging in that antibody (Ab) tests do not detect infection during acute infection when only HIV RNA or antigen (Ag) is present in blood (Figure 2).
Figure 2.
Sequence of appearance of laboratory markers of human immunodeficiency virus (HIV) infection. The figure is from the updated Centers for Disease Control and Prevention (CDC) guidelines for HIV testing and was adapted from prior publications [36–41].
Human immunodeficiency virus RNA testing is recommended in New York, San Francisco, and North Carolina for all seronegative persons at high-risk. Such testing reduces the risk that people with acute HIV infection might be falsely reassured by a negative Ab test and can lead to earlier treatment. Human immunodeficiency virus RNA tests are not universally available or affordable. Furthermore, the diagnostic yield of these tests is low (0.4% to 1%), depending on the underlying incidence of HIV infection in the population. Tests for HIV nucleic acids that are rapid and that can be conducted at the point of care have been developed, but they are not yet approved by the FDA for routine clinical use.
Starting PrEP during acute HIV infection is associated with selection for resistance to FTC [42, 43], although the benefits of PrEP for preventing HIV infection outweigh the relatively low risk of drug resistance [44]. Hence, using HIV Ag (or RNA) tests at the time of PrEP initiation is desirable, if such testing is available and affordable.
In situations in which RNA testing is not available, a fourth-generation HIV test that includes detection of HIV p24 Ag as well as anti-HIV immunoglobulin (Ig)M and IgG Abs is an alternative. These fourth-generation tests allow detection of infection 5 days earlier than third-generation Ab testing (that detects IgM and IgG) and 2 to 3 weeks earlier than second-generation Ab testing (that detects only IgG). A rapid CLIA-waived, point-of-care, fourth-generation test is available, although its sensitivity is less than fourth-generation tests conducted in the laboratory [36].
Clinical screening for signs and symptoms of an acute viral syndrome can guide use of more sensitive HIV Ag or RNA testing if such is not used routinely for all high-risk exposures. The majority of acute HIV infections are associated with symptoms of an acute viral syndrome including fever (75%), fatigue (68%), myalgia (49%), skin rash (48%), headache (45%), pharyngitis (40%), cervical adenopathy (39%), arthralgia (30%), night sweats (28%), and diarrhea (27%) [19]. However, these signs and symptoms are not specific to a diagnosis of acute HIV infection, so these symptoms should prompt testing for HIV Ag or nucleic acids, if available, or repeat Ab testing in 4 weeks.
CHOOSING AND TRANSITIONING AMONG ANTIRETROVIRAL STRATEGIES
When to Start Postexposure Prophylaxis Versus Pre-Exposure Prophylaxis
Postexposure prophylaxis should be started emergently within 72 hours of a mucosal exposure to fluids that are likely to be infected with HIV. There are differences in the regimens recommended for PEP vs PrEP: daily oral FTC/TDF is recommended for PrEP, whereas a regimen containing FTC/TDF and an integrase inhibitor is recommended in the United States for PEP. An advantage of the recommended 3-drug regimens is that they are fully active against established HIV infection, which can be difficult to rule out in the days between HIV exposure and HIV seroconversion. Ruling out active infection is especially challenging if the most recent exposure to possibly infected fluids is not the only recent exposure, such that HIV infection may already be present in the eclipse phase (in which RNA and Ab are both negative) or the RNA-positive and Ab-negative window period. The timing of the most recent sexual or injection exposure can also be difficult to discern by history due to anxiety, alcohol, and other substance use, or frequent exposures. The urgent circumstances after a recent exposure makes thorough evaluations even more difficult. Hence, it is recommended to start a 3-drug PEP regimen after a significant exposure in the past 72 hours before definitive HIV status test results are available [10, 11]. In addition, a 3-drug regimen can be started in people whose potential exposures occurred more than 72 hours before presentation, especially if they present with an acute viral syndrome and a history of frequent exposure such that prompt intervention is warranted.
Transitioning From Postexposure Prophylaxis to Pre-Exposure Prophylaxis
After completing a course of PEP, immediate transition to PrEP should be considered unless the viral exposure was reported to be an isolated event. Specific indications for PrEP have been published by the CDC [19] and the International AIDS Society-USA [45]. The indications include inconsistent or no condom use outside of a mutually monogamous relationship with a recently tested HIV-uninfected person, having used PEP more than twice in the past 12 months, having a sexual partner who is HIV infected, having had a recent sexually transmitted infection, sharing needles, or being a woman, including a transgender women, who has a male partner who has sex with men.
Because there is no evidence that prophylactic antiretroviral use delays seroconversion [13, 46, 47], and PEP is highly effective if taken as prescribed, there is no need for a gap between ending PEP and beginning PrEP to evaluate HIV infection status [48]. Such gaps create opportunities for HIV infection to occur, disrupt daily adherence habits, and create an opportunity for disengagement from care. It is rare for HIV infection to be present and undetected when starting PEP, and such infections would usually be detected by HIV testing performed after 4 weeks of PEP.
Testing for HIV infection after 4 weeks of PEP would ideally use a test that includes detection of both IgM and IgG Abs (ie, third- or fourth-generation tests), as such tests are expected to become positive within 4 weeks of infection (Figure. 2). If second-generation HIV Ab tests are used, such testing should occur at the time of the PEP to PrEP transition, and after 3 months of PrEP. Human immunodeficiency virus RNA tests may be suppressed by prophylactic antiretroviral use, although such was not observed in trials in which seroconversion occurred when drug concentrations were undetectable or low [13, 46].
Transitioning From Pre-Exposure Prophylaxis to Postexposure Prophylaxis
When taken daily or near daily, PrEP is highly effective for HIV prevention in persons with repeated HIV exposures [25, 49, 50]; therefore, initiating a PEP regimen is not indicated in people who are adherent to their PrEP regimen. When persons prescribed PrEP request PEP, it should be used as an opportunity to assess their adherence to PrEP. For those reporting they are taking PrEP sporadically and not within the week prior to the recent exposure, initiating a 28-day course of PEP may be warranted. Post-exposure evaluations and procedures should be offered, including Plan B contraception, evaluation for sexually transmitted infections, and review of vaccination status for human papillomavirus and hepatitis B virus.
Transitioning From Pre-Exposure Prophylaxis or Postexposure Prophylaxis to Treatment
Both PrEP and PEP require repeated HIV testing. In the case of PEP, testing is indicated at initiation, at the completion of the 28-day course, and at 3 months posttreatment [4]. For PrEP, HIV testing is required at initiation, and then every 3 months [19]. If an HIV RNA test is not performed at PrEP initiation, an additional Ab test can be performed after 1 month to identify clients who may have had acute infection when they started PrEP.
All PEP or PrEP patients whose HIV tests become reactive or positive should immediately be offered a suppressive antiretroviral treatment regimen [51]. Testing should be ordered to confirm HIV infection and to evaluate possible drug resistance [51]. If confirmatory testing is negative, antiretroviral therapy can be discontinued or scaled back to a PrEP regimen. Genotypic resistance testing is sufficient for assessing drug resistance among seroconverters [42, 46]. While awaiting the results from resistance tests, the DHHS recommends using a treatment regimen that contains a boosted protease inhibitor if infection occurs during or shortly after prophylactic use of antiretroviral medications [51]. Such boosted protease inhibitors have high barriers to drug resistance, which may be especially advantageous in people who had difficulty adhering to PrEP.
Resistance to tenofovir was extremely rare among people who became infected after receiving TDF alone or with FTC in PrEP clinical trials [17, 43], so TDF may be used in the treatment regimen. Emtricitabine resistance was observed more often [17, 42, 43, 46]. Continuing FTC in the treatment regimen can be justified because partial viral load suppression is expected [52] and maintaining FTC-selected viral mutations will increase viral susceptibility to TDF [46]. A treatment regimen consisting of FTC/TDF and a boosted protease inhibitor can be switched to a first-line regimen if initial resistance testing shows no resistance to tenofovir or FTC. Nearly all people who became infected after receiving PrEP regimens in trials had no evidence of drug resistance, as expected given their low or undetectable PrEP drug concentrations.
CONCLUSIONS
Transitions from PEP to PrEP and from prophylaxis to treatment can be clinically straightforward, with minimal risks and substantial benefits, including continuous HIV prophylaxis and earlier initiation of treatment. Integrating PEP, PrEP, and early treatment decisions using simple algorithms may address several common barriers to the uptake and use of these high-impact prevention interventions. Dissemination of information about an integrated approach may foster (1) expanded use of more sensitive diagnostics and (2) a greater number and diversity of healthcare providers who are knowledgeable about how to provide indicated antiretroviral medications for both prevention and treatment in a timely manner.
Acknowledgments
R. M. G. and D. K. S. were each responsible for writing sections of the paper and both contributed to editing and revising the text and Figure 1.
Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. R. M. G.'s effort to prepare this work was supported by the Gladstone Institutes and the National Institutes of Health (R01 AI118575-01 UM1 AI068619, and RO1 U01 AI064002).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveil Suppl Rep 2012;17 Available at: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf Accessed 12 March 2014. [Google Scholar]
- 2.Flash C, Krakower D, Mayer KH. The promise of antiretrovirals for HIV prevention. Curr Infect Dis Rep 2012; 14:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegfried N, Beanland RL, Ford N, Mayer KH. Formulating the future research agenda for postexposure prophylaxis for HIV: methodological challenges and potential approaches. Clin Infect Dis 2015; 60(suppl 3):S205–11. [DOI] [PubMed] [Google Scholar]
- 4.Smith DK, Grohskopf LA, Black RJ et al. . Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20. [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines on Post Exposure Prophylaxis for HIV: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2014. [Google Scholar]
- 6.Ford N, Mayer KH, World Health Organization Postexposure Prophylaxis Guideline Development Group. World Health Organization Guidelines on Postexposure Prophylaxis for HIV: Recommendations for a Public Health Approach. Clin Infect Dis 2015; 60(suppl 3):S161–4. [DOI] [PubMed] [Google Scholar]
- 7.Cardo DM, Culver DH, Ciesielski CA et al. . A case–control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med 1997; 337:1485–90. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JO, Martin JN, Roland ME et al. . Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: the San Francisco PEP Study. J Infect Dis 2001; 183:707–14. [DOI] [PubMed] [Google Scholar]
- 9.Schechter M, do Lago RF, Mendelsohn AB et al. . Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr 2004; 35:519–25. [DOI] [PubMed] [Google Scholar]
- 10.Kuhar DT, Henderson DK, Struble KA et al. . Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hospital Epidemiol 2013; 34:875–92. [DOI] [PubMed] [Google Scholar]
- 11.New York State AIDS Institute. HIV prophylaxis following non-occupational exposure, 2013. Available at: http://www.hivguidelines.org/wp-content/uploads/2014/12/hiv-prophylaxis-following-non-occupational-exposure.pdf Accessed 2 February 2015.
- 12.Krakower DS, Jain S, Mayer KH. Antiretrovirals for primary HIV prevention: the current status of pre- and post-exposure prophylaxis. Curr HIV/AIDS Rep 2015; 12:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, Sullivan AK, Clarke A, Reeves I, Schembri G, Mackie N, Bowman C, Lacey CJ, Apea V, Brady M, Fox J, Taylor S, Antonucci S, Khoo SH, Rooney J, Nardone A, Fisher M, McOwan A, Phillips AN, Johnson AM, Gazzard B, Gill ON. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet published online September 10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina J, Capitant C, Spire B et al. . On demand PrEP with oral TDF/FTC in MSM: results of the ANRS Ipergay trial. In: Conference on Retroviruses and Opportunistic Infections 2015; Seattle, WA. [Google Scholar]
- 16.Baeten JM, Donnell D, Ndase P et al. . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thigpen MC, Kebaabetswe PM, Paxton LA et al. . Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 18.Choopanya K, Martin M, Suntharasamai P et al. . Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2014: A Clinical Practice Guideline. 2014:1–67. Available at: http://www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf Accessed 28 May 2014.
- 20.World Health Organization Guideline on when to start ART and on PrEP for HIV, 2015. Available at http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ Accessed 19 August 2015.
- 21.Flash C, Landovitz R, Giler RM et al. . Two years of Truvada for pre-exposure prophylaxis utilization in the US, 2014. Available at: http://www.jiasociety.org/index.php/jias/article/view/19730/html. Accessed 19 August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush S, Ng L, Magnuson D et al. . Significant uptake of Truvada for pre-exposure prophylaxis (PrEP) utilization in the US in Late 2014 – 1Q 2015. In: IAPAC Treatment, Prevention, and Adherence Conference 2015; Miami, FL. [Google Scholar]
- 23.Grant R, Hecht J, Raymond H et al. . Scale-up of pre-exposure prophylaxis in San Francisco to impact HIV incidence. In: CROI 2015; Seattle, WA. [Google Scholar]
- 24.Liu A, Cohen S, Follansbee S et al. . Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med 2014; 11:e1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant RM, Anderson PL, McMahan V et al. . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu A, Cohen S, Vittinghoff E et al. . Adherence, sexual behavior and HIV/STI incidence among men who have sex with men (MSM) and transgender women (TGW) in the US PrEP demonstration (Demo) project. In: IAS 2015; Vancouver, BC, Canada. [Google Scholar]
- 27.Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS 2012; 7:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krakower D, Ware N, Mitty JA et al. . HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav 2014; 18:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarcho JA, Gandhi M, Gandhi RT. Single-pill combination regimens for treatment of HIV-1 infection. N Engl J Med 2014; 371:248–59. [DOI] [PubMed] [Google Scholar]
- 31.Saez-Cirion A, Bacchus C, Hocqueloux L et al. . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Eng J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner EM, McLees MP, Steiner JF et al. . The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwadz M, Applegate E, Cleland C et al. . HIV-infected individuals who delay, decline, or discontinue antiretroviral therapy: comparing clinic- and peer-recruited cohorts. Front Public Health 2014; 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–7. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention, Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: updated recommendations, 2014. Available at: http://stacks.cdc.gov/view/cdc/23447 Accessed 2 February 2015.
- 37.Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med 1997; 102:117–24. [DOI] [PubMed] [Google Scholar]
- 38.Fiebig EW, Wright DJ, Rawal BD et al. . Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
- 39.Owen SM, Yang C, Spira T et al. . Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol 2008; 46:1588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masciotra S, McDougal JS, Feldman J et al. . Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol 2011; 52:S17–22. [DOI] [PubMed] [Google Scholar]
- 41.Masciotra S, Smith AJ, Youngpairoj AS et al. . Evaluation of the CDC proposed laboratory HIV testing algorithm among men who have sex with men (MSM) from five US metropolitan statistical areas using specimens collected in 2011. J Clin Virol 2013; 58:e8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liegler T, Abdel-Mohsen M, Bentley LG et al. . HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis 2014; 210:1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehman DA, Baeten JM, McCoy CO et al. . Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single-or dual-agent preexposure prophylaxis. J Infect Dis 2015; 211:1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant RM, Liegler T. Weighing the risk of drug resistance with the benefits of HIV preexposure prophylaxis. J Infect Dis 2015; 211:1202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrazzo JM, del Rio C, Holtgrave DR et al. . HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014; 312:390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant RM, Liegler T, Defechereux P et al. . Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS 2015; 29:331–7. [DOI] [PubMed] [Google Scholar]
- 47.Curtis KA, Kennedy MS, Luckay A et al. . Delayed maturation of antibody avidity but not seroconversion in rhesus macaques infected with simian HIV during oral pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2011; 57:355–62. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, Krakower DS, Mayer KH. The transition from postexposure prophylaxis to preexposure prophylaxis: an emerging opportunity for biobehavioral HIV prevention. Clin Infect Dis 2015; 60(suppl 3):S200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson PL, Glidden DV, Liu A et al. . Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnell D, Baeten JM, Bumpus NN et al. . HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, 2014. Available at: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0 Accessed 2 February 2015.
- 52.Deeks SG, Hoh R, Neilands TB et al. . Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J Infect Dis 2005; 192:1537–44. [DOI] [PubMed] [Google Scholar]