Abstract
The presence of DNA in the cytoplasm of mammalian cells is perceived as a danger signal, alerting the host to the presence of microbial infection. In response to the detection of cytoplasmic DNA, the immune system mounts a programmed response that involves the transcription of anti-viral genes such as type I interferons and production of inflammatory cytokines such as interleukin (IL)-1β. The recent discovery of the cGAS-cGAMP second messenger pathway as well as IFI16 and additional sensors collectively provide critical insights into the molecular basis behind the sensing of cytoplasmic DNA. The insights obtained from these important discoveries could unveil new avenues to understand host-immunity, improve vaccine adjuvancy and allow development of new treatments for inflammatory diseases associated with abberrant sensing of DNA.
Introduction
Germline-encoded pattern recognition receptors are required for the generation of an efficacious host response to microbial infection [1–3]. These receptors monitor extracellular, endosomal and intracellular compartments for signs of infection. Molecular signatures characteristic of microbial infection (e.g. LPS) or those released from abnormal, damaged, or dying cells (e.g. ATP) engage distinct and overlapping sensors in these compartments [4–6]. Once pathogen-derived signals are detected, a rapid, relatively generic, innate immune response ensues, leading to the production of pro-inflammatory cytokines, type I interferons (IFNs) and chemokines. These events allow the host to curb growth and spread of infectious agents and clear them by activating adaptive immunity [1, 2].
Nucleic acids have been shown to be particularly potent molecular triggers of the innate immune response [7–9]. Microbe-derived nucleic acids commonly find their way into sub-cellular compartments of immune cells during infection [9, 10]. Immune cells are equipped with a plethora of nucleic acid receptors, each specific for a particular polynucleotide species and a specific expression pattern within cellular compartments. Examples of these receptors include RIG-I-like receptors (RLRs) such as RIG-I and MDA-5, which detect 5′ triphosphate RNA and dsRNA, respectively, in the cytosol; and Toll-like receptors (TLR) 3 (dsRNA sensitive); TLRs 7 and 8 (ssRNA sensitive); and TLR9 (CpG DNA sensitive) located in the endosomal compartment [7]. Signaling pathways of RNA sensing by TLRs and cytosolic RLRs have been studied extensively and reviewed recently in great detail [7, 11]. An area that has received particular focus in recent years is DNA sensing. Sensors of DNA include TLR9, which recognize unmethylated CpG in endosomes, as well as a number of more recently defined sensors including AIM2, IFI16, DDX41 and cGAS [10, 12]. Detection of cytosolic DNA results in two major types of pro-inflammatory responses. In one of these pathways, Absent in Melanoma-2 (AIM2) binds microbial DNA and recruits the adaptor protein ASC, facilitating the formation of a complex called the inflammasome [13–16]. This, in turn leads to activation of caspase-1, that subsequently mediates maturation of the pro-inflammatory cytokines IL-1β and IL-18. AIM2 is one of four proteins which constitute the PYHIN (PYD and HIN domain) containing proteins [17]. While the inflammasome is important in host-defense, the crucial response in nucleic acid sensing and antiviral immunity involves the transcriptional activation of type I IFN and other pro-inflammatory cytokine genes [12]. This activates phagocytic cells, such as macrophages and dendritic cells, and NK cells which destroy infected cells and reduce viral loads, thus bringing about the initial control of infection. In addition, type I IFNs induce the transcription of scores of interferon-stimulated genes (ISGs), whose products establish a general antiviral state by amplifying IFN responses and inhibiting viral replication [18, 19].
Understanding how DNA elicits the type I IFN response is important, since a range of pathogenic organisms appear to be detected by this pathway [12]. In addition, the DNA-sensing pathway is also important in DNA vaccination. Evidence from mouse studies in particular indicate that the adjuvancy of DNA vaccines rely on engagement of these mechanisms [20, 21]. Finally, a better understanding of these pathways has direct relevance for inflammatory disease. It has become clear over the past few years that host DNA present in the cytosol can also trigger an immune response, leading to debilitating inflammatory diseases, such as Aicardi-Goutieres syndrome (AGS), systemic lupus erythematosis (SLE) and other lupus-like diseases [3, 22]. In this review, we discuss recent progress in uncovering the mechanisms of DNA sensing in the cytosol with special emphasis on the role of cytosolic DNA receptors and associated signaling pathways resulting in type I IFN responses. We attempt to explore the importance of newly identified receptors all of which converge on a common adapter molecule called STING.
DNA sensing in the cytosol
The molecular basis of DNA sensing has been the focus of intense investigation for several years. Early studies showed that cells recognize DNA in the cytosol by TLR9-independent means, which ignited rapid and exciting progress in the field [23, 24]. DNA of microbial or host origin can gain access to the cytosol through different modes. During viral infection, genetic material from viral genomes or viral replication intermediates can access cytosolic compartments. Some bacterial pathogens such as Francisella tularensis replicate within the cytosol while others such as Mycobacterium tuberculosis introduce immunostimulatory DNA into the host cytosol through its type VII secretion system [25, 26]. These responses are not unique to viruses and bacteria, since protozoan DNA, too, has been shown to gain entry into the cytosol, as demonstrated in Plasmodium falciparum infections [27]. Failure to generate the requisite type I IFN can result in pervasive DNA virus infection, for example, the immune system fails to curb HSV-1 infection if these pathways are not engaged effectively [28–30].
The principal pathway of type I IFN induction is through activation of the stimulator of interferon gene (STING). STING, also known as TMEM173, MPYS, MITA and ERIS, is localized to the endoplasmic reticulum [31–33]. STING contains four transmembrane helices and a globular carboxy-terminal domain (CTD), which facilitates interaction of STING with the Inhibitor of kappa B kinase (IKK)-related kinase, called TANK binding kinase 1 (TBK1). TBK1 in turn phosphorylates and activates IRF3, an important transducer of IFN gene transcription [34]. Studies in mice lacking STING have revealed the importance of this protein both in recognition of cytosolic dsDNA and in controlling susceptibility to herpesvirus infections [28, 33]. In addition to its role as an adaptor in the DNA-sensing pathway, STING has also been described as a direct sensor of cyclic di-guanylate monophosphate (c-di-GMP) and cyclic-di-adenylate monophosphate (c-di-AMP) [35]. These cyclic dinucleotides regulate bacterial motility and biofilm formation. STING binds these small molecules through its CTD, leading to activation of TBK1 and IRF-3, which in turn results in transcription of IFN-β and associated genes [34]. STING is also subject to negative regulation by the E3 ligase, RING-finger protein 5 (RNF5). RNF5 ubiquitinates and degrades STING upon activation of antiviral response through STING, thus preventing excessive STING-mediated cytokine response [36]. STING has also been shown to coordinate activation of STAT6, which drives transcription of certain ISGs independently of IFN-β, which are important for antiviral immunity [37].
Although STING has been suggested to bind DNA, the prevailing paradigm of how the IFN response is initiated indicates that one or more upstream DNA-binding proteins couple DNA recognition to STING signaling [38]. Several cytosolic DNA-binding proteins have been identified and in a number of cases have been shown to function upstream of STING. These molecules are depicted in Figure 1 and include DNA-dependent activator of IRFs (DAI), DDX41, IFI16 and cGAS. DAI (also known as ZBP1), binds Z-form DNA and induces STING-mediated IFN-β response [39]. However, DAI was found to be dispensable for IFN-β induction, as the IFN-β response to cytosolic DNA was normal in in vitro studies in hematopoietic cell lines with DAI expression knocked down, and in in vivo studies using DAI-deficient mice, although the response was DAI-dependent in fibroblasts of human and murine origin [21, 39–41]. These data either imply DAI does not play a role in vivo and in cells of hematopoietic origin, or that redundant mechanisms come into play.
Figure 1. cGAS and other receptors of cytosolic DNA activate IFNβ transcription via activation of STING.
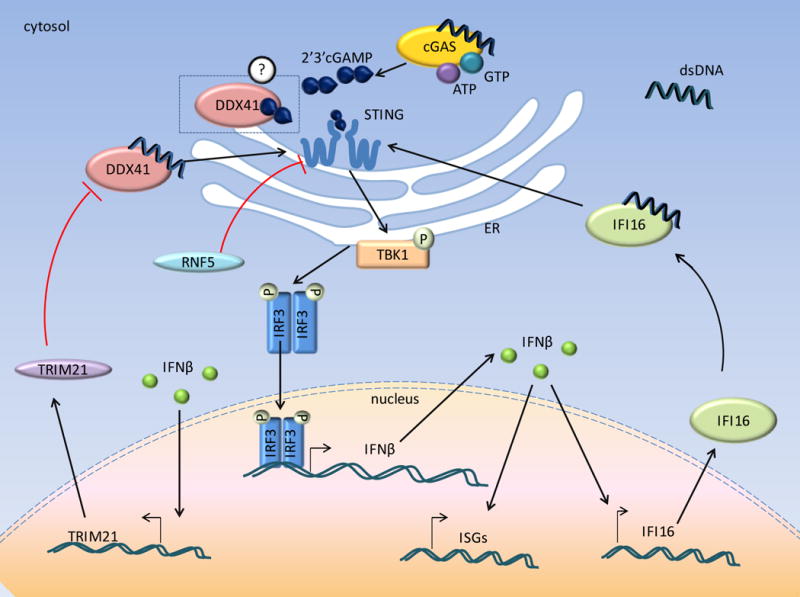
Cytosolic dsDNA binds cGAS and generates 2′3′cGAMP from the substrates ATP and GTP. 2′3′cGAMP in turn binds to and activates the STING dimer. This leads to phosphorylation of TBK-1, IRF3 which are translocated to the nucleus and activate IFN-β transcription. In addition, other receptors, such as, DDX41 and IFI16 also bind to cytosolic dsDNA and lead to IFN-β induction via the common STING pathway. IFN-β in turn induces expression of ISGs including IFI16. Different cytosolic receptors of dsDNA might be cell-type specific or specific to the stage of IFN-β induction.
The AIM2-like receptor, IFI16 and its mouse ortholog p204
Following the discovery of AIM2 and elucidation of its role as a DNA-binding receptor for inflammasome signaling, the related PYHIN proteins IFI16 (human) and p204 (murine) [17], which share a common domain organization, were both linked to DNA-induced IFN responses [14, 15, 42]. IFI16 is primarily a nuclear localized protein, previously linked to p53-mediated apoptosis and DNA damage signaling [43, 44]. IFI16 was identified in an affinity purification screen for cytosolic DNA binding proteins in THP-1 cells [42]. As depicted in Figure 1, IFI16 binds dsDNA, and studies in monocytic cells indicated that a pool of IFI16 localized in the cytosol co-localizes with dsDNA during HSV-1 infection. Structural studies confirmed that both IFI16 and AIM2 HIN domains bind the sugar-phosphate backbone of dsDNA through electrostatic interactions [45]. Whether STING binds IFI16 directly is currently unknown. Unlike AIM2, which engages ASC via PYD-PYD domain interactions, IFI16 was shown to form a complex with STING in monocytes and coordinate type I IFN responses in various cell types [29, 42, 46].
The murine PYHIN family comprises 13 family members [17]. One of these, p204 has also been linked to DNA-induced type I IFN responses [42]. The importance of IFI16 in mediating anti-viral responses to dsDNA is underscored by recent evidence indicating that viruses such as HSV1 encode proteins that target IFI16 for proteasomal degradation [47]. Nuclear IFI16 has been linked to recognition of HSV1 DNA during productive infection of human foreskin fibroblasts, leading to a type I IFN response. In this model, nuclear localized IFI16 detects nuclear HSV dsDNA and subsequently triggers a cytosolic STING pathway. Studies using p204 siRNA in mice have also linked p204 to control of HSV1 infection in an ocular model of HSV keratitis [29]. A broader analysis of the murine and human PYHIN proteins revealed that multiple members of this protein family can engage STING or ASC dependent pathways [48]. These findings further support a role for this family of PYHIN proteins as activators/regulators of the antiviral response.
DExD/H helicase DDX41
In addition to DAI and IFI16, DDX41, a DExD/H box helicase, was shown to induce IFN-β response upon stimulation with poly(dA:dT), HSV-1, Listeria monocytogenes, and adenovirus in macrophages as well as myeloid dendritic cell lines [49, 50]. Zhang et al. identified DDX41 by screening all DEAD box helicases for their role in this pathway [49]. Knockdown of DDX41 resulted in a profound decrease in IFN-β induction in mDCs upon stimulation by dsDNA ligands. This IFN-β induction by DDX41 was shown to occur via STING-mediated phosphorylation of TBK1 and IRF3. In another study, DDX41 was also found to bind to and control the IFN response to cyclic-dinucleotides (CDNs) such as cyclic-di-AMP and cyclic-di-GMP [51]. Biochemical studies indicate that DDX41 associates with STING through its DEADc domain [49]. Further evidence supporting a role for DDX41 in DNA sensing came from studies of TRIM21. TRIM21 is an E3 ligase that is induced in cells by IFN-β. TRIM21 ubiquitinates and subsequently degrades DDX41, providing the STING-mediated cytosolic surveillance pathway with a negative feedback mechanism, as shown in Figure 1 [52, 53].
OAS1-like nucleotidyl transferase, cGAS
Seminal recent studies identified a cyclic di-nucleotide called cyclic GMP-AMP in cells exposed to dsDNA and DNA viruses [54]. Using elegant biochemical purification and reconstitution strategies, the Chen lab identified this second messenger in the cytoplasm of cells exposed to DNA [54]. cGAMP is structurally similar to c-di-GMP and c-di-AMP. As with these c-di nucleotides, cGAMP binds STING and turns on TBK1-IRF3 signaling leading to type I IFN gene transcription. In addition to defining this second messenger, in a biochemical tour de force, the authors also used complementary protein purification strategies combined with quantitative mass spectrometry to identify the enzyme responsible for cGAMP generation. Their studies identified an uncharacterized mouse gene with significant structural homology to the catalytic domain of human oligoadenylate synthase (OAS1), a nucleotidyl transferase that is part of a larger family of NTases that include adenylate cyclase [55]. The authors named this enzyme cGMP-AMP synthase “cGAS”. cGAS binds DNA and catalyzes the synthesis of cGAMP from ATP and GTP via the mechanism outlined in Figure 2. The identification of cGAMP has reconciled prior observations that indicated STING could function both as an adapter for DNA sensing and a receptor for cyclic dinucleotides [35, 56].
Figure 2. Mechanism of cytosolic dsDNA sensing by cGAS.
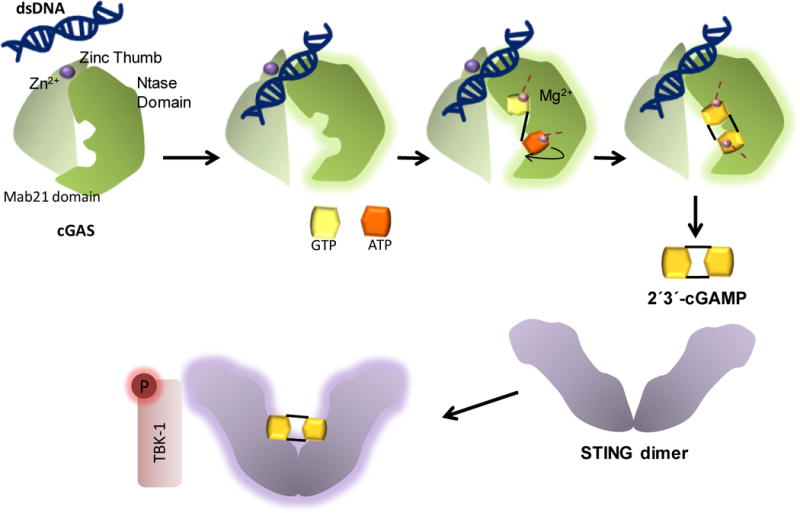
Cytosolic dsDNA binds cGAS via the basic surface of the bilobal scaffold of cGAS and the zinc thumb. Binding results in a conformational change in the NTase domain, allowing entry and binding of ATP and GTP to the catalytic core. Substrate binding coordinates two Mg2+ ions (depicted by purple spheres) to a specific tyrosine residue in the catalytic core causing a flip-over (shown by a curved arrow) in the linear intermediate of the enzymatic reaction between ATP and GTP, resulting in 2′3′cGAMP formation. 2′3′cGAMP binds to the inactive STING dimer at the dimer interface and triggers a conformational change which activates STING and leads to subsequent phosphorylation of TBK-1 and IRF3 thus activating transcription of IFN-β.
cGAS contains a nucleotidyl transferase (NTase) domain that partially overlaps with a C-terminal male abnormal 21 (Mab21) domain, as depicted in Figure 2. Recent structural insights into the enzyme have expanded our understanding of this unique second messenger system. Structures of the cGAS NTase and Mab21 domains reveal a bilobal scaffold, with an N-terminal NTase catalytic core that adopts a mixed α/β fold, and a C-terminal α-helical lobe. This bears significant structural similarity to 2′-5′-oligoadenylate synthase (OAS), with the exception of a unique “zinc thumb,” located between the two lobes of cGAS, that is essential for DNA binding. DNA binding to cGAS brings about a conformational change in the NTase domain of cGAS making it accessible to ATP and GTP in the cytosol, which are then utilized to generate cGAMP [54].
A series of follow-up biophysical and biochemical studies have since identified unique features of the endogenous cGAMP (generated by cGAS) that distinguish it from cGAMP and other c-di-nucleotides of microbial origin [54, 56–58]. The cGAMP produced by cGAS contains mixed phosphodiester linkages. A phosphodiester linkage between 2′-OH of GMP and 5′-phosphate of AMP and another between 3′-OH of AMP and 5′-phosphate of GMP constitutes endogenous or non-canonical cGAMP. This non-canonical molecule binds to STING with higher affinity than microbial cGAMPs or other cyclic di-nucleotides and elicits potent IFN-β responses. In further studies, Chen and colleagues generated cGAS-deficient mice [30]. Cells from cGAS-deficient (cGas−/−) mice, including fibroblasts, macrophages, and dendritic cells are compromised in their ability to produce type I interferons and other cytokines in response to DNA transfection or DNA virus infection [30]. cGas−/− mice were also shown to be more susceptible to lethal infection with herpes simplex virus 1 (HSV1) than wild-type mice. Finally, a follow-up study by the same group also implicated cGAS as a sensor for retroviruses including HIV, murine leukemia virus (MLV) and SIV [59]. cGAS-synthesized cGAMP can also be transferred from producing cells to neighboring cells through gap junctions, where it promotes STING activation and antiviral immunity independently of type I IFN signaling [60].
Significance of the STING-activating cytosolic DNA sensors
The discovery of the cGAS-cGAMP pathway and the recent structural, biophysical and genetic advances made in understanding how this second messenger system operates have profoundly increased our understanding of the mechanisms of DNA sensing and host responses to DNA viruses. It is likely that these discoveries will pave the way for future insights related to DNA-driven immune responses. As with all innate immune pathways, a network of regulatory checkpoints must exist to tightly regulate these pathways to prevent excessive inflammation arising from unchecked signaling. Unlike other microbial products, such as LPS, which is unique to bacteria, nucleic acids are not distinct from their host counterparts. Therefore, sensing of nucleic acids needs to be carefully regulated such that the delicate balance between protective responses against pathogens and destructive over-reaction to self-nucleic acids or their intermediates is not perturbed. Endogenous DNA from damaged or dying cells that are engulfed by phagocytes should normally be degraded inside cellular phagosomes. However, if the enzymes such as DNase II, which normally degrade this DNA are inefficient or impaired, this DNA is inefficiently degraded and appears to leak into cytosolic compartments to drive IFN dependent pathology[61]. DNA that accumulates from endogenous retroelements can also pose a serious problem if this is not cleared properly. AGS is with a case in point, where mutations in TREX1 prevent the clearance of DNA from endogenous retroelements which then triggers STING dependent IFN responses, resulting in lupus-like symptoms [3, 22, 62]. Small molecule inhibitors targeting the cGAS pathway could prove very useful in treating this and potentially other diseases. Given that cGAS is an enzyme, therapeutic targeting of this pathway should be feasible.
Despite the compelling evidence indicating essential roles for cGAS in DNA driven immunity, the role of the other STING associated sensors still remains to be resolved. It will be important to determine if IFI16, DDX41 and perhaps some of the other molecules function in the context of cGAS or play roles in distinct cell types or cellular locations. It is plausible that these sensors function in a cell type specific fashion, or in temporally distinct stages of the IFN-β response. Future research endeavors might uncover important cross-talk between these disparate components.
Acknowledgments
The authors would like to thank Drs. Vijay A. Rathinam and Shruti Sharma for critical reading of this manuscript and acknowledge grant support from the NIH (AI093752 to K.A.F).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Medzhitov R, Janeway CA., Jr Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol. 1999;64:429–435. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 5.De Nardo D, De Nardo CM, Latz E. New Insights into Mechanisms Controlling the NLRP3 Inflammasome and Its Role in Lung Disease. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii KJ, Akira S. Innate immune recognition of nucleic acids: beyond toll-like receptors. Int J Cancer. 2005;117:517–523. doi: 10.1002/ijc.21402. [DOI] [PubMed] [Google Scholar]
- 9.Goubau D, Deddouche S, Reis ESC. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathinam VA, Fitzgerald KA. Cytosolic surveillance and antiviral immunity. Curr Opin Virol. 2011;1:455–462. doi: 10.1016/j.coviro.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Atianand MK, Fitzgerald KA. Molecular basis of DNA recognition in the immune system. J Immunol. 2013;190:1911–1918. doi: 10.4049/jimmunol.1203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 17.Schattgen SA, Fitzgerald KA. The PYHIN protein family as mediators of host defenses. Immunol Rev. 2011;243:109–118. doi: 10.1111/j.1600-065X.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 18.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. The Capsids of HIV-1 and HIV-2 Determine Immune Detection of the Viral cDNA by the Innate Sensor cGAS in Dendritic Cells. Immunity. 2013 doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 22.Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 23.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 25.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 26.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, Barber GN, Gazzinelli RT, Fitzgerald KA, Golenbock DT. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5:173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong B, Zhang L, Lei C, Li Y, Mao AP, Yang Y, Wang YY, Zhang XL, Shu HB. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity. 2009;30:397–407. doi: 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, Xia W, Gu J, Ishikawa H, Gutman D, Barber G, Qin Z, Jiang Z. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 40.Lippmann J, Rothenburg S, Deigendesch N, Eitel J, Meixenberger K, van Laak V, Slevogt H, N’Guessan PD, Hippenstiel S, Chakraborty T, Flieger A, Suttorp N, Opitz B. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 41.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouchi M, Ouchi T. Role of IFI16 in DNA damage and checkpoint. Front Biosci. 2008;13:236–239. doi: 10.2741/2673. [DOI] [PubMed] [Google Scholar]
- 44.Kwak JC, Ongusaha PP, Ouchi T, Lee SW. IFI16 as a negative regulator in the regulation of p53 and p21(Waf1) J Biol Chem. 2003;278:40899–40904. doi: 10.1074/jbc.M308012200. [DOI] [PubMed] [Google Scholar]
- 45.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, Xiao TS. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horan KA, Hansen K, Jakobsen MR, Holm CK, Soby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, Ellermann-Eriksen S, Kurt-Jones E, Landolfo S, Damania B, Melchjorsen J, Bowie AG, Fitzgerald KA, Paludan SR. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J Immunol. 2013;190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A. 2012;109:E3008–3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein SC, Falck-Pedersen E. Sensing adenovirus infection: activation of interferon regulatory factor 3 in RAW 264.7 cells. J Virol. 2012;86:4527–4537. doi: 10.1128/JVI.07071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Bao M, Lu N, Weng L, Yuan B, Liu YJ. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat Immunol. 2013;14:172–178. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, Sjostrand M, Eloranta ML, Ni Gabhann J, Winqvist O, Sundelin B, Jefferies CA, Rozell B, Kuchroo VK, Wahren-Herlenius M. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206:1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, Patel DJ. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 62.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]


