Abstract
Radical gastrectomy with extended lymph node dissection and prophylactic resection of the omentum, peritoneum over the posterior lesser sac, pancreas and/or spleen was advocated at the beginning of the 1960s in Japan. In time, prophylactic routine resections of the pancreas and/or spleen were abandoned because of the high incidence of postoperative complications. However, omentectomy and bursectomy continued to be standard parts of traditional radical gastrectomy. The bursa omentalis was thought to be a natural barrier against invasion of cancer cells into the posterior part of the stomach. The theoretical rationale for bursectomy was to reduce the risk of peritoneal recurrences by eliminating the peritoneum over the lesser sac, which might include free cancer cells or micrometastases. Over time, the indication for bursectomy was gradually reduced to only patients with posterior gastric wall tumors penetrating the serosa. Despite its theoretical advantages, its benefit for recurrence or survival has not been proven yet. The possible reasons for this inconsistency are discussed in this review. In conclusion, the value of bursectomy in the treatment of gastric cancer is still under debate and large-scale randomized studies are necessary. Until clear evidence of patient benefit is obtained, its routine use cannot be recommended.
Keywords: Gastric cancer, Gastrectomy, Bursectomy, Omentum, Pancreas
Core tip: Components of radical gastrectomy have decreased over time but bursectomy has been still accepted as an integral part of radical gastrectomy by Far East surgeons but not world-wide. More large-scale comparative studies are necessary to determine its benefits for cancer recurrence and patient survival. Until patient benefits are demonstrated by future studies, its routine application cannot be justified.
INTRODUCTION
The top three causes of cancer deaths in the world are lung cancers (1.4 million deaths/year), stomach cancers (738000 deaths/year), and liver cancers (695900 deaths/year), respectively[1]. Stomach cancer refers to several different histological types of stomach tumors (stromal, carcinoid, lymphoma) but more than 90% of stomach cancers arise from the gastric mucosa as adenocarcinoma. The incidence of gastric adenocarcinoma shows a certain geographic distribution and it is highest in the Far East. A gender difference is also present and it is almost twice as common in men as in women. Surgery with curative intent such as radical gastrectomy and regional lymph node dissection produces the best treatment outcomes for advanced (beyond the submucosa) gastric adenocarcinomas. The extent of surgical resection includes gastrectomy (total or subtotal), lymphadenectomy (D1, D2 or D3), and prophylactic or therapeutic resection of the surrounding organs or tissues (e.g., omentum, peritoneum, pancreas, spleen, colon, liver). Prophylactic or therapeutic peritonectomy over the lesser sac during radical gastrectomy is called bursectomy. The value of bursectomy in the treatment of gastric cancer is still under debate. This surgical technique is usually preferred by Far East surgeons[2] but is not accepted in the rest of the world. The aim of this article is to review the current data about the role of bursectomy in the treatment of gastric cancers.
HISTORY AND LOGIC OF BURSECTOMY
Radical gastrectomy with extended lymph node dissection was advocated at the beginning of the 1960s in Japan by Jinnai[3]. At that time, additional prophylactic resection of the omentum, the peritoneum over the posterior lesser sac, the pancreas and/or spleen had been justified as the standard procedure to perform during complete radical gastectomy. In time, prophylactic routine resections of the pancreas and/or spleen were abandoned because of the high incidence of postoperative complications[4]. However, omentectomy and bursectomy continued to be standard parts of traditional radical gastrectomy.
The omental bursa, also known as the lesser sac, is a posterior cavity in the abdomen and is demarcated anteriorly by the liver, stomach, and omentum. Posteriorly it is marked by the pancreas, left surrenal, and kidney (Figure 1). It is connected with the main anterior peritoneal cavity via the foramen of Winslow. The bursa omentalis was thought to be a natural barrier against invasive of cancer cells at the posterior part of the stomach and resection of the peritoneum lining over this cavity as bursectomy was accepted as a integral part of radical gastrectomy. The theoretical rationale for this procedure was to reduce the risk of peritoneal recurrences by eliminating the peritoneum over the lesser sac that might have included free cancer cells or micrometastases[5].
Figure 1.
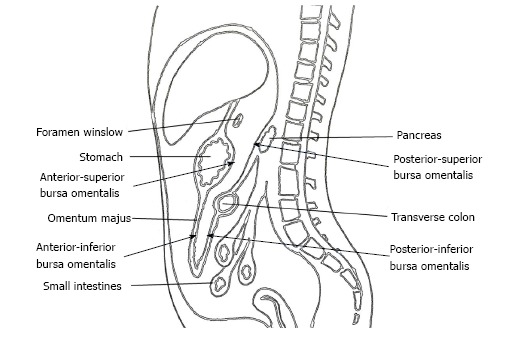
Anatomy of the bursa omentalis.
Bursectomy includes the removal of the peritoneal lining covering the pancreas (anterior pancreatic capsule) and the anterior plane of the transverse mesocolon along with a total omentectomy. Omentectomy has two objectives in a radical gastrectomy. First, it eliminates the perigastric lymph nodes along the greater curvature of the stomach, and second, it provides for the excision of the gastrocolic ligament that covers the anterior/inferior part of the lesser sac (Figure 1). The anterior/superior part of the lesser sac is removed by the gastrectomy itself. The posterior wall of the bursectomy is completed by removing the peritoneal sheath over the transverse mesocolon and the pancreas.
This Japanese-originated surgical technique has been known for 50 years and is mainly accepted by Far East surgeons but also by some other groups[6-9]. It was routinely recommended in the Japanese Gastric Cancer Treatment Guidelines as a part of radical surgery for gastric cancer without any supporting evidence, but was included due to traditional acceptance (version 1, 2001)[10]. The Japanese Gastric Cancer Association revised the gastric cancer treatment guidelines three years after the first version and recommended bursectomy only for serosa-invading tumors (version 2, 2004)[11]. Recently, they changed the guidelines again and this time they limited the indication of bursectomy only to posterior gastric wall tumors penetrating the serosa (version 3, 2010)[12].
WEAKNESSES OF BURSECTOMY
Gastric cancers can penetrate the serosa at the anterior or posterior gastric walls. Penetrating tumors can cause seeding of the micrometastatic tumor cells to the free peritoneal surfaces. Anterior-wall-located serosal invasions can cause implantation into the entire intraperitoneal abdominopelvic cavity (greater sac) and prophylactic peritonectomy of all of the peritoneum is not justified. In theory, the risk of posteriorly located serosa-invading cancers may be reduced by peritonectomy of the lesser sac (bursectomy) and the posterior location itself can provide an advantage for controlling the tumor cells.
In 2004, Yoshikawa et al[13] analyzed the clinical records of patients who underwent radical gastrectomy with bursectomy for gastric cancer invading the serosa, with special reference to the location of tumor invasion. A total of 134 patients were divided into two groups, which included patients with serosa positive tumors that invaded only the posterior or anterior gastric walls. Survival rates at 3 and 5 years were 67.3% and 53.0% for the posterior group and 68.8% and 53.8% for the anterior group, respectively. There was no significant difference in survival between the two groups and multivariate analyses demonstrated that the significant independent factor for survival was the stage of the tumor, not the location as anterior or posterior. They suggested that bursectomy for posterior-located serosa invading tumors did not provide any survival benefit over their anterior counterparts. This was one of the first studies to raise doubts about the bursa omentalis being a natural barrier against implanted cancer cells and the role of bursectomy.
Histopathological confirmation of invisible tumor deposits in the retro-gastric cavity and on the peritoneum of the lesser sac can be good supporting evidence for prophylactic bursectomy. To study this, we sent bursectomy specimens (the anterior layer of the mesocolon and the pancreas) from 40 gastric cancer patients separately from the main gastrectomy specimens for pathological examination[14]. We also examined the cytology of bursa omentalis wash-out of these patients. Only four bursectomy specimens (10%) demonstrated positive cancer cells, and all of these patients already had macroscopic tumors on the peritoneal surfaces of the transverse mesocolon or pancreas. The cytology of bursa omentalis wash-out results was parallel to these pathological reports. Therefore, we failed to demonstrate invisible tumor cells in or on the lesser sac by conventional histopathology.
Anatomically, the cavity of the bursa omentalis is not a closed space and it is connected with the greater sac via the foramen of Winslow. Demonstration of the migration of tumor cells from the lesser sac to the greater sac or the contrary demonstration of the restriction of tumor cells to the lesser sac are important issues for the justification of bursectomy.
Yamamura et al[15] in 2007 examined the cytology of the peritoneal washes obtained from the Douglas pouch, left subphrenic cavity, and the inside of the omental bursa in 136 patients by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. Cancer-related cells were detected in one or more samples from the three different sites of peritoneal washes in 43 (31.6%) patients. In 14 patients, these tumor cells were detected in the samples obtained from the bursa omentalis and in 12 (85.7%) of these 14 patients, cancer-related cells were also detected in the samples taken from the Douglas pouch or subpherenic cavity. This study demostrated that viable cancer cells disseminated into the bursa omentalis and did not remain restricted to this cavity. The authors suggested that these cells are unlikely to be optimal targets for surgical removal, and the emergence of more effective locoregional therapy is urgently needed to improve the survival of serosa-positive patients.
Lastly, quality control of the complete en-bloc bursectomy is not easy. It depends on both the experience of the surgeon and the patient’s mesenteric fat content. While bursectomy is technically more comfortable in slim patients, finding the right plane over the transverse mesocolon in fatty patients can be troublesome. In some fatty patients, we tried to inject normal saline between the peritoneum and the mesenteric fat of the transverse colon to provide an easier bursectomy technique; however, this hydrodissection method failed.
TECHNIQUE OF BURSECTOMY
We usually prefer to begin bursectomy with the entrance to the avascular plane between the greater omentum and the transverse mesocolon in the midline. To facilitate finding the correct plane, the first assistant should hang the greater omentum up for retraction and the transverse colon should be retracted to the opposite site by the surgeon (Figure 2). Diathermy can be used for entering the embryonic avascular plane just over the colon and can improve these dissections. Care must be taken not to damage the appendicular arteries of the colon and dissections should be skipped over these arteries. Once entered into the avascular plane, it is easier to extend the dissections to the hepatic and splenic flexure of the mesocolon. During this peritoneal peeling, continuous counter-traction to both sides of the mesocolon and to the omentum is mandatory. We usually prefer sharp dissections but sometimes gentle blunt dissection can provide an easy and fast peritonectomy. En-bloc resection without any window on the peritoneum is desired, but it is not always possible. If there is a tear in the peritoneum over the mesocolon, patiently going back a few steps to work on removal from the free edge of the torn peritoneum should be the preferred approach. Care should also be taken to not damage the mesocolic vessels at the bottom. When the procedure reaches the lower border of the pancreas, the dissection should be extended over it lengthwise. The entire posterior leaf of the peritoneum covering the lesser sac over the transverse mesocolon and the pancreas should be excised en-bloc.
Figure 2.
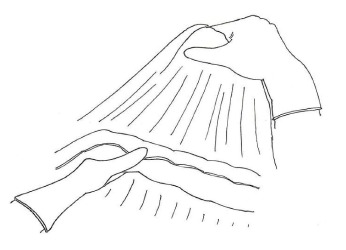
Traction and counter-traction of the omentum, stomach and colon.
CLINICAL RESULTS OF BURSECTOMY
There are only a limited number of studies that analyzed the influence of bursectomy on the survival of patients with gastric cancers[5,13,16-20]. Three studies are from Japan, one from South Korea, and one from the Ukraine. One of the early studies by Yoshikawa et al[13] compared the outcomes of bursectomy and non-bursectomy groups in a total of 134 serosa-positive gastric cancers. They suggested that there was no survival benefit of bursectomy in patients with gastric cancer[13]. In 2012, Fujita et al[5] reported the first results of their randomized study including 210 patients with T2-T3 gastric cancers. They found that bursectomy could improve survival and should not be abandoned as a futile procedure until more definitive data can be obtained[5].
Recently, the same group reported their updated results with the same conclusions[17]. However, their study included only 48 serosa-positive gastric cancers and there were no data about the comparability of the serosa-positive patients between groups. Cox multivariate analysis of the overall survival in that study pointed out that the most important independent factor for survival was the stage of the tumor (T stage, P < 0.001). Although nonbursectomy was found to be an independent risk factor (P = 0.034), male sex was also determined to be an independent risk factor in the same multivariated analysis (P = 0.032). These findings indicate that there were too few patients in that study to allow for clear conclusions.
The third study from Japan by Kochi et al[18] had a similar deficit in that only 41 of 254 patients had serosa-positive gastric cancers, and these authors found no survival benefit of bursectomy. In 2013, a congress abstract reported from the Ukraine that included 108 patients (T1-4) with gastric cancers concluded that the bursectomy group had a better 5-year survival, but the details of this study have not yet been published[19]. Eom et al[20] from South Korea compared bursectomy and nonbursectomy patients in a total of 381 serosa positive gastric cancers (nonbursectomy = 284 vs bursectomy = 97) and found in multivariate analyses that bursectomy was not a significant independent factor for survival.
CONCLUSION
Recently a meta-analysis that included all published studies on prophylactic bursectomy at radical gastrectomy was published[21]. According to the available data, the bursectomy did not show superiority to non-bursectomy in terms of survival in gastric cancer patients. Although the subgroup analyses suggested that bursectomy may improve survival in serosa-positive patients, this was not statistically significant and a definitive conclusion could not be made[21]. Because of the risk of potential morbidities[22], unless the exact benefits are demonstrated by forthcoming studies, its routine application cannot be justified. A large-scale multicentric Phase III trial is currently underway for macroscopically subserosa or serosa-positive gastric cancer in Japan (JCOG 1001)[23]. This study included only patients from Japan, and it has already closed patient enrollment[23]. The long-term outcomes of this study will provide important information about the role of bursectomy at radical gastrectomy.
Footnotes
P- Reviewer: Nakayama Y S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Conflict-of-interest statement: Author declares no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: July 25, 2015
Article in press: August 21, 2015
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535–e547. doi: 10.1016/S1470-2045(13)70436-4. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T. Historical Review of Research and Treatment of Gastric Cancer in Japan: Clinical Aspect. The Diversity of Gastric Carcinoma: Pathogenesis, Diagnosis, and Therapy. In: Kaminishi M, Takubo K, Mafune K, editors. Springer; 2005. pp. 29–47. [Google Scholar]
- 4.Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 5.Fujita J, Kurokawa Y, Sugimoto T, Miyashiro I, Iijima S, Kimura Y, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer. 2012;15:42–48. doi: 10.1007/s10120-011-0058-9. [DOI] [PubMed] [Google Scholar]
- 6.Vural V, Saylam B, Çomçalı B, Düzgün AP, Özer MV, Coşkun F. D1 versus D2 dissection in gastric carcinoma: Evaluation of postoperative mortality and complications. Ulus Cerrahi Derg. 2013;29:1–6. doi: 10.5152/UCD.2013.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostanci EB, Kayaalp C, Ozogul Y, Aydin C, Atalay F, Akoglu M. Comparison of complications after D2 and D3 dissection for gastric cancer. Eur J Surg Oncol. 2004;30:20–25. doi: 10.1016/j.ejso.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Kung CH, Lindblad M, Nilsson M, Rouvelas I, Kumagai K, Lundell L, Tsai JA. Postoperative pancreatic fistula formation according to ISGPF criteria after D2 gastrectomy in Western patients. Gastric Cancer. 2014;17:571–577. doi: 10.1007/s10120-013-0307-1. [DOI] [PubMed] [Google Scholar]
- 9.Blouhos K, Boulas KA, Hatzigeorgiadis A. Bursectomy in gastric cancer surgery: surgical technique and operative safety. Updates Surg. 2013;65:95–101. doi: 10.1007/s13304-013-0210-7. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Commentary of gastric cancer treatment guidelines (in Japanese) Tokyo: JGCA; 2001. [Google Scholar]
- 11.Japanese Gastric Cancer Association. Gastric cancer treatment guidelines (in Japanese) Tokyo: Kanehara; 2004. pp. 9–10. [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Hasegawa S, Ishiwa N, Morinaga S, Noguchi Y, Yamamoto Y, et al. Is bursectomy necessary for patients with gastric cancer invading the serosa? Hepatogastroenterology. 2004;51:1524–1526. [PubMed] [Google Scholar]
- 14.Kayaalp C, Olmez A, Piskin T. Prophylactic bursectomy at radical gastrectomy for gastric cancer. Gastric Cancer. 2011;14:399–400. doi: 10.1007/s10120-011-0084-7. [DOI] [PubMed] [Google Scholar]
- 15.Yamamura Y, Ito S, Mochizuki Y, Nakanishi H, Tatematsu M, Kodera Y. Distribution of free cancer cells in the abdominal cavity suggests limitations of bursectomy as an essential component of radical surgery for gastric carcinoma. Gastric Cancer. 2007;10:24–28. doi: 10.1007/s10120-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 16.Imamura H, Kurokawa Y, Kawada J, Tsujinaka T, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Influence of bursectomy on operative morbidity and mortality after radical gastrectomy for gastric cancer: results of a randomized controlled trial. World J Surg. 2011;35:625–630. doi: 10.1007/s00268-010-0914-5. [DOI] [PubMed] [Google Scholar]
- 17.Hirao M, Kurokawa Y, Fujita J, Imamura H, Fujiwara Y, Kimura Y, Takiguchi S, Mori M, Doki Y. Long-term outcomes after prophylactic bursectomy in patients with resectable gastric cancer: Final analysis of a multicenter randomized controlled trial. Surgery. 2015;157:1099–1105. doi: 10.1016/j.surg.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Kochi M, Fujii M, Kanamori N, Kaiga T, Mihara Y, Funada T, Tamegai H, Takayama Y, Yoshida N, Takayama T. D2 gastrectomy with versus without bursectomy for gastric cancer. Am J Clin Oncol. 2014;37:222–226. doi: 10.1097/COC.0b013e31825eb734. [DOI] [PubMed] [Google Scholar]
- 19.Shchepotin I, Kolesnik O, Lukashenko A, Rozumiy D, Burlaka A. D2 Gastrectmoy with versus without bursectomy for gastric cancer: Results of single center randomized trial. Ann Oncol. 2013;24:P–0103. [Google Scholar]
- 20.Eom BW, Joo J, Kim YW, Bae JM, Park KB, Lee JH, Ryu KW, Kook MC. Role of bursectomy for advanced gastric cancer: result of a case-control study from a large volume hospital. Eur J Surg Oncol. 2013;39:1407–1414. doi: 10.1016/j.ejso.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Shen WS, Xi HQ, Wei B, Chen L. Effect of gastrectomy with bursectomy on prognosis of gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:14986–14991. doi: 10.3748/wjg.v20.i40.14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayaalp C, Piskin T, Olmez A. Complications of bursectomy after radical gastrectomy for gastric cancer. World J Surg. 2012;36:229; author reply 230. doi: 10.1007/s00268-011-1218-0. [DOI] [PubMed] [Google Scholar]
- 23.Japan Clinical Oncology Group. Clinical Trials. Available from: http: //www.jcog.jp/en/trials/index.html.


