Abstract
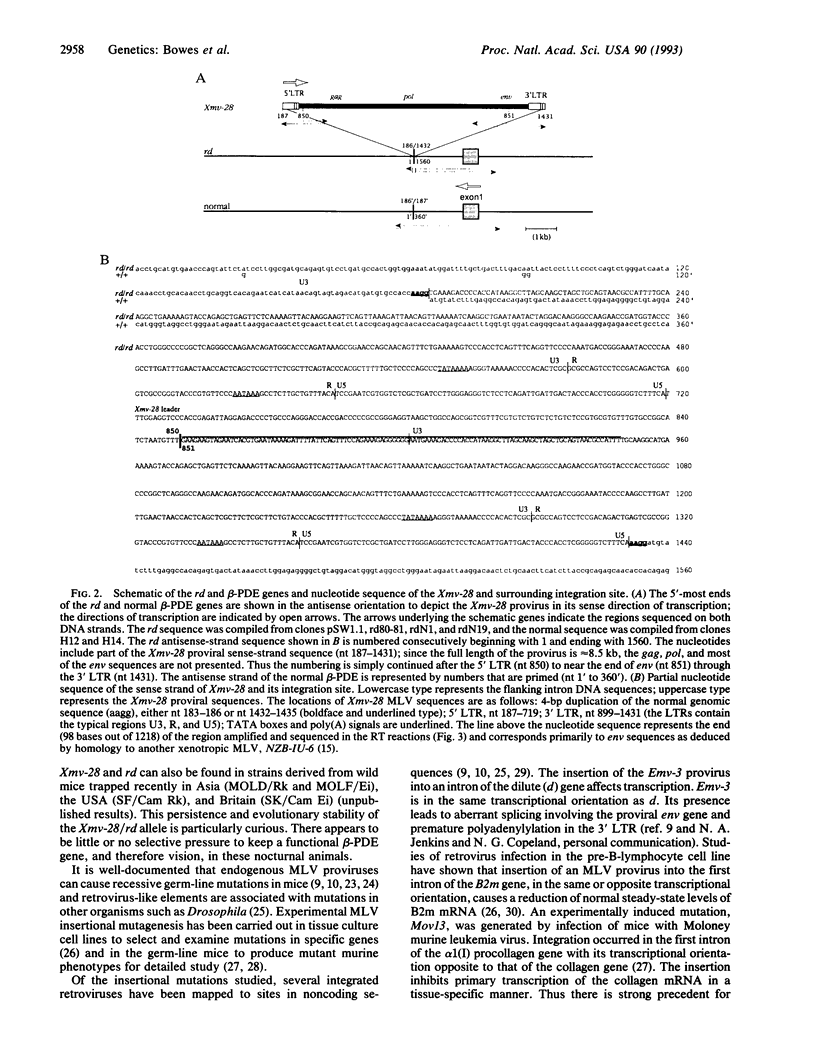
Retinal degeneration in the rd mouse is inherited as an autosomal recessive trait and is caused by a defect in the gene encoding the beta subunit of cGMP phosphodiesterase. Recently, a close genetic association of the rd gene with an endogenous xenotropic murine leukemia virus (Xmv-28) was established by linkage analysis using recombinant inbred strains of mice. In this study, genomic DNA mapping and sequence analyses clarify the position of the proviral sequences in relation to the rd gene. We find that the Xmv-28 provirus is integrated into intron I of the rd gene 1511 bp downstream of the exon-intron boundary. The transcriptional orientation of the provirus is opposite to that of the gene for the beta subunit of cGMP phosphodiesterase. Reverse transcription-PCR demonstrates that the integrated Xmv-28 sequences are transcribed in the retina. The provirus is present in every strain of rd mouse tested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowes C., Danciger M., Kozak C. A., Farber D. B. Isolation of a candidate cDNA for the gene causing retinal degeneration in the rd mouse. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9722–9726. doi: 10.1073/pnas.86.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Danciger M., Bowes C., Kozak C. A., LaVail M. M., Farber D. B. Fine mapping of a putative rd cDNA and its co-segregation with rd expression. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1427–1432. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Potter T. A., Rajan T. V. Effect of proviral insertion on transcription of the murine B2mb gene. J Virol. 1989 Jun;63(6):2623–2628. doi: 10.1128/jvi.63.6.2623-2628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J Virol. 1989 Apr;63(4):1763–1774. doi: 10.1128/jvi.63.4.1763-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W., Potter T. A., Rosenberg N., Lenz J., Rajan T. V. Retroviral insertional mutagenesis of a target allele in a heterozygous murine cell line. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6600–6604. doi: 10.1073/pnas.82.19.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Kuehn M., Delius H., Jaenisch R. Insertion of retrovirus into the first intron of alpha 1(I) collagen gene to embryonic lethal mutation in mice. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1504–1508. doi: 10.1073/pnas.81.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., O'Neill R. R., Kozak C. A. Nonecotropic murine leukemia viruses in BALB/c and NFS/N mice: characterization of the BALB/c Bxv-1 provirus and the single NFS endogenous xenotrope. J Virol. 1986 Dec;60(3):980–986. doi: 10.1128/jvi.60.3.980-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Sidman R. L. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974 May;91(5):394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- Lamont C., Culp P., Talbott R. L., Phillips T. R., Trauger R. J., Frankel W. N., Wilson M. C., Coffin J. M., Elder J. H. Characterization of endogenous and recombinant proviral elements of a highly tumorigenic AKR cell line. J Virol. 1991 Sep;65(9):4619–4628. doi: 10.1128/jvi.65.9.4619-4628.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J., Flannery J. G., Li T., Applebury M. L., Farber D. B., Simon M. I. Retinal degeneration is rescued in transgenic rd mice by expression of the cGMP phosphodiesterase beta subunit. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Spence S. E., Gilbert D. J., Swing D. A., Copeland N. G., Jenkins N. A. Spontaneous germ line virus infection and retroviral insertional mutagenesis in eighteen transgenic Srev lines of mice. Mol Cell Biol. 1989 Jan;9(1):177–184. doi: 10.1128/mcb.9.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Fenner S., Greenoak G. E., Moran C., Coffin J. M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988 Jul 29;54(3):383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]