Abstract
Polycystic kidney disease (PKD) protein 2 Like 1 (PKD2L1), also called transient receptor potential polycystin-3 (TRPP3), regulates Ca2+-dependent hedgehog signalling in primary cilia, intestinal development and sour tasting but with an unclear mechanism. PKD2L1 is a Ca2+-permeable cation channel that is activated by extracellular Ca2+ (on-response) in Xenopus oocytes. PKD2L1 co-expressed with PKD protein 1 Like 3 (PKD1L3) exhibits extracellular acid-induced activation (off-response, i.e., activation following acid removal) but whether PKD1L3 participates in acid sensing remains unclear. Here we used the two-microelectrode voltage-clamp, site directed mutagenesis, Western blotting, reverse transcriptase-polymerase chain reaction (RT-PCR) and immunofluorescence, and showed that PKD2L1 expressed in oocytes exhibits sustained off-response currents in the absence of PKD1L3. PKD1L3 co-expression augmented the PKD2L1 plasma membrane localization but did not alter the observed properties of the off-response. PKD2L1 off-response was inhibited by an increase in intracellular Ca2+. We also identified two intra-membrane residues aspartic acid 349 (D349) and glutamic acid 356 (E356) in the third transmembrane domain that are critical for PKD2L1 channel function. Our study suggests that PKD2L1 may itself sense acids and defines off-response properties in the absence of PKD1L3.
Polycystic kidney disease (PKD) protein 2 Like 1 (PKD2L1) belongs to the transient receptor potential (TRP) superfamily of which members are implicated in a number of sensory functions, such as detection of light, force, osmolarity, temperature, odor, taste and pain1,2. Human PKD2L1 gene is a homologue of PKD protein 2 (PKD2) and was first identified in 19983. While mutations in PKD protein 1 (PKD1) and PKD2 account for about 85% and 15% of the cases in autosomal dominant PKD (ADPKD)4, respectively, PKD2L1 does not seem to be involved in the disease. In 1999, Chen et al. discovered the Ca2+-regulated non-selective cation permeability of PKD2L15. We previously reported that human PKD2L1 expressed alone in Xenopus oocytes targets to the plasma membrane (PM) and displays the regulation of Ca2+-induced channel activation (on-response) by a number of factors including Ca2+, voltage, pH, amiloride analogs, large monovalent organic cations, troponin I, α-actinin, and receptor for activated protein kinase 1 (RACK1)5,6,7,8,9,10,11,12. In mammalian cells, outward rectifying cation permeability of PKD2L1, with or without co-expression of PKD protein 1 Like 1 (PKD1L1, a homologue of PKD1), was recently reported13. This group of researchers found that PKD2L1, present in primary cilia, regulates the ciliary Ca2+ concentration and sonic hedgehog signalling. Lack of PKD2L1 in mice was associated with defective hedgehog signalling and development of inverted colon13,14.
In mammalian cells and oocytes, PKD2L1, in complex with PKD protein 1 Like 3 (PKD1L3, another PKD1 homologue), was activated by acids in an off-response manner, i.e., the PKD2L1/PKD1L3 channel complex mediated a large current only after the removal of the extracellular acid15,16. These and subsequent studies17,18 indicated that PKD2L1 or PKD1L3 forms a novel extracellular acid sensor. Forming a candidate for sour taste sensation, PKD2L1 was shown to interact with PKD1L318 in murine taste receptor type III cells. PKD2L1 and PKD1L3 were co-expressed around the pore region of taste buds in mouse circumvallate and foliate papillae19, but only PKD2L1 was found in other areas, including the fungiform and palate taste buds15,18. The involvement of the PKD2L1/PKD1L3 complex in sour tasting remains controversial as studies using knockout mice showed that PKD2L1, but not PKD1L3, is involved in sour tasting and acid sensing17,20,21. Further, these taste buds, regardless of whether expressing PKD1L3 or not, still have similar PKD2L1 distribution patterns with a specific localization to the pore region18,21. The PM localization of PKD2L1 alone was reported in both HEK cells and Xenopus oocytes by immunofluorescence, surface biotinylation and/or electrophysiology5,12,13,16,22. Co-expression of PKD1L3 substantially increased the PKD2L1 PM targeting, which presumably allowed the channel function to be observed more easily15,16,23,24.
PKD2L1/PKD1L3 channel complex responded to both weak and strong acids. It was reported that weak acids, such as citric acid, induce larger off-response currents in HEK cells expressing the PKD2L1/PKD1L3 complex than strong acids, such as HCl, at the same pH. All these acids possessed similar activation threshold values of around pH 3.018. Controversially, it was reported later that the induced off-response for PKD2L1/PKD1L3 by strong (HCl and sulphuric) and weak (citric, malic, phosphoric, succinic and tartaric) acids are not significantly different in HEK cells15. In intact biological systems, PKD2L1-expressing cells were associated with activation thresholds of higher pH values. For example, the threshold value for taste receptor cells (TRCs) was found to be around pH 5.020,25. Murine cerebrospinal fluid contacting neurons (CSF-cN), expressing endogenous PKD2L1, were very sensitive to changes in extracellular pH, in the pH range of 6.5–7.420. Moreover, two negatively charged residues in the PKD2L1 pore loop, D52326 and D52516, have been identified to affect Ca2+ permeation and cation selectivity of the PKD2L1/PKD1L3 channel complex, and may thus be involved in channel gating and selectivity.
Despite recent significant progresses towards understanding the biological and physiological roles of PKD2L1 and PKD1L3, how PKD2L1 and/or PKD1L3 sense acid is not fully understood. In the current study, we employed Xenopus oocyte expression model to examine how acids and PKD1L3 modulate PKD2L1 channel activity. In addition, we examined roles of Ca2+ and intra-membrane acidic residues in this modulation. For this we utilized two-microelectrode voltage clamp in combination with mutagenesis, Western blotting, RT-PCR and immunofluorescence.
Results
Acid-induced off-response currents in oocytes expressing PKD2L1 with or without PKD1L3
Although reported acid-dependent off-response properties have so far all been associated with the co-expression of PKD2L1 and PKD1L315,18,23,24,25,26,27,28, it has remained unclear as to the role of PKD2L1 in acid sensing and induction of the off-response. We employed the two-microelectrode voltage clamp electrophysiology to investigate how PKD2L1 expressed alone in Xenopus oocytes responds to acidic stimuli. In the absence of extracellular Ca2+ (to avoid the on-response effect of Ca2+ on PKD2L1), we found that increasing extracellular acidity (from pH 7.5 to pH 2.5–4.5) to PKD2L1-expressing or control oocytes does not induce appreciable currents. However, in PKD2L1-expressing, but not water-injected control, oocytes voltage clamped at −50 mV a large inward current was elicited following substitution of an acidic extracellular solution with a control solution at pH 7.5 (Fig. 1A). This large current induced in an off-response manner resembled those associated with PKD2L1/PKD1L3 co-expressed in HEK cells, oocytes and TRCs15,16,18,25. This indicates that PKD2L1 alone is able to exhibit off-response activation. We also voltage clamped oocytes at other membrane potentials using a voltage ramp protocol, which produced the acid-elicited off-response currents as function of the membrane potential (I-V curves) (Fig. 1B).
Figure 1. Acid-induced off-response currents in Xenopus laevis oocytes over-expressing human PKD2L1.
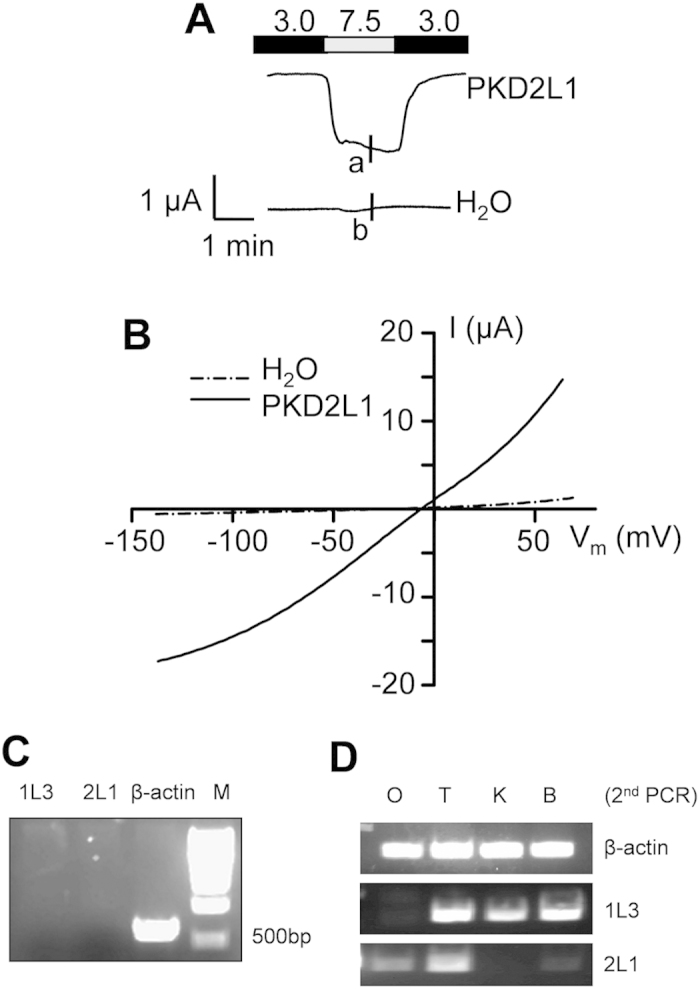
(A) Representative currents recorded by the two-microelectrode voltage clamp at −50 mV from an oocyte expressing human PKD2L1 or a water-injected oocyte (as a control). An off-response current is defined as the difference between the current amplitudes at pH 7.5 and pH 3.0. The pH values of the standard extracellular solutions are indicated. (B) Representative current–voltage relationship curves obtained using a voltage ramp protocol at the time points indicated by ‘a’ and ‘b’ in the panel A tracings. (C) RT-PCR using Xenopus oocytes detecting the RNA signals of PKD2L1 (2L1), PKD1L3 (1L3) and β-actin. The predicted sizes are 658 bp, 706 bp and 718 bp for β-actin, PKD1L3 and PKD2L1, respectively. (D) Second (nested) PCR detecting PKD1L3 and PKD2L1 signals using Xenopus oocytes (O), tongue (T), kidney (K) and brain (B). β-actin signals served as positive controls.
To examine whether any oocyte endogenous PKD1L3 contributed to our observed off-response currents we performed RT-PCR assays using total RNAs isolated from oocytes and various Xenopus tissues including the tongue, kidney and brain. No PKD1L3 or PKD2L1 signal was detected in oocytes in the first round of RT-PCR (Fig. 1C). We followed up with nested PCR assays and revealed a weak PKD2L1 signal in oocytes and brain, but still no detectable PKD1L3 signal in oocytes (Fig. 1C,D). Thus, the off-response observed in PKD2L1-expressing oocytes is independent of Xenopus PKD1L3.
We next examined the effect of PKD1L3 co-expression on PKD2L1-induced off-responses. In oocytes injected with a small amount (4 ng per oocyte) of PKD2L1 mRNA the off-response was observed only in the presence of PKD1L3 co-expression (injected at 25 ng mRNA per oocyte) (Fig. 2A,B). Our immunofluorescence experiments showed that PKD1L3 co-expression increases the PM localization of PKD2L1 (Fig. 2C). These data are in agreement with a previous report in which similar amounts of mRNAs were injected16. In oocytes injected with a larger amount (25 ng per oocyte) of PKD2L1 mRNA, co-expression of PKD1L3 still increased off-response currents and PKD2L1 PM localization (Fig. 2). Thus, taken together, our data indicated that PKD2L1 exhibits the off-response when its PM localization exceeds a certain threshold level and that PKD1L3 enhances the off-response amplitude, likely through increasing the PM population of PKD2L1.
Figure 2. Off-response currents in oocytes expressing PKD2L1 with or without PKD1L3.
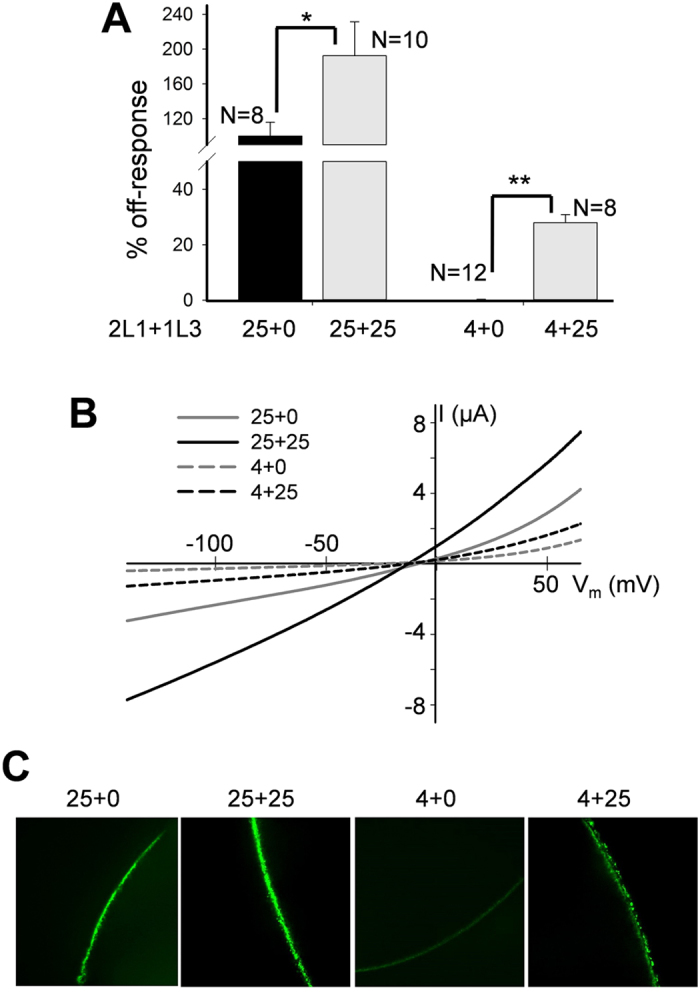
(A) Averaged and normalized off-response currents elicited by extracellular pH 3.0 from oocytes injected with 4 or 25 ng of PKD2L1 (2L1) mRNA alone or co-injected with 25 ng of PKD1L3 (1L3) mRNA, and voltage clamped at −50 mV. Shown are off-response currents averaged from different numbers of oocyte, as indicated. *p = 0.05 and **p = 0.004, unpaired t-test. (B) Representative off-response I-V curves obtained using a ramp protocol under the same conditions as in panel A. (C) Representative immunofluorescence data showing the PM intensity of PKD2L1 protein in oocytes injected with different amounts of mRNAs, as indicated.
Effects of acid dose, application time, and type on the PKD2L1 off-response
Our voltage clamp data showed that the PKD2L1-associated off-response is dose-dependent with half maximal activation value of pH 3.5 ± 0.5 (N = 5–9) in PKD2L1-expressing oocytes voltage clamped at −50 mV (Fig. 3A,B). Previous studies also found dose-dependent off-responses, with cell-type dependent half maximal activation pH values. PKD2L1/PKD1L3 expressed in HEK cells responded to pH 3.0 or lower15,18,24, taste cells responded to solutions of pH 5.0 or lower19,20, while mouse CSF-cN neurons responded to changes in extracellular pH in the 6.5 to 7.4 range20.
Figure 3. Dose dependence of the acid-induced off-response.
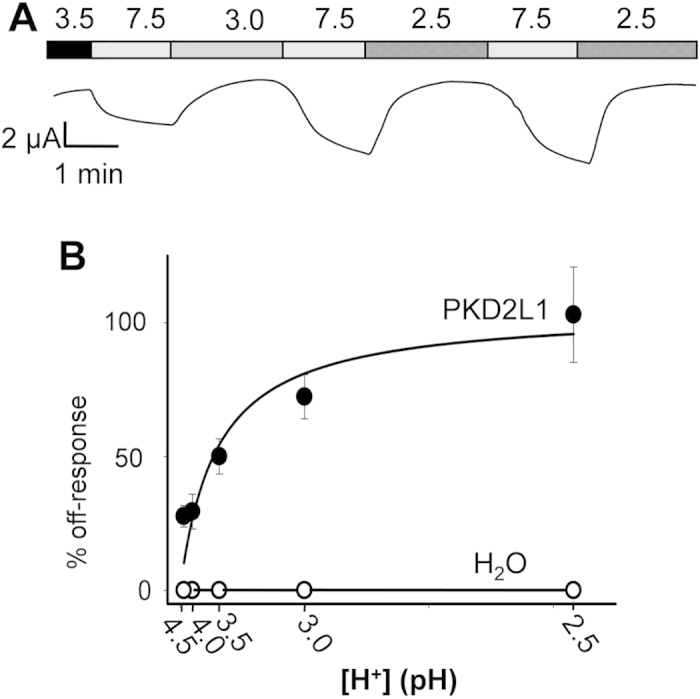
(A) representative current recorded at −50 mV and different extracellular pH, as indicated, from a PKD2L1-expressing oocyte. (B) pH dose dependence of the off-response currents for oocytes injected with PKD2L1 mRNA or water. [H+] indicates protons concentration. Data points represent average values from N = 5–9 with the standard error of the mean (SEM) and were fitted to the Michaelis Menton Equation, which produced a Km value of pH 3.5 ± 0.5.
In order to determine whether the off-response is sensitive to the duration of acid application, we applied a solution of pH 3.0 for different periods of time. We found that oocytes over-expressing PKD2L1 exhibit similar off-response amplitudes for different application durations, ranging from 10 sec to 1 min (Fig. 4A). Our data indicated that acid application time is rather not a determinant of the off-response amplitude, resembling the previously reported duration-independent characteristic of the PKD2L1/PKD1L3 channel complex15.
Figure 4. Effects of acid application time and type on the off-response.
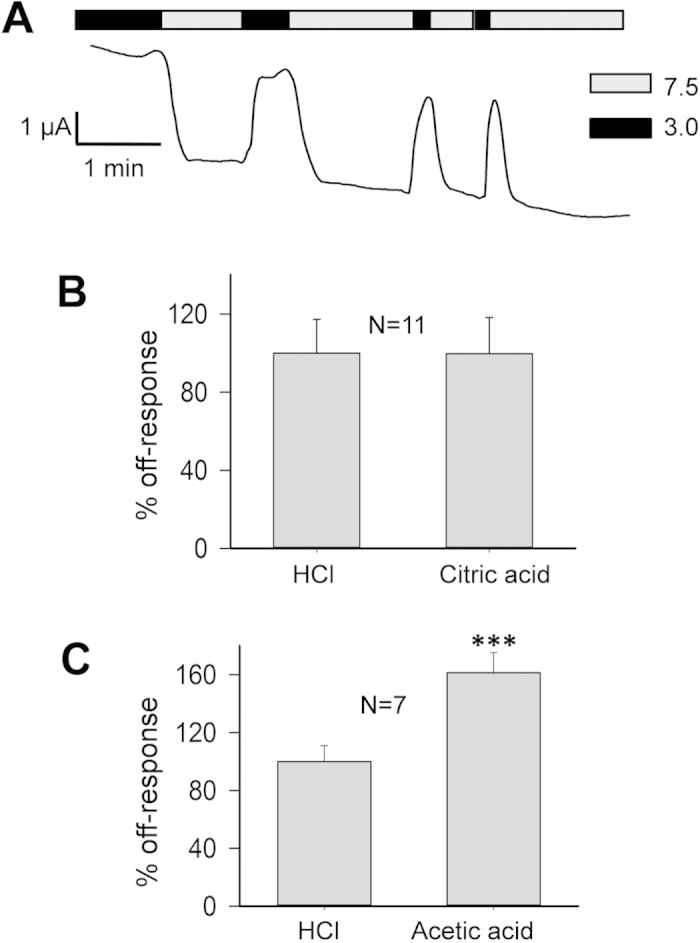
(A) Representative current recorded at −50 mV and with the application of the standard solution at pH 3.0 for different time intervals in an oocyte expressing PKD2L1. (B) Averaged and normalized off-response currents elicited by HCl or citric acid at pH 3.0 were compared from the same oocytes. (C) Averaged and normalized off-response currents elicited by HCl or acetic acid at pH 3.0 were compared from the same oocytes (***p = 0.0004, paired t-test).
Further, we investigated whether there is any difference between weak and strong acids with respect to the amplitude of off-response current. For this we compared the effects of acetic and citric acids with HCl, using 50 mM Na-acetate or Na-citrate to replace 50 mM NaCl in the standard solution. These solutions were adjusted to pH 3.0 by titration with HCl. Considering the pKa values of both acids, the resulting solutions should contain 49.1 and 12.9 mM of the undissociated membrane-permeable form of acetic and citric acids, respectively. Additionally, we found that the off-response current induced by acetic acid, but not citric acid, is significantly larger than that induced by HCl (60%, N = 7, p = 0.0004, paired t-test) (Fig. 4B,C). This pattern of increase is comparable to the response in the chorda tympani of rat TRCs by acetic, citric and hydrochloric acids at the same pH29.
Roles of extracellular protons in modulating PKD2L1 on-response
It was previously reported that application of extracellular Ca2+ induces PKD2L1 channel activation followed by an ensuing channel inactivation5. In contrast, an acid-induced off-response did not display an ensuing inactivation under Ca2+-free conditions (Fig. 5A). Thus, the same channel expressed in oocytes exhibits two apparently distinct properties: Ca2+-induced on-response with inactivation and acid-induced off-response (in the absence of Ca2+) without inactivation.
Figure 5. Acid-induced vs Ca2+-induced channel activation of PKD2L1.
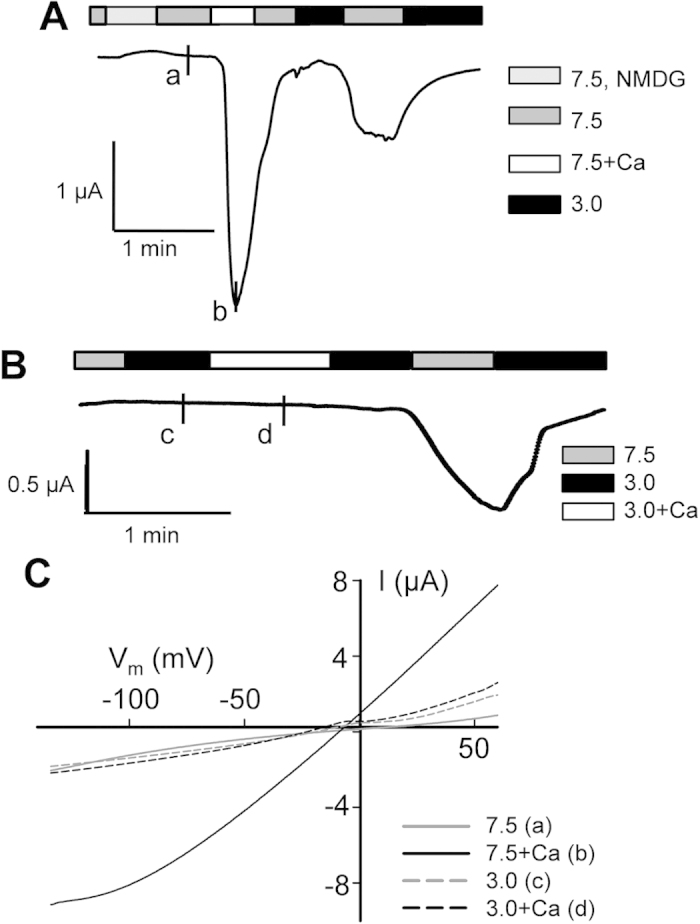
(A) Representative current recorded at −50 mV in a PKD2L1-expressing oocyte to show the on-response induced by 5 mM extracellular Ca2+ (at pH 7.5) and the subsequent off-response induced by extracellular pH 3.0 (no extracellular Ca2+). (B) Representative current recorded at −50 mV in a PKD2L1-expressing oocyte to show the on-response induced by 5 mM extracellular Ca2+ (at pH 3.0) and the subsequent off-response induced by extracellular pH 3.0 (no extracellular Ca2+). (C) I–V curves generated at the time points (‘a’–‘d’) indicated in panels A and B.
Extracellular low pH was previously found to significantly reduce Ca2+-induced PKD2L1 activation5. Consistently, here we found that the Ca2+-induced activation is completely abolished when extracellular pH drops to pH 3.0 (Fig. 5B). Similar conclusion can be made by comparing between the on-response current-voltage relationships obtained at pH 7.5 and 3.0 (Fig. 5C). Thus, the off-response activator, acid, played an inhibitory role in the on-response.
Roles of Ca2+ ion in modulating PKD2L1 off-response
Reversely, we wondered whether and how the on-response activator Ca2+ plays a role in the off-response. So far, we have performed all the off-response experiments in Ca2+-free extracellular environments, in part to avoid the Ca2+-induced on-response. Interestingly, in the presence of extracellular Ca2+ (5 mM), PKD2L1-expressing oocytes responded with a robust off-response current followed by channel inactivation, forming a spike of current (Fig. 6A) that has a similar shape as those previously published using HEK cells expressing PKD2L1/PKD1L3 in the presence of Ca2+ 15. Thus, compared with the presence of a sustained off-response current plateau in the absence of Ca2+ (see Figs 1A and 4A), our data together suggest that the off-response inactivation is attributed to Ca2+. Indeed, consistently, we found that sustained off-response currents were abolished by addition of Ca2+ (Fig. 6B). Thus, the on-response activator Ca2+ played an inhibitory role in the off-response.
Figure 6. Roles of Ca2+ ions in the PKD2L1 off-response.
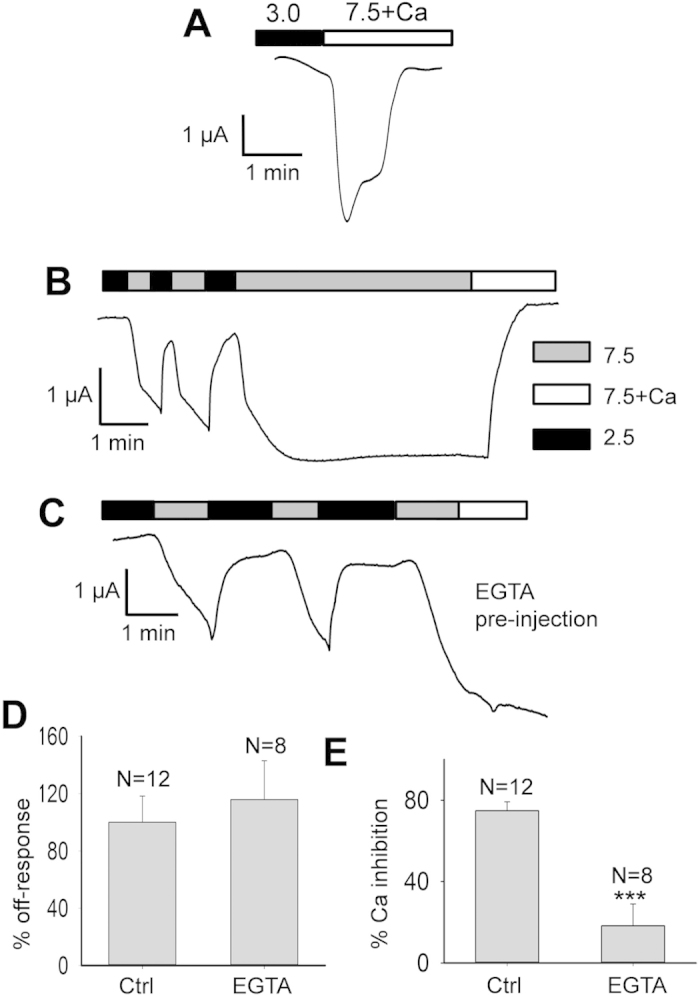
(A) Representative tracing recorded at −50 mV, showing the off-response activation and ensuing inactivation in the presence of extracellular Ca2+ (5 mM). (B) Representative current recorded at −50 mV in a PKD2L1-expressing oocyte, showing the inhibition of the off-response current by extracellular Ca2+ (5 mM). (C) Representative current recorded at −50 mV in a PKD2L1-expressing oocyte 1 hr after injection of 50 nL of 50 mM EGTA, showing the effect of extracellular Ca2+ (5 mM). (D) Averaged and normalized off-response currents with EGTA or water (Ctrl) pre-injection. (E) Averaged percentage of the off-response current inhibition by extracellular Ca2+ with EGTA or water pre-injection. ***p = 0.0007, unpaired t-test.
Because of the permeability of PKD2L1 to Ca2+ we wondered whether the Ca2+-induced inhibition/inactivation of the off-response is due to an increase in the intracellular Ca2+ concentration ([Ca2+]i). For this, we pre-injected Xenopus oocytes over-expressing PKD2L1 with 50 nL of 50 mM ethylene glycol tetra-acetic acid (EGTA) tetra -sodium salt solution 1 hr before the same electrophysiological measurements as those for Fig. 6B, to chelate the intracellular Ca2+. We found that while EGTA pre-injection does not prevent PKD2L1 from exhibiting the off-response, it substantially reduces the ability of extracellular Ca2+ to inhibit the off-response (Fig. 6C–E). In average, EGTA pre-injection resulted in similar off-response currents compared with the control condition (115.8 ± 27.1%, N = 8–12, p = 0.6), while it reduced the Ca2+ inhibition ability to 23.1 ± 12.0% (N = 8–12, p = 0.0007, unpaired t-test) (Fig. 6D,E). These results suggest that the increase in [Ca2+]i is critical for the extracellular Ca2+-induced inhibition, but not for the activation, of the off-response.
We also used EGTA to chelate extracellular Ca2+. We added 1 mM EGTA to our extracellular solutions to chelate any trace amounts of Ca2+ ion and, consistently, found a 36.7 ± 3.8% (N = 11, p = 0.04, unpaired t-test) increase in the off-response current (Fig. 7A,B). We went further to determine the Ki value of the extracellular Ca2+ concentration ([Ca2+]o) for the Ca2+ inhibition by applying different [Ca2+]o values, as shown in Fig. 7A. We found that 1 mM CaCl2 is sufficient to completely inhibit the off-response current and that Ki for [Ca2+]o is equal to 167 ± 33 μM (N = 11) for the off-response current generated from pH 3.0 to 7.5 (Fig. 7C). This Ki value was obtained by fitting experimental data to the equation
Figure 7. Role of extracellular Ca2+ ions in the PKD2L1 off-response.
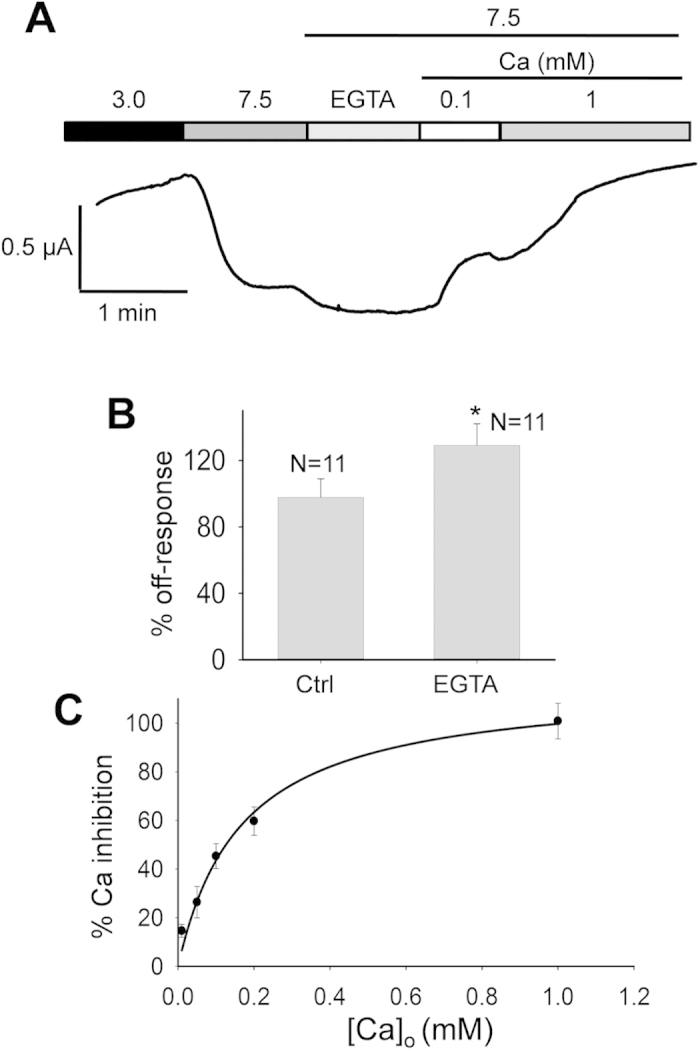
(A) Representative current recorded at −50 mV in a PKD2L1-expressing oocyte under various extracellular conditions, as indicated. (B) Based on experiments described in panel A, averaged data to show the effect of extracellular EGTA (1 mM). *p = 0.04, unpaired t-test. (C) Dose-dependence of the relative Ca2+ inhibition of the off-response current. Data were averaged from 11 oocytes and the curve is a fit of the data to Equation 1, with Ki value of 167 ± 33 μM.
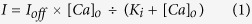 |
where Ioff represents the off-response current and I is the inhibited fraction of Ioff. Of note, we think that the presence of trace amounts of ‘contaminating’ Ca2+ in extracellular solutions (~5 μM, based on data sheets of MgCl2, NaCl and KCl) may account, at least in part, for the difference in the off-response current with and without extracellular EGTA (Fig. 7A).
Roles of acidic residues in PKD2L1 on- and off-responses
Previous reports found the importance of aspartic acid residue D523 in human PKD2L1 for the Ca2+ permeability13 as well as for the off-response of the PKD2L1/PKD1L3 complex16,26. We wanted to explore whether this mutation affects the Ca2+-induced on-response as well. We found that the D-to-N mutation inhibits the on- and off-response currents by 104.0 ± 1.6% (N = 20, p = 3.0e-6, unpaired t-test) and 77.2 ± 2.8% (N = 8, p = 0.002, unpaired t-test), respectively (Fig. 8A,B). We verified by Western blotting and immunofluorescence that the mutant is expressed on the PM to a similar extent as the wild type (WT) PKD2L1 (Fig. 8C,D). Thus, our studies found that residue D523 is essential for both on- and off-responses of PKD2L1.
Figure 8. Effects of mutation D523N on the PKD2L1 on- and off-responses.
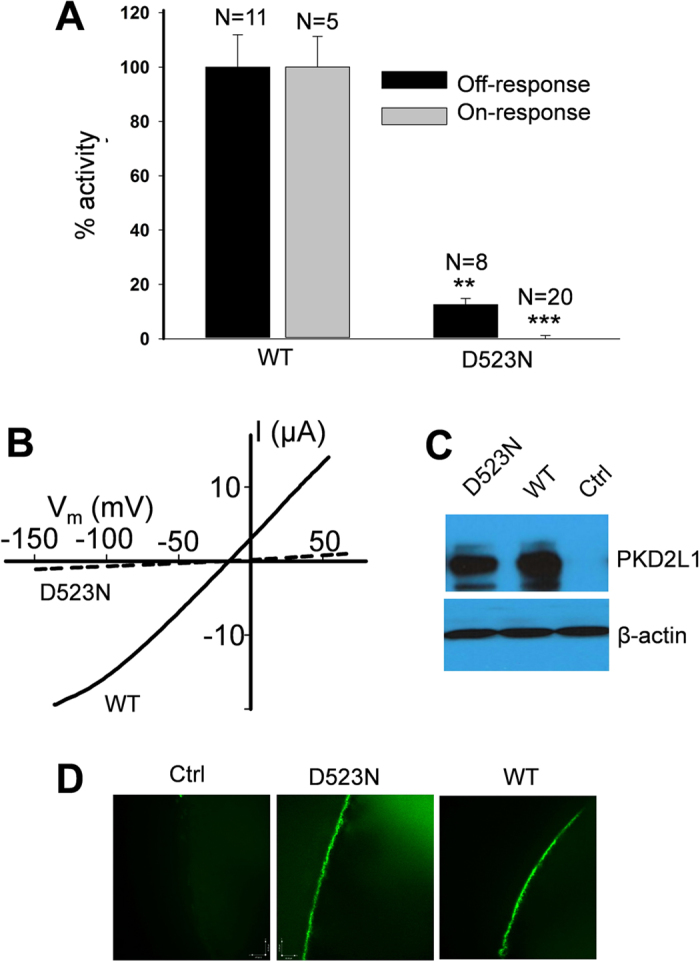
(A) Averaged and normalized on- and off-response currents elicited by extracellular 5 mM Ca2+ and pH 3.0, respectively, from oocytes expressing WT or mutant PKD2L1 voltage clamped at −50 mV. Shown are on- (***p = 3.0e-6, unpaired t-test) and off-response currents (**p = 0.002, unpaired t-test) averaged from different numbers of oocyte, as indicated. (B) Representative off-response I-V curves obtained by a ramp protocol under the same condition as in panel A. (C) Western blotting data showing the protein expression of WT and mutant PKD2L1 in expressing or water-injected (Ctrl) oocytes. (D) Representative immunofluorescence data showing the PM localization of WT and mutant PKD2L1 in oocytes.
We next examined the importance of intra-membrane acidic residues for PKD2L1 on- or off-response properties. For this we mutated eight such residues into their corresponding neutral residues: E103Q and D113N in TM1, D349N, E356N, E369Q and E370Q in TM3, D390N in TM4, and D476N in TM5, and then examined their expression and channel function in oocytes. We found that mutants E369Q, D390N and D476N have no detectable protein expression although their mRNA is normal (Fig. 9A), possibly rapidly degraded due to mis-folding. While the function of mutants E103Q, D113N and E370Q were similar to that of the WT channel, we found that the function of mutants D349N and E356N is significantly reduced (Fig. 9B). As our immunofluorescence data indicated that their PM levels are not affected (Fig. 9C), our electrophysiology data indicated that residues D349 and E356 in TM3 are critical for both the Ca2+-induced on-response and H+-induced off-response, possibly through interaction with Ca2+ or protons. Of note, the loss of function by point mutations D523N, D349N and E356N further supports the specificity of the off-response currents observed for WT PKD2L1 expressed alone in oocytes.
Figure 9. Effects of negatively charged intramembrane residues on the PKD2L1 on- and off-responses.
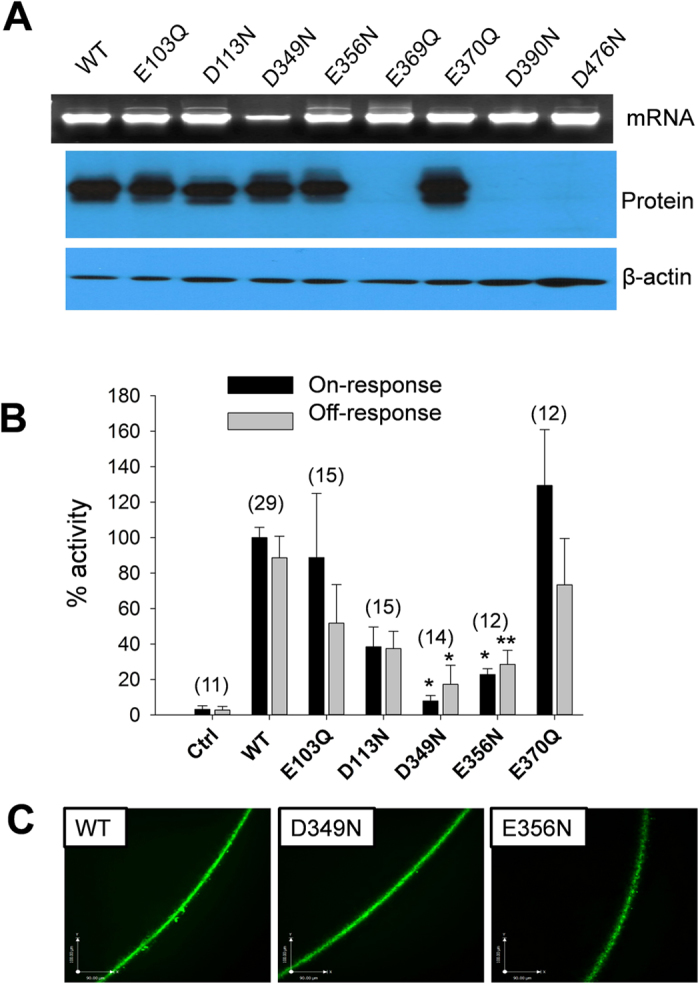
(A) Representative data showing mRNA and protein bands that were revealed in 1% agarose gel and 8% SDS-PAGE, respectively. (B) Averaged and normalized on- and off-response currents elicited by extracellular 5 mM Ca2+ and pH 3.0, respectively. Oocytes expressing WT, mutant or water-injected oocytes (Ctrl) were voltage clamped at −50 mV. The number of each group is indicated in a bracket. On-response currents were significantly reduced, with *p = 0.03 and 0.05 (unpaired t-test) for D349N and E356N, respectively. Off-response currents were also significantly reduced, with *p = 0.05 and **p = 0.01 (unpaired t-test) for D349N and E356N, respectively. (C) Representative immunofluorescence data showing the PM localization of PKD2L1 WT, D349N and E356N expressed in oocytes.
Discussion
In the present study we have examined acid-dependent properties of human PKD2L1 expressed in Xenopus oocytes using the two-microelectrode voltage clamp electrophysiology, together with molecular biology and mutagenesis techniques. We found that PKD2L1 over-expressed alone in oocytes exhibits acid-induced off-response characteristics, such as pH dependence, activation by strong and weak acids, and I-V relationships, that are comparable to those previously reported for the PKD2L1/PKD1L3 complex in HEK cells and oocytes. Given the non-detectable PKD1L3 in oocytes, this indicates that PKD1L3 is not required for acid sensation of PKD2L1. PKD2L1 off-response was inhibited and regulated by an increase in intracellular Ca2+. Moreover, the intra-membrane residues D349 and E356 were found to be essential for channel function.
Previous reports showed that PKD1L3 is essential for the off-response through increasing the targeting of PKD2L1 to the PM15,16,23,24. Nonetheless, the functional roles of PKD1L3 in the channel activity of the PM PKD2L1/PKD1L3 channel complex remain unclear. It was proposed that the PKD1L3 long extracellular N-terminus is involved in sensing extracellular pH15. While PKD2L1 knockout mice have defective sour tasting, PKD1L3 knockout mice do not exhibit tasting abnormality17,21. This fact appears to be controversial and argues that PKD1L3 does not play a significant role in the PKD2L1/PKD1L3 complex in terms of sensing acid in the tongue. Studies by Yu and colleagues16, nicely supported our finding that PKD2L1 alone in oocytes is expressed on the PM. However, injection of ~4 ng mRNA of PKD2L1 into each oocyte did not result in measurable PKD2L1-associated currents in our study as well as in a previous report16. This is likely due to an insufficient PM population of PKD2L1. Co-expression of PKD1L3 much increased the PM level of PKD2L1, allowing PKD2L1 to exhibit measurable off-response whole-cell currents16. In fact, this report showed that tetrameric PKD2L1 is present on the PM even with a small mRNA amount injected and in the absence of PKD1L3. In addition, the same report indicated that PKD1L3 alone does not target to the PM or exhibit channel activity16. Therefore, these data together suggest that PKD2L1, rather than PKD1L3, may possess an acid sensing capability and forms an off-response channel. However, we could not exclude the possibility that an endogenous partner protein participates in the acid sensing in cells expressing PKD2L1 alone or complex PKD2L1/PKD1L3. In addition to the chaperone role, it is nevertheless possible that PKD1L3 modulates PKD2L1 channel properties. It is noted that another PKD1 homologue PKD1L1 indeed modulates the single-channel conductance of PKD2L1 in mammalian cells, though PKD2L1 alone exhibits channel function on the PM13.
PKD2L1 was first reported in 1999 to be a non-selective cation channel activated by and highly permeable to Ca2+ in Xenopus oocytes5, a property that we call here a Ca2+-induced on-response. Its Ca2+ permeability in mammalian cilium membrane is important for regulating ciliary Ca2+ concentration and Ca2+-dependent hedgehog signalling. However, distinct channel properties were observed in the primary cilium, such as outward rectification and absence of Ca2+-induced channel activation13,14. Whether the Ca2+-induced on-response and acid-induced off-response share part of the molecular mechanisms remains unknown. However, some different properties between the on- and off-responses are noted. For example, after an on-response a waiting/recovery period of 5–8 minute was required for the induction of a new on-response5. In contrast, as shown here, the off-responses (at least under Ca2+-free conditions) can be repeatedly induced immediately one after another. Further, Ca2+-induced channel activation was followed by channel inactivation while acid-induced channel activation was sustained without inactivation in the absence of Ca2+. These data also suggest that the Ca2+-dependent on-response and acid-dependent off-response are not completely governed by the same mechanism.
In fact, acid-induced off-response activation in PKD2L1-expressing oocytes occurred in the absence of extracellular Ca2+ and reached a plateau, similar to reported data using oocytes16. In fact, in the presence of extracellular Ca2+, the off-response was still present but was followed by an ensuing inactivation, producing a current spike that is similar to the reported observations in HEK cells for PKD2L1/PKD1L3 under a comparable condition15. Furthermore, if Ca2+ was added during an off-response activation, then an immediate inhibition of the off-response current was observed, with a Ki value (50% inhibition) for [Ca2+]o of only 0.17 mM. These data together indicated that extracellular Ca2+ regulates (somehow inhibits) the off-response currents. In contrast, other previous reports using HEK cells expressing PKD2L1 and PKD1L3 stated that the acid-induced off-response requires the presence of extracellular Ca2+ and results in an increase in [Ca2+]i 25. The discrepancy in the role of extracellular Ca2+ will have to be clarified in further studies.
Interestingly, while preloading oocytes with Ca2+ chelator did not significantly alter the off-response, it abolished the ability of extracellular Ca2+ to inhibit the off-response. This suggests that an increase in the intracellular free Ca2+ is required for Ca2+ to inhibit the off-response activation. On the other hand, we previously reported that an increase in intracellular Ca2+ is required for both the Ca2+-induced on-response activation and the ensuing inactivation5. In terms of the effect of intracellular protons, our data and previous report29 using weak and strong acids support that acid-induced off-responses positively correlate with increased intracellular H+ concentrations. Putting all data together, although Ca2+ and acid distinctly induce PKD2L1 channel activations they seem to share the same or similar way of inactivation, i.e., by an increase in [Ca2+]i.
It has remained unclear as to how Ca2+ and proton ions trigger channel activations although Ca2+ plays roles in both phenomena. It is worthy to note that the PKD2L1 C-terminus possesses an EF hand, a coiled-coil domain, a cytoplasmic regulatory domain (CRD, aa 561–805) and phosphorylation sites30,31. PKD2L1 CRD was shown to be related to channel inactivation32, maybe through direct binding to Ca2+, or through activating downstream signalling molecules. More recently, CRD binding to Ca2+ was reported to change the α-helical property of the C-terminus30, which may explain the desensitization/inactivation phenomenon that follows the Ca2+-induced activation. However, whether the same domain is involved in the Ca2+ inhibition associated with acid-dependent off-response needs further studies. On the other hand, we found two negatively charged residues D349 and E356 to be critical for both on- and off-response functions. Because aspartate and glutamate residues have their pKa values of pH 3.7 and pH 4.0, respectively, which are well within the pH range in which PKD2L1 exhibits the off-response, our work suggests that protons may interact with these residues as part of the off-response activation process. Further, because of their importance for the Ca2+-induced on-response activation, these acidic residues may also interact with Ca2+ during the on-response process.
In summary, in the present study we discovered that PKD2L1 expressed alone in Xenopus oocytes exhibits extracellular acid-induced off-responses with characteristics resembling those associated with PKD2L1/PKD1L3 and that this off-response is inhibited by an increase in the intracellular Ca2+. Further studies will help better understanding how PKD1L3 and Ca2+ modulate the PKD2L1 channel function.
Materials and Methods
Preparation of PKD2L1 plasmid constructs
Human PKD2L1 gene was cloned as previously described6. Mutations of aspartic and glutamic acid residues to the neutral residues asparagine or glutamine were performed using QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies, La Jolla, CA, USA). Primer design and procedure were performed following the manufacturer’s protocol.
mRNA preparation and micro-injection into Xenopus laevis oocytes
Plasmids pCHGF harbouring WT or a mutant PKD2L1 cDNA were linearized with Mlu I, followed by phenol/chloroform purification and ethanol precipitation.
Linearized plasmids were used to in vitro synthesize capped mRNAs using the T7 mMessage mMachineTM kit (Ambion, Austin, TX, USA). Stage V-VI oocytes were isolated from Xenopus laevis under an approved institutional protocol. Defolliculation of oocytes was performed through incubation in Barth’s solution6 containing Type 2 collagenase (2 mg/mL) (Worthington, Lakewood, NJ, USA) at room temperature for 1.5 hours (hr). Oocytes were then incubated at 16–18 °C in Barth’s solution supplemented with 1% antibiotics (penicillin/streptomycin) (GIBCO™, Life Technologies, Burlington, ON, Canada) for at least 3 hr before injection of 50 nL H2O containing 25–50 ng mRNAs or as stated in the experiment. An equal volume of H2O was injected into control oocytes. Injected oocytes were incubated at 16–18 °C in Barth’s solution, for 2–4 days prior to experiments. For intracellular Ca2+ chelation studies, oocytes were injected with 50 nL of 50 mM EGTA (Sigma-Aldrich, Oakville, ON, Canada) to allow a final intracellular concentration of ~5 mM 1 hr prior to recordings.
Western blotting
Western blotting was performed as previously described6. Briefly, 10–20 μg of purified oocyte proteins were resolved on SDS PAGE (8%) gel, transferred to nitrocellulose membrane. Membrane was blocked with 3–5% skim milk in Tris Buffered Saline, 1% Tween 20 (TBST) for 1 hr at room temperature. This was followed by a 4 °C overnight incubation with rabbit anti-PKD2L1 polyclonal antibody (cat# PAB5914, Abnova, Taipei, Taiwan) diluted 1:1000 in the blocking buffer. Loading control β-actin was detected using a mouse monoclonal antibody (cat# sc-47778, Santa Cruz Biotechnology, Dallas, TX, USA). Secondary horseradish peroxidase (HRP) -coupled anti-mouse and anti-rabbit were purchased from GE Healthcare (Baie d’Urfe, QC, Canada).
Immunofluorescence
Xenopus oocytes were washed in PBS, fixed in 3% paraformaldehyde for 15 min, and washed 3 times in PBS plus 50 mM NH4Cl, and then permeabilized with 0.1% Triton X-100 for 4 min. Oocytes were then washed 3 times in PBS for 5 min each time, blocked in 3% skim milk in PBS for 30 min, and then incubated overnight with the PKD2L1 polyclonal antibody. This was followed by 3 times 10 min washes in PBS. The oocytes were then incubated with a secondary AlexaFluor 488-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 30 min, followed by 3 times washes in PBS and mounting in Vectashield (Vector labs, Burlington, ON, Canada). The slides were examined on an AIVI spinning disc confocal microscopy (Cell Imaging Facility, Faculty of Medicine and Dentistry, University of Alberta).
Solution preparation
The standard extracellular solution contained (in mM): 100 NaCl, 2 KCl, 1 MgCl2, 10 HEPES adjusted to pH 7.5 by TRIS base. Low pH (2.5–4.5) standard solutions were prepared from the standard solution adjusted by HCl. Solutions containing extracellular Ca2+ were prepared from the standard solution with the addition of CaCl2.
Electrophysiology
Current and voltage signals were measured with the conventional two-microelectrode voltage clamp technique with a commercial amplifier (TEV-200A, Dagan, Minneapolis, MN, USA). Electrodes were fabricated from borosilicate glass (Warner Instruments, Hamden, CT, USA) by a micropipette puller (P-87, Sutter Instruments, Novato, CA, USA) and filled with 3 M KCl with typical tip resistance of 0.5–3 MΩ. Digidata 1320A converter and pClamp 9.2 (Axon Instruments, Union City, CA, USA) were used for data acquisition and analysis. In experiments using a ramp or gapfree protocol5, current/voltage signals were digitized at 200 ms/sample. Recorded tracings were then analyzed using pClamp 9.2 and plotted using SigmaPlot 12 (Systat Software, San Jose, CA, USA). Absolute amplitudes of channel activity were collected and normalized to the average activity of WTPKD2L1 obtained under the same day, condition and group. Normalized values were then expressed as a percentage value and plotted using SigmaPlot 12.
Reverse Transcriptase –Polymerase Chain Reaction (RT-PCR)
Total RNAs were prepared from female Xenopus laevis oocytes, tongue, kidney, and brain tissues. Oocytes were collected and defolliculated as described above. Tissues were washed in PBS and kept on ice. RNAs were then extracted at room temperature with TRIzol reagent (Invitrogen Life Technologies, Burlington, ON, Canada). According to the manufacturer’s manual, 1 mL TRIzol was used to homegenize 50–100 mg of tissue. Chloroform was used to isolate the RNA followed by precipitation, wash and resuspension steps. One-step RT-PCR was carried out using SuperScript®III One-step RT-PCR kit (Invitrogen Life Technologies) by following the instruction manual. The oligonucleotide primers for Xenopus laevis β-actin and PKD1L3 for the first PCR were as follows: β-actin, sense GAGATGAAGCTCAAAGCAAAAG and antisense CAGGATTCCATACCAAGGAAAG; PKD1L3 sense GCAGATTGTGAGGAAGAAAGG and antisense TGCTGAGAGCTGGTAGGGTAGT. 10X diluted first PKD1L3 PCR products were used as templates to run the second (nested) PCR using inner primers to increase PCR specificity and efficiency. Sequences of the PKD1L3 inner primers were: sense AGATTGTGAGGAAGAAAGGGGG and antisense TTTGCTAAAGTCTGGTGGGTTG.
Statistical Analysis
Data were presented as mean ± (SEM). All data were collected from at least three different experiments. Student’s t-test was used to compare two groups of data for statistical significance indicated by a p value. P values of less than 0.05 and 0.01 were considered significant and very significant, respectively.
Additional Information
How to cite this article: Hussein, S. et al. Acid-induced off-response of PKD2L1 channel in Xenopus oocytes and its regulation by Ca2+. Sci. Rep. 5, 15752; doi: 10.1038/srep15752 (2015).
Acknowledgments
Mouse HA-tagged PKD1L3 plasmid was a generous gift of J. Yang (Columbia University) and H. Matsunami (Duke University). Funding: This work was supported by the Canadian Institutes of Health Research (to X.-Z.C.; C.D.; J.Y. and J.T.), the Natural Sciences and Engineering Research Council of Canada, and the Kidney Foundation of Canada (to X.-Z.C.). S.H. and W.Z. were supported by Alberta Innovates Technology Futures (AITF) Graduate Scholarship. Q.W. was supported by the Alberta Innovates Health Solutions (AIHS) Graduate Scholarship. F.Z. and Y.C. were supported by Nature Science Foundation of China.
Footnotes
Author Contributions S.H. designed and performed the major parts of the experiments, analyzed the data and wrote the first version of the manuscript and revisions. W.Z. performed RT-PCR experiments, analyzed the data and revised the manuscript. C.D. performed some of the electrophysiology experiments. Q.W. performed parts of the experiments and analyzed data. J.Y. performed parts of the experiments and analyzed the data. F.Z. analyzed part of the data and participated in paper revision. J.T. participated in experiments and paper revision. Y.C. participated in design of experiments and paper revision. X.-Z.C. conceived or designed the experiments, helped analyzing the data and revised the manuscript. All authors gave final approval for publication.
References
- Naziroglu M., Dikici D. M. & Dursun S. Role of oxidative stress and Ca(2)(+) signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem. Res. 37, 2065–2075 (2012). [DOI] [PubMed] [Google Scholar]
- Venkatachalam K. & Montell C. TRP channels. Annu. Rev. Biochem. 76, 387–417 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H. et al. Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J. Biol. Chem. 273, 25967–25973 (1998). [DOI] [PubMed] [Google Scholar]
- Pei Y. Molecular genetics of autosomal dominant polycystic kidney disease. Clin. Invest Med. 26, 252–258 (2003). [PubMed] [Google Scholar]
- Chen X. Z. et al. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401, 383–386 (1999). [DOI] [PubMed] [Google Scholar]
- Yang J. et al. Receptor for activated C kinase 1 (RACK1) inhibits function of transient receptor potential (TRP)-type channel Pkd2L1 through physical interaction. J. Biol. Chem. 287, 6551–6561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Modulation of the human polycystin-L channel by voltage and divalent cations. FEBS Lett. 525, 71–76 (2002). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Troponin I binds polycystin-L and inhibits its calcium-induced channel activation. Biochemistry 42, 7618–7625 (2003). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Direct binding of alpha-actinin enhances TRPP3 channel activity. J. Neurochem. 103, 2391–2400 (2007). [DOI] [PubMed] [Google Scholar]
- Dai X. Q., Karpinski E. & Chen X. Z. Permeation and inhibition of polycystin-L channel by monovalent organic cations. Biochim. Biophys. Acta 1758, 197–205 (2006). [DOI] [PubMed] [Google Scholar]
- Dai X. Q. et al. Inhibition of TRPP3 channel by amiloride and analogs. Mol. Pharmacol. 72, 1576–1585 (2007). [DOI] [PubMed] [Google Scholar]
- Shimizu T., Janssens A., Voets T. & Nilius B. Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch. 457, 795–807 (2009). [DOI] [PubMed] [Google Scholar]
- DeCaen P. G., Delling M., Vien T. N. & Clapham D. E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M., DeCaen P. G., Doerner J. F., Febvay S. & Clapham D. E. Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H. et al. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 9, 690–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. et al. Molecular mechanism of the assembly of an acid-sensing receptor ion channel complex. Nat. Commun. 3, 1252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio N. et al. Sour taste responses in mice lacking PKD channels. PLoS. One. 6, e20007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y. et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 103, 12569–12574 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S. et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses 33, 243–254 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L. et al. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. M. et al. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem. Senses 35, 565–577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Higuchi T., Fujii T., Nilius B. & Sakai H. Bimodal effect of alkalization on the polycystin transient receptor potential channel, PKD2L1. Pflugers Arch. 461, 507–513 (2011). [DOI] [PubMed] [Google Scholar]
- Ishimaru Y. et al. Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J. 24, 4058–4067 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S. et al. Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem. Biophys. Res. Commun. 385, 346–350 (2009). [DOI] [PubMed] [Google Scholar]
- Kawaguchi H. et al. Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J. Biol. Chem. 285, 17277–17281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto C. et al. The single pore residue Asp523 in PKD2L1 determines Ca2+ permeation of the PKD1L3/PKD2L1 complex. Biochem. Biophys. Res. Commun. 404, 946–951 (2011). [DOI] [PubMed] [Google Scholar]
- Lopezjimenez N. D. et al. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 98, 68–77 (2006). [DOI] [PubMed] [Google Scholar]
- Ishii S. et al. The response of PKD1L3/PKD2L1 to acid stimuli is inhibited by capsaicin and its pungent analogs. FEBS J. 279, 1857–1870 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V. et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am. J. Physiol Cell Physiol 281, C1005–C1013 (2001). [DOI] [PubMed] [Google Scholar]
- Molland K. L., Paul L. N. & Yernool D. A. Crystal structure and characterization of coiled-coil domain of the transient receptor potential channel PKD2L1. Biochim. Biophys. Acta 1824, 413–421 (2012). [DOI] [PubMed] [Google Scholar]
- Murakami M. et al. Genomic organization and functional analysis of murine PKD2L1. J. Biol. Chem. 280, 5626–5635 (2005). [DOI] [PubMed] [Google Scholar]
- Li Q., Liu Y., Zhao W. & Chen X. Z. The calcium-binding EF-hand in polycystin-L is not a domain for channel activation and ensuing inactivation. FEBS Lett. 516, 270–278 (2002). [DOI] [PubMed] [Google Scholar]


