Abstract
Background and aims:
Methotrexate (MTX) is sometimes used as part of combination therapy for the treatment of inflammatory bowel disease [IBD]; however, the optimal MTX dose for combination therapy has not been established. This study compared the efficacy of lower-dose and higher-dose MTX with anti tumor necrosis factor alpha (anti-TNF) therapy among IBD patients.
Methods:
Retrospective chart review was performed of 88 IBD patients at our center between 2010 and 2013. Low-dose MTX was defined as ≤ 12.5mg/week and high-dose MTX as 15–25mg/week. Patients who met the criteria for clinical remission [Harvey-Bradshaw Index ≤ 4, Simple Clinical Colitis Activity Index ≤ 2] at baseline were followed for up to 42 months. Chart review occurred in 6-month intervals. The primary outcome was consecutive months in remission prior to relapse. Secondary outcomes included other indicators of worsening disease [endoscopic inflammation, steroid use, therapy escalation/addition, or surgery] and adverse events. Regression analysis and Kaplan–Meier survival analysis were completed.
Results:
We identified 73 [83%] dual-therapy patients, of whom 32 low-dose and 14 high-dose individuals achieved remission. When compared with high-dose patients, low-dose patients were more likely to relapse [log-rank test, p < 0.01]. Secondary indicators of worsening disease occurred during 34.4% of low-dose review periods and 31.4% of high-dose review periods [p = 0.67]; 3/52 [6%] low-dose patients and 3/21 [14%] high-dose patients [p = 0.34] discontinued MTX therapy due to adverse events.
Conclusions:
When combined with anti-TNF therapy, MTX at doses of >12.5mg/week was more effective at maintaining clinical remission than lower doses. These findings will guide management of combination therapy in IBD patients.
Keywords: Methotrexate, anti-TNF therapy, combination therapy, maintenance of remission, inflammatory bowel disease, infliximab, adalimumab, certolizumab-pegol
1. Introduction
Sixty years after it initially gained Food and Drug Administration (FDA) approval for the treatment of leukemia, methotrexate (MTX) is now one of the primary immunomodulator therapeutic agents used as ‘off-label’ therapy for the induction and maintenance of IBD treatment.1 Although it has become more commonly used in current clinical practice, its utility in IBD was not made apparent until it was used to treat leukemia in patients with comorbid autoimmune disease. These patients demonstrated parallel improvements in both hematological malignancy and autoimmune activity, thus providing initial evidence for the immunomodulatory capabilities of MTX.2 In rheumatoid arthritis, MTX has become one of the main treatments for achieving remission, both as monotherapy and, importantly, as combination therapy with anti tumor necrosis factor alpha (anti-TNF) agents.34,5,6
For the past two decades MTX has been increasingly used for the treatment of IBD, particularly among patients with Crohn’s disease (CD). Clinical trials have demonstrated efficacy of MTX monotherapy for the induction [25mg intramuscularly weekly] and maintenance [15mg intramuscularly weekly] of CD treatment. In addition, smaller cohort studies have suggested potential therapeutic efficacy among ulcerative colitis (UC) patients, and randomized controlled clinical trials [RCTs] that assess the role of MTX in management of UC are ongoing but have not yet yielded results [eg MERIT-UC NCT01393405 and METEOR NCT00498589].7,8,9,10,11
In addition to MTX’s primary efficacy in CD and possibly UC, there has been increasing interest in its utility as a combination therapy with anti-TNF biologic treatments. A recent study within the IBD patient population has demonstrated that concurrent MTX use prevents formation of antibodies in CD patients.12 Whereas the complementary nature of the methotrexate and biologic medication is clear, the precise efficacy and optimal dosing of dual therapy in IBD has yet to be established.456 In addition, it is unknown whether oral or parenteral administration of MTX as combination therapy is more effective. In contrast, among patients with rheumatoid arthritis, lower doses of oral MTX are recommended at induction and are escalated when required based on response and tolerability.13
The aims of this study were to assess and compare the duration of remission and tolerability between different dosing regimens of MTX in IBD patients who were concomitantly prescribed anti-TNF therapy.
2. Materials and Methods
2.1. Patient selection and ethical statement
A retrospective chart review of the practice of a single physician [DTR] identified a total of 88 patients who were administered MTX for the primary treatment of IBD and who had at least one follow-up visit between October 2010 and October 2013. Inclusion criteria included: age of 16 years or older and a diagnosis of CD, UC or indeterminate colitis [IC] with active disease (Harvey-Bradshaw Index [HBI] > 4, Simple Clinical Colitis Activity Index [SCCAI] > 2 or by endoscopic report). Patients were excluded if they had been administered MTX for another primary diagnosis. The study was approved by our institutional review board.
2.2. Data collection and outcome measures
Retrospective chart review was conducted and reviewed electronic clinic notes, laboratory data [including anti-TNF drug levels and antibodies], and histologic appearance. We defined low-dose MTX [LD-MTX] as ≤ 12.5mg/week and high-dose MTX [HD-MTX] as 15-25mg/week. Collected information included general demographic information, smoking behavior, disease diagnosis and phenotype [Montreal classification], use of other therapy to target IBD, disease activity per 6-month period, remission status, and history of surgery prior to or during MTX treatment or hospitalizations related to IBD. Clinical remission was defined as a HBI ≤ 4, SCCAI ≤ 2.
The primary outcome of duration of remission maintenance was defined as consecutive months in clinical remission until clinical relapse. Secondary outcomes included indicators of worsening disease within a 6-month period [indicated by endoscopic inflammation, corticosteroid use, therapy escalation, addition or escalation of concomitant therapy, or surgery] and occurrence of adverse events.
2.3. Statistical considerations
The primary outcome of consecutive months in clinical remission until relapse was assessed by Kaplan–Meier survival analysis. A log-rank test was used to assess the difference between the two groups. Objective measures of disease-worsening were registered and dummy-coded [with a 1 for worsening and 0 for stable disease]. The total number of disease-worsening events were calculated per regimen category and compared by chi-square analysis. Logistic regression was used to assess for factors that could predict LD versus HD regimens. Adverse events were assessed based on whether dose change such as de-escalation or withdrawal was necessary, and descriptives were calculated by chi-square analyses and Fisher exact test where indicated. Predictors for adverse events were assessed by regression analysis.
All statistical analysis were based on a 2-sided tail model with p < 0.05. Statistical analysis was performed with IBM SPSS Statistics version 20.0 [Armonk, NY: IBM] and Graphpad Prism version 5.00 [GraphPad Software, San Diego, CA].
3. Results
3.1. Baseline demographics and overall response rates
Of the 88 patients with IBD who received MTX, 73 received this as combination with anti-TNF therapy. Of the 73 patients, 69% [n = 52] were males, 71% [n = 51] were prescribed LD-MTX, and oral administration was designated in 75% [n = 55] of patients. Baseline patient characteristics were comparable between patients achieving remission [responders] and patients not achieving clinical remission during the induction phase [non-responders] [Table 1].
Table 1.
Patient characteristics.
| Characteristics | Total [n=73]a | Responderd [n=46]a | Non-responder [n=27]a | p Valuec |
|---|---|---|---|---|
| Age at MTX initiation [years]d | 30 [14-67] | 28 [14-65] | 35 [15-52] | 0.57 |
| Median duration of disease at MTX initiation [years]d | 5 [0-43] | 5 [0-32] | 5 [2-43] | 0.93 |
| Median duration of MTX use [months]d | 17 [0-112] | 17 [2-112] | 6 [0-18] | 0.91 |
| Male sex | 50 [69%] | 34 [74%] | 16 [59%] | 0.30 |
| Smokers, former smokers | 7 [10%], 9 [12%] | 6 [13%], 5 [11%] | 1 [46%], 4 [15%] | 0.25; 0.72 |
| Crohn’s disease | 54 [74%] | 35 [76%] | 19 [70%] | 0.79 |
| Age at diagnosis [years] [A1, A2, A3]e | 18 [33%], 31 [57%] 5 [9%] | 12 [34%], 20 [57%], 3 [9%] | 6 [32%], 11 [58%], 2 [11%] | 0.78; 0.82; 1.00 |
| Location [L1, L2, L3]f | 10 [18%], 8 [15%], 36 [67%] | 7 [20%], 3 [9%], 25 [71%] | 3 [16%], 5 [26%], 11 [58%] | 0.74; 0.14; 0.38 |
| Addition of L4f | 11 [20%] | 7 [20%] | 4 [21%] | 1.00 |
| Crohn’s disease | ||||
| Behavior [B1, B2, B3]g | 18 [33%], 15 [28%] 21 [39%] | 12 [34%], 9 [36%] 14 [40%] | 6 [32%], 6 [32%] 7 [37%] | 0.93; 0.79; 0.89 |
| Perianal disease | 19 [35%] | 13 [37%] | 6 [32%] | 0.77 |
| Ulcerative colitis | 16 [22%] | 8 [17%] | 8 [30%] | 0.36 |
| Extent | ||||
| - Left-sided | 6 [38%] | 3 [38%] | 3 [38%] | 0.66 |
| - Extensive | 10 [62%] | 5 [63%] | 5 [63%] | 0.57 |
| Indeterminate colitis | 3 [4%] | 3 [7%] | 0 | 0.29 |
| Low-dose MTXh | 51 [71%] | 32 [70%] | 19 [70%] | 0.94 |
| Oral MTX route | 55 [75%] | 35 [76%] | 20 [74%] | 0.85 |
| Concomitant anti-TNF agent | ||||
| - Adalimumab | 36 [49%] | 24 [52%] | 12 [44%] | 0.56 |
| - Infliximab | 29 [40%] | 20 [43%] | 9 [33%] | 0.54 |
| - Certolizumab pegol | 8 [11%] | 2 [4%] | 6 [22%] | 0.05 |
MTX, methotrexate; anti-TNF, anti tumor necrosis factor.
a% relates to the total n per column.
bResponder, achieved remission after induction of MTX therapy.
cLow dose equals ≤ 12.5 mg.
dAge and duration are expressed as median [range].
eA1 <16 years; A2 17-49 years; A3 >40 years.
fL1 ileal; L2 colonic; L3 ileocolonic; L4 upper GI.
gB1 non-stricturing, non-penetrating; B2 stricturing; B3 penetrating.
hCalculated for responders versus non-responders.
Of the 73 patients on combination therapy, 62% [n = 46] achieved clinical remission. The remaining 37% [n = 27] did not achieve remission during induction phase and were subsequently excluded from our analysis [Supplementary Figure 1, available as Supplementary data at JCC online].
3.2. Adverse events
Of the 73 patients in our cohort who were prescribed MTX combination therapy, 18% [13] reported an adverse event during follow-up. Of the total cohort of patients who achieved remission, this particular patient cohort comprised 13%. Adverse events were more frequently reported in HD-MTX patients (33% [n = 7]) than LD-MTX patients (12% [n = 6]), but this difference was not statistically significant [p = 0.13]. In these 13 patients there were 17 adverse events. Nausea and/or vomiting was the most common [n = 6] followed by abnormal liver chemistry (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] defined as more than twice the upper limit of normal) [n = 4]. Other adverse events included fatigue, low-grade fever, headache, general malaise, rash, and joint pain as illustrated by [ Table 2 ].
Table 2.
Number of adverse events of MTX per treatment modality in 13 patients.
| Event type | Low [≤12.5mg] | High [15-25mg] | Total: | ||
|---|---|---|---|---|---|
| Oral | Injection | Oral | Injection | ||
| Increased ALT/AST > x 2 | 1 | 1 | 2 | 4 | |
| Nausea ± vomiting | 3 | 1 | 2 | 6 | |
| Fatigue | 1 | 1 | |||
| Low-grade fever | 1 | 1 | |||
| Headache | 1 | 1 | 2 | ||
| General malaise | 1 | 1 | |||
| Rash | 1 | 1 | |||
| Joint pain | 1 | 1 | |||
| Total events: | 6 | 1 | 4 | 6 | 17 |
MTX, methotrexate; ALT, alanine aminotransferase; AST; aspartate aminotransferase.
Eight patients elected to change therapy due to adverse events. Two of these patients de-escalated MTX dose and the remaining 6 discontinued MTX therapy completely. Discontinuation occurred in 6% [n = 3] of LD-MTX patients and 14% [n = 3] HD-MTX patients [p = 0.34]. Discontinuation due to adverse events was similar for both the responder [7%, n = 3/46] and non-responder groups, with 7% and 11% of patients terminating therapy, respectively [p = 0.66]. Table 3 illustrates the details of adverse events between HD-MTX and LD-MTX patients.
Table 3.
Adverse events compared between low- and high-dose regimens
| LD-MTX [n=52] | HD-MTX [n=21] | |
|---|---|---|
| Adverse events* | 6 [12%] | 7 [33%] |
| - Adalimumab | 5 | 5 |
| - Infliximab | 1 | 1 |
| - CTZ | 0 | 1 |
| Change due to events** | 4 [2%] | 4 [19%] |
| - Discontinued | 3 | 3 |
| - De-escalated | 1 | 1 |
| No change | 2 | 3 |
LD-MTX, low-dose methotrexate; HD-MTX, high-dose methotrexate; CTZ, certolizumab-pegol.
*p = 0.13;** p = 0.22.
3.3. Patients in remission
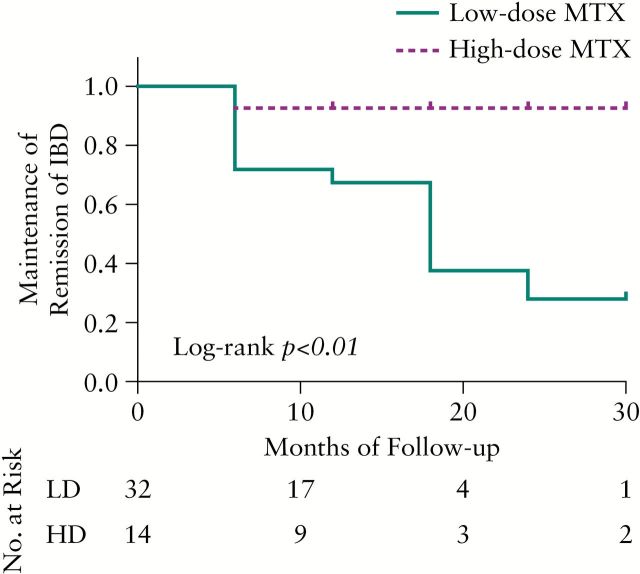
Of the patients on combination therapy, 62% [n = 46] achieved clinical remission. In 70% [n = 32] of patients, concurrent biologic therapy was started at the same time at MTX induction. MTX was added after the initiation of anti-TNF therapy in 26% [n = 12] of patients. Finally, anti-TNF therapy was started after the induction of MTX in the remaining 4% of patients [n = 2]. There was no difference in MTX induction time between LD-MTX and HD-MTX dosing regimens (75% LD-MTX versus 70% LD-MTX [p = 0.73], respectively). Of these patients, 70% were prescribed LD-MTX therapy. Patients receiving HD-MTX [n = 14; 30%] faired significantly better in the maintenance of clinical remission compared with LD-MTX [n = 32; 70%] patients [log-rank test p < 0.01]. The Kaplan–Meier survival analysis of duration of remission maintenance is plotted in Figure 1. We found no differences in these observations between combination therapy with adalimumab or with infliximab [log rank test p = 0.58]. The sample of patients that achieved and maintained remission on certolizumab pegol was too small to analyze [n = 2].
Figure 1.
Kaplan–Meier curve of maintenance of remission of low-dose [[LD] vs high-dose [HD] methotrexate [MTX] in combination with anti-tumor necrosis factor-alpha [TNF] therapy. [LD-MTX = ≤ 12.5mg/week; HD-MTX = 15–25mg/week].
Patients who achieved remission on methotrexate and concomitant anti-TNF agents were observed for a total of 198 6-month review periods. During this interval, an increase in incidents of worsening disease [as indicated by endoscopic inflammation, corticosteroid use, therapy escalation, addition or escalation of concomitant therapy, or surgery] occurred in 34.4% of LD-MTX periods and 31.4% of HD-MTX periods (crude odds ratio [OR] 1.14; 95% confidence interval [CI] 0.61, 2.13, p = 0.67). This remained the same when stratified for 6-month periods with the Mantel–Haenszel method. Patients with CD were more likely to receive HD-MTX with anti-TNF than patients with UC. No other patient demographics or disease characteristics predicted prescribed dosage [LD- versus HD-MTX][Table 4 ].
Table 4.
Logistic regression analysis of factors associated with use of high dose MTX.
| Factors | Odds ratio [CI] | p-Value |
|---|---|---|
| Age | 1.00 [0.97 to 1.04] | 0.87 |
| Female | 2.22 [0.75 to 6.62] | 0.15 |
| Smoking | 0.60 [0.08 to 4.71] | 0.63 |
| CD [vs UC] | 4.46 [1.02 to 19.4] | <0.05 |
| Prior surgery | 1.24 [0.47 to 3.28] | 0.66 |
| IBD duration | 1.00 [1.00 to 1.00] | 0.90 |
| CD | ||
| Location | ||
| Colonic | 1.78 [0.28 to 11.35] | 0.55 |
| Ileocolonic | 2.82 [0.58 to 13.84] | 0.20 |
| Upper GI involvement | 2.83 [0.44 to 18.18] | 0.10 |
| Behavior | ||
| Stenotic | 0.59 [0.15 to 2.24] | 0.43 |
| Penetrating | 0.35 [0.10 to 1.24] | 0.10 |
| Perianal disease | 1.01 [0.38 to 2.68] | 0.98 |
| UC | ||
| Location | ||
| Pancolitis | 4 * 10^8 [0.00 to 0.00] | 1.00 |
| Severity | ||
| Moderate | 0 [0 to 0] | 1.00 |
| Severe | 3 [0.08 to 107.45] | 0.55 |
[CI], confidence interval; CD, Crohn’s disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; GI, gastrointestinal.
Predictors of high dose MTX. Cox and Snell R2 p = 0.09; Nagelkerke R2 = 0.13.
3.3.1. Mode of administration
Of the patients who achieved remission and entered the maintenance phase, 76% [n = 35] were prescribed oral MTX in addition to their anti-TNF agent, and 24%[n = 11] were prescribed parenteral MTX. Whereas 37%[n = 13] of oral patients relapsed during the maintenance phase, 27% [n = 3] of parenteral patients relapsed during this time period. Despite this variation, this difference was not significant when plotted by Kaplan–Meier analysis [log-rank test p = 0.56] [ Supplementary Figure 2, available as Supplementary data at JCC online ].
3.3.2. Maintenance by diagnosis
Overall, 34% [12/35] of patients with CD relapsed during maintenance therapy compared with 25% [2/8] of patients with UC. When these disease entities were compared against each other, there was no difference seen in maintenance of remission [log rank test p = 0.78] [ Supplementary Figure 3 ]. Kaplan–Meier analysis was completed again after excluding patients with UC, to compare lower versus higher doses among patients with CD only. Among these patients, maintenance of remission curves for HD-MTX was significantly superior to that for LD-MTX [log rank test p < 0.01] [ Supplementary Figure 4A, available as Supplementary data at JCC online ]. The difference between oral versus parenteral administration did not reach statistical significance [log rank test p = 0.29] [ Supplementary Figure 4B, available as Supplementary data at JCC online ].
4. Discussion
To our knowledge, this is the largest study of clinical outcomes using concomitant MTX with anti-TNF therapy in a real-world IBD practice to date. We found that MTX doses of 15–25mg/week in combination with anti-TNF biologic therapy are more likely to maintain clinical remission than MTX of ≤ 12.5mg/week in combination therapy. However, dose of MTX was not associated with secondary measures of disease control, including anti-TNF dose change, corticosteroid use, endoscopic inflammation, or surgery.
Randomized controlled trials of LD-MTX monotherapy in both CD and UC previously have failed to demonstrate any greater benefit in prevention of flares compared with HD-MTX.14,15 A recently published combination therapy study of infliximab and HD-MTX administered subcutaneously [up to 25mg /week] versus infliximab plus placebo did not report improved treatment efficacy. However, this study was limited in that both groups received corticosteroids during infliximab infusions.12 However, patients in the combination therapy group had a lower rate of immunogenicity and higher trough levels. Notably, endoscopic activity was not assessed in this study.
Additional studies that assessed combination therapy in IBD and included MTX as one of the agents are sparse, and are limited to assessment with infliximab only. At this time, there have only been two studies that compared azacitidine [AZA] to MTX [on 15-mg/maintenance dose] while these medications were used as part of combination therapy. Sokol and colleagues found that combination therapy of AZA, but not MTX, was associated with decreased occurrence of abdominal surgery or switch to adalimumab.16 Vermeire et al. found that MTX and AZA prevented antibodies in combination therapy equally well. Clinical outcome was not assessed.17
In our cohort, the rate of side effects was higher among patients receiving HD-MTX. The side effect profile of MTX is well known to be dose-related, so this result is not surprising, but may have influenced the clinical interpretation of efficacy as well. Despite this, there was no difference between the two groups in the need for a regimen change due to adverse events. Of note, adverse events were only documented when they occurred while a patient was concurrently prescribed folic acid and ondansetron in addition to MTX. Previous clinical trials have demonstrated high withdrawal rates [up to 17%] due to adverse events among MTX-prescribed IBD patients.7 Among patients receiving 15–25mg, the withdrawal rate due to adverse events [14%] in our MTX cohort was comparable with this finding. The adverse event rate within this cohort was 18% overall, and up to 33% in the HD regimen group. A meta-analysis by Valentino et al. found that all [paediatric] studies that reported hepatotoxicity during MTX administration had a pooled rate of 10.2% for hepatotoxicity prevalence, requiring dose-reduction in 6.4% and discontinuation in 4.5%.18 In our cohort, 5% [n = 4] of patients were found to have increased transaminases of more than twice the upper limit. Of these patients, two [1 LD and 1 HD] had to discontinue MTX and one [1 HD] underwent change of dosing.
This study has several methodological limitations. It was a retrospective analysis that was completed in a tertiary center among patients who have relatively complex IBD. Therefore, patients who failed previous anti-TNF therapy and had prior surgeries were included and comprised a greater percentage of patients than might exist within a community sample. In addition, as many of the measures included in this study are subjective, outcomes may have been influenced by the report bias of the physician or patients. It is also possible that the dosing regimens utilized by the patients included within the study may not be indicative of dosing trends within the population at large. Whereas we believe that the dosing trends within this institution reflect standard-of-care clinical practice, it is possible that they may reflect a biased patient sample population that reflects the disease severity of the patient cohort. We argue that the trend for lower MTX dose does not reflect a less severe patient cohort and instead is indicative of merging clinical practice in the field. To the same extent, one might argue that a subset of these patients may have had less severe disease if the prescribing physician initially started the patient on MTX monotherapy and added concomitant anti-TNF therapy at a later point in treatment. This does not appear to be the case—when examining at our primary outcome group [patients who continued therapy during the maintenance phase], only 2 patients received anti-TNF therapy after induction with MTX therapy. These included 1 patient on LD-MTX and 1 on HD-MTX. As such, we may conclude that disease-severity at inclusion did not plausibly influence our primary outcome measures.
Another limitation to this analysis is the lack of available therapeutic drug levels in all patients, which could have provided additional objective information about disease control. However, additional information about therapeutic drug levels would not have affected our primary outcome, as clinical remission is defined by the clinical disease indices HBI or SCCAI and not by the presence of therapeutic drug levels.
Of note is the mix of parenteral and oral dosing regimens with MTX. The total number of parenteral patients was too small to characterize the MTX route of effective administration, but prior research suggests relative bioequivalence and clinical outcomes of oral and subcutaneous MTX in patients with CD.19–21 However, there are conflicting data about the bioavailability of HD-MTX [25mg or more].22 Bioavailability is an important index of quality control, but serum levels themselves may lack the sensitivity and/or specificity to adequately compare outcomes. More studies that explore therapeutic drug monitoring and the relationship between drug levels and clinical outcomes are needed. These may require the inclusion of MTX substrates such as intracellular polyglutamates.23
Future research should include a prospective trial that compares combination therapy of an anti-TNF agent with MTX [in low, high, ora,l and injectable regimens] versus anti-TNF combination therapy with a different immunomodulator class. Additionally, it would also be of great interest to compare the effectiveness of immunogenicity prevention among different immunosuppressive agents.
In conclusion, we found that combination therapy of anti-TNF biologics with MTX at doses of 15–25mg/week [either orally or subcutaneously administered] are superior in maintaining clinical remission compared with MTX at lower doses, but found no differences in secondary measures of disease activity. Our finding of more adverse events in HD-MTX supports the need to balance efficacy with tolerability, to optimize management of IBD patients receiving anti-TNF therapy.
As the management of IBD continues to support combination therapy approaches, the findings of this study have important implications for the successful use of MTX as concomitant therapy for our IBD patients.
Supplementary Data
Supplementary data are available at JCC online.
Conflict of interest
None declared. xlink:href="http://ecco-jcc.oxfordjournals.org/lookup/suppl/doi:10.1093/ecco-jcc/jjv027/-/DC1
Acknowledgments
This manuscript was supported in part by the Digestive Diseases Research Core Center [DDRCC] at the University of Chicago [NIDDK P30DK42086].
Both authors contributed to study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, and drafting of the manuscript. RJC and DTR both approved the final draft submitted.
Data of this manuscript were presented in part at the Annual Meeting of the American College of Gastroenterology, Philadelphia, PA, October 2014 [No. P1052].
References
- 1. Lahiff C, Kane S, Moss AC. Drug development in inflammatory bowel disease: the role of the FDA. Inflamm Bowel Dis 2011;17:2585–93. [DOI] [PubMed] [Google Scholar]
- 2. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787–93. [DOI] [PubMed] [Google Scholar]
- 3. Saag KG, Yazdany J, Alexander C, et al. Defining quality of care in rheumatology: the American College of Rheumatology white paper on quality measurement. Arthritis Care Res [Hoboken] 2011;63:2–9. [DOI] [PubMed] [Google Scholar]
- 4. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. [DOI] [PubMed] [Google Scholar]
- 5. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 6. St Clair EW, van der Heijde DM, Smolen JS, et al. ; Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med 1995;332:292–7. [DOI] [PubMed] [Google Scholar]
- 8. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000;342:1627–32. [DOI] [PubMed] [Google Scholar]
- 9. Baron TH, Truss CD, Elson CO. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci 1993;38:1851–6. [DOI] [PubMed] [Google Scholar]
- 10. Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med 1989;110:353–6. [DOI] [PubMed] [Google Scholar]
- 11. Cummings JR, Herrlinger KR, Travis SP, Gorard DA, McIntyre AS, Jewell DP. Oral methotrexate in ulcerative colitis. Aliment Pharmacol Ther 2005;21:385–9. [DOI] [PubMed] [Google Scholar]
- 12. Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–8.e1. [DOI] [PubMed] [Google Scholar]
- 13. Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009;68:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maté-Jiménez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol 2000;12:1227–33. [DOI] [PubMed] [Google Scholar]
- 15. Oren R, Moshkowitz M, Odes S, et al. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol 1997;92:2203–9. [PubMed] [Google Scholar]
- 16. Sokol H, Seksik P, Carrat F. et al. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut 2010;59:1363–8. [DOI] [PubMed] [Google Scholar]
- 17. Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valentino PL, Church PC, Shah PS. et al. Hepatotoxicity caused by methotrexate therapy in children with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:47–59. [DOI] [PubMed] [Google Scholar]
- 19. Kurnik D, Loebstein R, Fishbein E. et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2003;18:57–63. [DOI] [PubMed] [Google Scholar]
- 20. Turner D, Doveh E, Cohen A, et al. Efficacy of oral methotrexate in paediatric Crohn’s disease: a multicentre propensity score study. Gut 2014, 21 Nov. doi: 10.1136/gutjnl-2014-307964. [DOI] [PubMed] [Google Scholar]
- 21. Wilson A, Patel V, Chande N, et al. Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2013;37:340–5. [DOI] [PubMed] [Google Scholar]
- 22. Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol 2004;31:645–8. [PubMed] [Google Scholar]
- 23. Chabner BA, Allegra CJ, Curt GA, et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest 1985;76:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]