Abstract
AIM: To further characterize the possible relationship between the molecular changes and prognosis of ESC and to elucidate the possible mechanisms involved.
METHODS: 114 specimens of ESC were collected from Linzhou city, and all patients were followed up for more than 5 years after resection. Histopathological analysis and immunohistochemical staining (ABC) were employed to detect the alteration of MUC1.
RESULTS: The positive immunostaining rate for MUC1 was 79% (90/114), and the high-expression rate was 63% (72/114). The mean survival periods (months) of those with high- and low-expression rates of MUC1 were 41 (95%CI: 35, 47) and 52 (95%CI: 45, 59), respectively. Patients in the low-expression group obviously survived longer than those in high-expression group, and the difference was significant (P < 0.05). The expression of MUC1 protein in the esophageal carcinoma specimens with metastasis was stronger than those without metastasis, the difference was also significant (P < 0.05). The stepwise multivariate analysis showed that “differentiation”, “expression of MUC1” and “TNM staging” were the most important factors affecting the prognosis of esophageal carcinoma patients (P < 0.05).
CONCLUSION: A good correlation between the alteration of MUC1 and the regional lymph node metastasis was observed. Furthermore, high-expression of MUC1 was associated with poor prognosis for esophageal cancer patients. These results indicated that MUC1 is a promising biomarker for predicting lymph node metastasis and prognosis in esophageal cancer.
INTRODUCTION
Esophageal squamous-cell carcinoma (ESC) is one of the most common malignant diseases in northern China, and Linzhou city (formerly Linxian) had being the highest incidence area[1,2]. The five-year survival rate for early esophageal cancer patients is more than 90%. However, for the patients at late or advanced stage, the five year survival rate is only 10%-15%[1,2]. So far, the conventional traditional prognostic markers, such as cancer stage based on metastasis and pathological grade are still used to evaluate the prognosis of esophageal cancer patients. But, it has been well recognized that there is discordance between the conventional prognosis biomarkers and the actual prognosis. For example the patients with well differentiated cancer may have a worse prognosis than those with poorly differentiated ones, indicating the limitation of those markers for predicating.
With the development of molecular biotechnology, many new measurements have been applied in cancer prognosis research. Studies on ESC prognosis have been expanded in recent years; however, the molecular mechanisms involved in prognosis of esophageal cancer, especially the survival analysis on whom from high-incidence area of esophageal carcinoma was very limited. We followed up the ESC patients from Linzhou city and determined the alteration of MUC1 expression and its relationship to the prognosis, to further characterize the possible relationship between them so as to elucidate the possible mechanisms of ESC carcinogenesis, and to determine the alteration of MUC1 and prognosis with histopathological and immunohistochemical methods.
MATERIALS AND METHODS
Patients
One hundred and fourteen patients with ESC, who had undergone esophagectomy at the Esophageal Carcinoma Hospital of Linzhou City between 1993 and 1996 were enrolled in this study. All the patients were local residents of Linzhou city and had not received radiation therapy or chemotherapy prior to the surgery. There were 67 men and 49 women. The mean age was 53.5 ± 8.1 (range 37-72) years for males and 53.6 ± 7.8 (range 40-69) years for females, respectively. All specimens were confirmed by pathology as ESC.
Follow-up
All patients were followed up until March 2001, at which the patients had survived for more than 5 years or died within that period after surgical treatment. 57 patients survived less than 5 years died of recurrence or metastasis.
Tissues processing
All tumor specimens were fixed with formalin and embedded with paraffin. Each block was sectioned serially at 5 um, one of which was stained with hematoxylin and eosin for histopathological analysis by two pathologists and the others were used for immunostaining.
Histopathological analysis
Histopathological diagnoses were made according to the previously established criteria[3].
Immunohistochemical staining
Anti-MUC1 antibody was a mouse monoclonal anti-serum directed at a hexapeptide in the tandem repeat region of the protein core of MUC1 (clone Ma552; Novocastra, Burlingame, CA), which was kindly provided by Dr. Yongqin Li (College of Medicine, Harvard University). The avidin-biotin-peroxidase complex (ABC) method was used for MUC1 immunostaining. In brief, after dewaxing, quenching endogenous peroxidase activity with 3% H2O2, and blocking cross-reactivity with normal serum (Vectastain Elite Kit; Vector, Burlingame, CA), the tissues were incubated overnight at 4 °C with primary antibodies (1:400 for MUC1). Location of the primary antibodies was achieved by subsequent use of a biotinylated anti-primary antibody, an avidin-biotin complex conjugated to horseradish peroxidase, and 3’,5’ - diaminobenzidine (Vectasitain Elite Kit). Normal serum blocking and omission of the primary antibody were used as negative controls.
Evaluation of immunostaining
Clear cytoplasm and cell membrane staining was the criterion for a positive reaction. The staining was graded by the percentage of positively stained neoplastic cells as follows: -, < 5%; +, 5%-50%; ++, > 50% of the neoplastic cells stained. For statistical analysis, the examined cases were divided into 2 groups: the low-expression group, composed of the “-” and “+” groups (less than 50% of neoplastic cells stained) and the high-expression group, the “++” group (over 50% of the neoplastic cells stained)[4].
Statistical analysis
Chi-squared test was performed to evaluate the relevance of regional lymph node metastasis and expression of MUC1 protein, Kaplan-Meier was used for survival analysis, and multivariate analysis for screening prognostic factors. The significant difference was considered when the P value was less than 0.05.
RESULTS
Results of follow up
Among the follow up of 114 ESC patients followed up, 33% (33/114) survived 5 years, 50% (57/114) died within 5 years after surgical treatment and 17% (20/114) cases were censored during the follow-up.
Expression of MUC1 and its relationship with survival of ESC
Among the 114 surgically resected ESC specimens examined, the positive immunostaining for MUC1 was observed in 90 cases (78.9%), and high-expression was seen in 72 cases (63.2%) and low-expression was in 42 cases (36.8%). The mean survival period (months) and 95 % confidence interval of esophageal carcinoma patients with high- and low-expression of MUC1 were 41(35, 47) and 52(45, 59), respectively. Patients in the low-expression group obviously survived longer than those in high-expression group, and the difference was significant (P < 0.05, Table 1 and Figure 1).
Table 1.
Survival analysis of high-expression and low-expres-sion of MUC1 in ESC
| Expression of MUC1 | No. of specimens examined | No. of death | Mean survival period (month) x (95%CI*) |
| Low-expression | 42 | 17 | 52 (45, 59) |
| High-expression | 72 | 40 | 41 (35, 47) |
| Total | 114 | 57 |
*: Confidence Interval; Log-rank: χ2 = 5.11, P = 0.0238.
Figure 1.
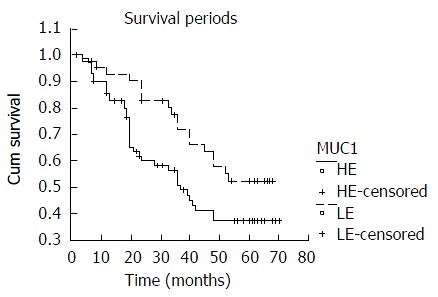
Comparison of survival period between high-expres-sion and low-expression of MUC1 in ESC. HE: High-expression; LE: Low-expression.
Relationship between the expression of MUC1 and regional lymph node metastasis
According to the status of the regional lymph nodes with or without metastasis, all specimens were divided into two groups, with metastasis and without metastasis. The expression of MUC1 protein in the ESC specimens with metastasis was obviously stronger than those without metastasis, and the difference was significant (P < 0.05, Table 2).
Table 2.
Relationship between the expression of MUC1 and regional lymph node metastasis
| Group | No. of specimen examined |
Expression of MUC1 protein |
|||
| - n(%) | + n(%) | ++ n(%) | +++ n(%) | ||
| Without metastasis | 77 | 19 (24.7) | 29 (37.7) | 7 (9.1) | 22 (28.5) |
| With Metastasis | 37 | 0 (0) | 4 (10.8) | 5 (13.5) | 28 (75.7) |
| Total | 114 | 19 | 33 | 12 | 50 |
Chi-squared test: χ2 =27.4693, P < 0.05.
Cox model analysis
Ten parameterss, including sex, age, invading depth, regional lymph node metastasis, metastasis of other organs, TNM stage, differentiation and MUC1, were used as independent variables, survival periods of ESC patients were used as dependent variables, and all variables were ranked into Cox model analysis (Table 3). The stepwise multivariate analysis showed that “differentiation”, “expression of MUC1” and “TNM staging”, were the most important factors affecting the prognosis of ESC patients (P < 0.05), RR values for each parameter were 2.2382, 1.9409 and 1.8621, respectively (Table 4).
Table 3.
All factors employed by Cox regression model
| Factors | Variable | |
| Sex | X1 | 0 = male, 1 = female |
| Age | X2 | 0 ≤ 45 years, 1 = 45~55 years, 2 ≥ 55 years |
| Invading depth | X3 | 0 = lamina propria or submucosa, 1 = muscularis propria, 2 = adventitia, 3 = adjacent structures |
| Regional lymph node metastasis | X4 | 0 = no, 1 = yes |
| Distant metastasis | X5 | 0 = no, 1 = yes |
| TNM stage | X6 | 0 = 0, 1 = I, 2 = II, 3 = III, 4 = IV |
| Differentiation | X7 | 0 = high, 2 = moderate, 3 = low |
| Expression of MUC1 | X8 | 0 = low, 1 = high |
Table 4.
Results of Cox model stepwise regression analysis
| Variable | Parameter Estimated | Standard error | R | RR |
| X6 | 0.6217 | 0.1549 | 0.1717 | 1.8621 |
| X7 | 0.8057 | 0.1961 | 0.1763 | 2.2382 |
| X8 | 0.6632 | 0.3075 | 0.0311 | 1.9409 |
DISCUSSION
Both patients and doctors monitored the patients’ survival period after surgery. So far, it is still controversial about evaluating. Out study on the expression of MUC1 in ESC tissues showed that the patients in the low-expression group obviously survived longer than those in high-expression group, and the difference was significant (P < 0.05). It is therefore indicated that detective of expression of MUC1 may be of value in assessing the prognosis of ESC patients.
The epithelial mucin coded by the MUC1 gene is a transmembrane molecule, which is expressed in most glandular epithelial cells. The molecule was first identified in human milk, as a large molecular weight glycoprotein rich in serine, threonine and proline carrying a high percentage of O-linked carbohydrate[5]. MUC1 is widely expressed by normal glandular epithelial cells, and the expression is dramatically increased when the cells become malignant[6,7], and its relationship with the prognosis of several carcinomas have been already reported[4,8-11]. Changes in the expression levels of MUC1 have also been described in esophageal lesions[12]. Our finding showed that MUC1 was expressed in all surgical specimens with lymph node metastasis. Its high-expression rate reached 89%, and was significantly different from the specimens without lymph node metastasis (P < 0.05). The lymph node and lymphatic vessel invasion has been reported as poor prognosis factor[13,14].
Mucins are heavily glycosylated glycoproteins that have protective and lubricating functions[15,16]. MUC1 expressed in tumors may function as an anti-adhesion molecule, which inhibits cell-cell adhesion, inducing a release of cells from tumor nests. Thus, MUC1 expression may be related to invasion or metastasis of carcinoma cells[17-19]. MUC1 can down-regulate the expression of E-cadherin, which is a calcium-dependent adhesion molecule, functioning in the cell-cell adhesion, while the low-expression of E-cadherin increased the invading ability of tumor cells[20-22]. Our previous studies found that MUC1 was expressed in 50 primary ESC cells and the metastasized cancer cells of the matched lymph nodes. In addition, it was found that the coincidence of positive immunostaining between primary tumor and its matching lymph node was observed in 28 cases (56.0%), while the coincidence of negative immunostaining was observed only in 2 cases (1.0%)[23]. It therefore indicated that the expression of MUC1 might play an important role in the invasion or metastasis of ESC cells, which might be one of the mechanisms involving in poor prognosis. But a discordant view was held by Japanese authors who argued that the expression of MUC1 was not significantly associated with metastasis of human esophageal carcinomas[24]. Further study is still necessary.
Considering the complexity in cancer, it is difficult to define it useful prognostic and predictive factors[25-28]. In the present study, we use multivariate analytic method for screening the prognostic factors in combination with clinical data. The stepwise multivariate analysis shows that “differentiation”, “expression of MUC1” and “TNM staging” are the most important factors affecting the prognosis of ESC patients (P < 0.05), our findings also indicate that high expression of MUC1 is related to poor prognosis of ESC.
Footnotes
Supported by National Outstanding Young Scientist Award of China , NO. 30025016; NCI CA65871 (U.S.A.)
Edited by Ma JY
References
- 1.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 2.Lu JB, Yang WX, Zu SK, Chang QL, Sun XB, Lu WQ, Quan PL, Qin YM. Cancer mortality and mortality trends in Henan, China, 1974-1985. Cancer Detect Prev. 1988;13:167–173. [PubMed] [Google Scholar]
- 3.Wang LD, Shi ST, Zhou Q, Goldstein S, Hong JY, Shao P, Qiu SL, Yang CS. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric-cardia carcinogenesis. Int J Cancer. 1994;59:514–519. doi: 10.1002/ijc.2910590414. [DOI] [PubMed] [Google Scholar]
- 4.Sagara M, Yonezawa S, Nagata K, Tezuka Y, Natsugoe S, Xing PX, McKenzie IF, Aikou T, Sato E. Expression of mucin 1 (MUC1) in esophageal squamous-cell carcinoma: its relationship with prognosis. Int J Cancer. 1999;84:251–257. doi: 10.1002/(sici)1097-0215(19990621)84:3<251::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim Biophys Acta. 1999;1455:301–313. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 6.Hirasawa Y, Kohno N, Yokoyama A, Kondo K, Hiwada K, Miyake M. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Respir Crit Care Med. 2000;161:589–594. doi: 10.1164/ajrccm.161.2.9905028. [DOI] [PubMed] [Google Scholar]
- 7.Baldus SE, Zirbes TK, Glossmann J, Fromm S, Hanisch FG, Mönig SP, Schröder W, Schneider PM, Flucke U, Karsten U, et al. Immunoreactivity of monoclonal antibody BW835 represents a marker of progression and prognosis in early gastric cancer. Oncology. 2001;61:147–155. doi: 10.1159/000055366. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Kimura N, Yoshida R, Lin H, Yoshinaga K. Immunohistochemical study of Muc1, Muc2 and human gastric mucin in breast carcinoma: relationship with prognostic factors. Oncol Rep. 2001;8:1177–1182. doi: 10.3892/or.8.5.1177. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer. 2001;92:1427–1434. doi: 10.1002/1097-0142(20010915)92:6<1427::aid-cncr1466>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Kraus S, Abel PD, Nachtmann C, Linsenmann HJ, Weidner W, Stamp GW, Chaudhary KS, Mitchell SE, Franke FE, Lalani el-N. MUC1 mucin and trefoil factor 1 protein expression in renal cell carcinoma: correlation with prognosis. Hum Pathol. 2002;33:60–67. doi: 10.1053/hupa.2002.29682. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwagi H, Kijima H, Dowaki S, Ohtani Y, Tobita K, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, Inokuchi S, et al. MUC1 and MUC2 expression in human gallbladder carcinoma: a clinicopathological study and relationship with prognosis. Oncol Rep. 2001;8:485–489. doi: 10.3892/or.8.3.485. [DOI] [PubMed] [Google Scholar]
- 12.Guillem P, Billeret V, Buisine MP, Flejou JF, Lecomte-Houcke M, Degand P, Aubert JP, Triboulet JP, Porchet N. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int J Cancer. 2000;88:856–861. doi: 10.1002/1097-0215(20001215)88:6<856::aid-ijc3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Brücher BL, Stein HJ, Werner M, Siewert JR. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. 2001;92:2228–2233. doi: 10.1002/1097-0142(20011015)92:8<2228::aid-cncr1567>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 15.Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998;58:5582–5589. [PubMed] [Google Scholar]
- 16.Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, Mandel U, Clausen H, Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 17.Kam JL, Regimbald LH, Hilgers JH, Hoffman P, Krantz MJ, Longenecker BM, Hugh JC. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–5581. [PubMed] [Google Scholar]
- 18.1 Takao S, Uchikura K, Yonezawa S, Shinchi H, Aikou T. Mucin core protein expression in extrahepatic bile duct carcinoma is associated with metastases to the liver and poor prognosis. Cancer. 1999;86:1966–1975. doi: 10.1002/(sici)1097-0142(19991115)86:10<1966::aid-cncr13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Konishi K, Arakawa H, Oonishi I, Kaji M, Maeda K, Yabushita K, Tsuji M, Miwa A. Number of lymph node metastases influences survival in patients with thoracic esophageal carcinoma: therapeutic value of radiation treatment for recurrence. Dis Esophagus. 1999;12:205–208. doi: 10.1046/j.1442-2050.1999.00049.x. [DOI] [PubMed] [Google Scholar]
- 20.Kondo K, Kohno N, Yokoyama A, Hiwada K. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–2019. [PubMed] [Google Scholar]
- 21.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si HX, Tsao SW, Lam KY, Srivastava G, Liu Y, Wong YC, Shen ZY, Cheung AL. E-cadherin expression is commonly downregulated by CpG island hypermethylation in esophageal carcinoma cells. Cancer Lett. 2001;173:71–78. doi: 10.1016/s0304-3835(01)00646-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang ZH, Wang LD, Gao SS, Fan ZM, Song ZB, Qi YJ, Li YJ, Li JX. Expression of MUC1 in esophageal and gastric cardiac carci-noma tissues from high-incidence area for esophageal cancer in Henan. Zhenzhou Daxue Xuebao. 2002;37:774–777. [Google Scholar]
- 24.Kijima H, Chino O, Oshiba G, Tanaka H, Kenmochi T, Kise Y, Shimada H, Abe Y, Tokunaga T, Yamazaki H, et al. Immunohistochemical MUC1 (DF3 antigen) expression of human esophageal squamous cell carcinoma. Anticancer Res. 2001;21:1285–1289. [PubMed] [Google Scholar]
- 25.Hall PA, Going JJ. Predicting the future: a critical appraisal of cancer prognosis studies. Histopathology. 1999;35:489–494. doi: 10.1046/j.1365-2559.1999.00862.x. [DOI] [PubMed] [Google Scholar]
- 26.Song ZB, Gao SS, Wang LD, Li JL, Fan ZM, Guo HQ, Yi XN. Correlation between p53, PCNA and the prognosis of esophageal carcinoma from the subjects in high-incidence area. Henan Yixue Yanjiu. 2002;11:97–100. [Google Scholar]
- 27.Xiong XD, Xu LY, Shen ZY, Cai WJ, Luo JM, Han YL, Li EM. Identification of differentially expressed proteins between human esophageal immortalized and carcinomatous cell lines by two-dimensional electrophoresis and MALDI-TOF-mass spectrometry. World J Gastroenterol. 2002;8:777–781. doi: 10.3748/wjg.v8.i5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng WJ, Liu GY, Xu J, Zhou XD, Zhang YE, Zhang N. Pathological characteristics, PCNA labeling index and DNA index in prognostic evaluation of patients with moderately differentiated hepatocellular carcinoma. World J Gastroenterol. 2002;8:1040–1044. doi: 10.3748/wjg.v8.i6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]


