Abstract
AIM: To study the difference of gene expression between esophageal carcinoma and its pericancerous epithelium and to screen novel associated genes in the early stage of esophageal carcinogenesis by cDNA microarray.
METHODS: Total RNA was extracted with the original single step way from esophageal carcinoma, its pericancerous epithelial tissue and normal esophageal epithelium far from the tumor. The cDNA retro-transcribed from equal quantity of mRNA was labeled with Cy5 and Cy3 fluorescence functioning as probes. The mixed probes were hybridized with two pieces of BioDoor 4096 double dot human whole gene chip. Fluorescence signals were scanned by ScanArray 3000 laser scanner and farther analyzed by ImaGene 3.0 software with the digital computer.
RESULTS: (1) A total of 135 genes were screened out, in which 85 and 50 genes whose the gene expression levels (fluorescence intensity) in esophageal carcinoma were more than 2 times and less than 0.5 times respectively compared with the normal esophageal epithelium. (2) There were also total 31 genes, among then 27 and 4 whose expressions in pericancerous tissue were 2-fold up-regulated and 0.5-fold down-regulated respectively compared with normal esophageal epithelium. (3) There were 13 genes appeared simultaneously in both pericancerous epithelium and esophageal carcinoma, while another 18 genes existed in pericancerous epithelium only.
CONCLUSION: With the parallel comparison among these three gene profiles, it was shown that (1). A total of 135 genes, Whose expression difference manifested as fluorescence intensity were more than 2 times between esophageal carcinoma and normal esophageal epithelium, were probably related to the occurrence and development of the esophageal carcinoma. (2). The 31 genes showing expression difference more than 2 times between pericancerous and normal esophageal epithelium might be relate to the promotion of esophageal pericancerosis and its progress. The present study illustrated that by using the gene chip to detect the difference of gene expression profiles might be of benefit to the gene diagnosis, treatment and prevention of esophageal carcinoma.
INTRODUCTION
The differentially expressed genes in different specimens may be detected with parallel analysis by gene chips which has greatly improved the traditional experiment in that only a single or several genes expression can be observed for each test. More and more cDNA microarray methods are applied nowadays in the study of gene expression. In this paper, gene chip technique was used to analysis the difference of gene expression patterns between the esophageal carcinoma, its pericancerous tissue and normal epithelium of esophagus, in order to explore the tumor-associated gene-clusters and their functions in the process of occurrence and development of esophageal carcinoma, which will be helpful to understand comprehensively the molecular mechanism of cell transformation and provide molecular markers and target genes for clinical diagnosis, prevention, susceptibility forecast and treatment of esophageal carcinoma.
MATERIALS AND METHODS
Materials
All the tissue specimens including esophageal carcinoma, its pericancerous epithelium and normal epithelium of esophagus that serves as control were taken from 11 patients being operated in our hospital from July 17 to September 7, 2000. For each sample, one part was cut to be frozen in liquid nitrogen immediately after surgical resection, and the other part was used for histopathological examination to ensure all the pericancerous and control esophageal epithelia without cancer cells but with their corresponding histological appearance. The clinical and pathological data of these patients were show in Table 1.
Table 1.
Clinical and pathological data of 11 cases with esophageal carcinoma
| No. of in-P | Name | Sex | Age | Pathological diagnosis | Lymph metastasis | Clinical stage |
| 106811 | Xu_ | F | 65 | Middle esophageal, marrow type, well-middle d. | ||
| Scc, exterior layer invasion with tumor embolus | 0/18 | II | ||||
| 106850 | Xu _ | F | 58 | Middle esophageal, marrow type, Poor d. Scc with | ||
| Exterior layer invasion | 1/8 | III | ||||
| 106839 | Yu _ | M | 60 | Middle-inferior esophageal, marrow type, well-middle d. | ||
| Scc, exterior layer invasion with tumor embolus | 0/26 | II | ||||
| 107001 | Zhou _ | M | 53 | Middle esophageal, ulcer type, middle d. Scc, | ||
| Exterior layer invasion with tumor embolus | 4/19 | III | ||||
| 107151 | Wang_ | M | 42 | Middle-up esophageal, marrow type, middle d. | ||
| Scc with exterior layer invasion | 0/8 | II | ||||
| 107326 | Gu _ | M | 51 | Middle- inferior esophageal, marrow type, | ||
| Middle-Poor d. Scc with exterior layer invasion | 2/14 | III | ||||
| 107546 | Zhao_ | F | 63 | Middle- inferior esophageal, ulcer type, well- | ||
| Middle d. Scc with shallow muscle layer invasion | 0/15 | II | ||||
| 107564 | Lu_ | M | 61 | Middle- inferior esophageal, marrow type, | ||
| Middle d. Scc with deep muscle layer invasion | 1/32 | III | ||||
| 107146 | Chen_ | F | 65 | Superior esophageal, ulcer type, well d. | ||
| Scc with exterior layer invasion | 0/17 | II | ||||
| 107527 | Xu_ | M | 49 | Inferior esophageal, ulcer type, middle d. | ||
| Scc with shallow muscle layer invasion | 1/18 | III | ||||
| 107531 | Guan_ | M | 56 | Middle esophageal, marrow type, middle d. | ||
| Scc with deep muscle layer invasion | 0/26 | II |
d.: differentiated Scc: squamous cell carcinoma.
Methods
Chip preparation Four thousand and ninety six target cDNA clones used in cDNA chip were provided by United Gene Ltd. and cooperative fellows. These genes were amplified with PCR using universal primers and then purified with standard method. The quality of PCR was monitored by agarose electrophoresis. The obtained genes were dissolved in 3 × SSC spotting solution and then spotted on silylated slides (Telechem. Inc) by Cartesian 7500 spotting Robotics (Cartesian, Inc). Each target gene was dotted twice. After spotting, the slides were hydrated (2 h) and dried (0.5 h, room temperature.). The samples were cross-linked with UV light and treated with 0.2% SDS, H2O and 0.2% NaNBH4 for 10 min respectively. Then the slides were dried in cold condition and ready for use.
Probe preparation Total sample RNA was extracted by single step method[1]. Briefly, after taking out from liquid Nitrogen specimens were ground completely into tiny powder while adding liquid Nitrogen in ceramic mortar and then homogenized in D solution plus 1% mercaptoethanol. After centrifugation, the supernatant was extracted with phenol: chloroform (1:1), NaAC and acidic phenol: chloroform (5:1) respectively. The aqueous phase was precipitated by equal volume of isopropanol and centrifuged. The precipitate was dissolved with Millie-Q H2O. After further purification by LiCl precipitating method obtained RNA sample was demenstrated, good in quality with UV analysis and electrophoresis. mRNAs were isolated and purified with Oligotex mRNA Midi Kit (Quagen, Inc.). The fluorescent-labeled cDNA probe was prepared through retro-transcription, referring to the method of Schena[2]. The probes from normal epithelium were labeled with Cy3-dUTP, while those from cancer tissue and pericancerous epithelium with Cy5-dUTP respectively. The probes were mixed (Cy3-dUTP control + Cy5-dUTP esophageal carcinoma, Cy3-dUTP control + Cy5-dUTP pericancerous epithelium) and precipitated by ethanol, and then resolved in 20 μl hybridization solution (5 × SSC + 0.2% SDS).
Hybridization and washing Probes and chip were denatured respectively in 95 °C bath for 5 min, then the probes were added on the chip. They were hybridized in sealed chamber at 60 °C for 15-17 h and washed in turn with solutions of 2 × SSC + 0.2% SDS, 0.1× SSC + 0.2% SDS and 0.1% SSC 10 min each, then dried at room temperature.
Fluorescent scanning and results analysis The chip was read by Scan Array 3000 Scanner (General Scanning Inc). The overall intensities of Cy3 and Cy5 were normalized and corrected by a coefficient according to the ratios of the located 40 housekeeping genes. The acquired image was further analyzed by ImeGene 3.0 Software with digital computer to obtain the intensities of fluorescent signals and the Cy3/Cy5 ratio. The data were taken on an average of the two repeated spots. The differentially expressed gene were defined as: (1) The absolute value of the Cy5/Cy3 natural logarithm was more than 0.69 (the variation of gene expression was more than 2-fold). (2) Either Cy3 or Cy5 signal value was required for more than 600. (3) The PCR results were satisfactory.
RESULTS
Scatter-plot of hybridization signals on gene chip
The scatter plot that was plotted with Cy3 and Cy5 fluorescent signal values displayed a quite disperses pattern in distribution. Most of spots gathered around a 45º diagonal line, in which blue spots represented the area where the signal intensities varied between 0.5 to 2- fold compared with that of the control. Some red spots distributed beyond or far from 45° diagonal line were indicated the existence of abnormal gene expression in esophageal carcinoma and in pericancerous epithelium. Their signal intesities were 2 times more than that of the control. (Figure 1).
Figure 1.
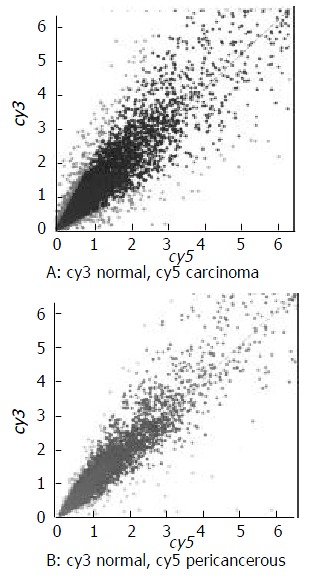
The scatter plots of gene expression pattern. (A) Carcinoma; (B) Pericancerous epithelium.
Results and gene expression pattern by scanning analysis
The fluorescent scanning profile of gene expression was showed in Figure 2 A and B.
Figure 2.
Scanning results of hybridizing signals on gene chip. (A) Carcinoma and the control epithelium; (B) Pericancerous and the control epithelium.
cDNA probes that labeled with Cy3 from control epithelium and that labeled with Cy5 from esophageal carcinoma or that labeled with Cy5 from pericancerous epithelium were hybridized through microarray. Hybridization results were obtained in parallel by comparing with these sample gene expression patterns demonstrated. In carcinoma tissues, A total of 41 genes were found having expression variations more than three times from the normal control, the up-and down-regulated genes were 28 and 13 respectively (Table 2). There were also 135 genes that manifested expression variations more than two times from the control and the up-and down-regulated genes were 85 and 50 respectively. Besides, there were nine genes found in the esophageal carcinoma having not been recorded in genbank. Their functions remain to be studied.
Table 2.
Genes with expression difference > 3 or < 0.33 on average ratio between esophageal carcinoma and normal epithelium
| Genbank-ID | Fcf | Gene name | R1 | R2 | AR |
| hsu67963 | Human lysophospholipase homolog(HU-K5)mRNA, complete cds. | 0.11 | 0.16 | 0.14 | |
| Hslimdp | 8 | H.sapiens mRNA for ZNF185 gene. | 0.15 | 0.23 | 0.19 |
| 4505166 | 0.13 | 0.26 | 0.19 | ||
| d87451 | 15 | Human mRNA for KIAA0262gene,complete cds | 0.13 | 0.31 | 0.22 |
| hsy15409 | 11 | Homo sapiens mRNA for putative glucose 6-phosphate translocase. | 0.28 | 0.23 | 0.25 |
| hsu16996 | 12 | Human protein tyrosine phosphatase mRNA, complete cds. | 0.31 | 0.26 | 0.29 |
| hsu30255 | 12 | Human phosphogluconate dehydrogenase (hPGDH) gene, complete cds. | 0.29 | 0.30 | 0.29 |
| 4504714 | 0.47 | 0.16 | 0.31 | ||
| ab002349 | 15 | Human mRNA for KIAA0351 gene, complete cds. | 0.14 | 0.50 | 0.32 |
| humpai2 | 13 | Human placental plasminogen activator inhibitor mRNA, complete cds. | 0.28 | 0.35 | 0.32 |
| hsu73824 | Human p97 mRNA, complete cds. | 0.30 | 0.34 | 0.32 | |
| hsa132270 | 2 | Homo sapiens mRNA for integral type I protein p24B (p26). | 0.31 | 0.34 | 0.33 |
| Humorfkg1f | 11 | Human mRNA for KIAA0058 gene,complete cds. | 0.31 | 0.36 | 0.33 |
| s74452 | 3 | Cyclin B1{promoter region}[human,cervical carcinoma cell line, HeLa cells,Genomic, 351 nt]. | 3.21 | 2.79 | 3.00 |
| hspros27 | 10 | H.sapiens PROS-27 mRNA. | 3.29 | 2.85 | 3.07 |
| Hstrr | 10 | Human mRNA for transferrin receptor. | 2.70 | 3.48 | 3.09 |
| Humprion | 15 | Homo sapiens mRNA for prion protein, complete cds. | 3.07 | 3.19 | 3.13 |
| hshr21spa | 13 | H.sapiens mRNA for protein involved in DNA double-strand break repair. | 2.29 | 4.01 | 3.15 |
| AB00109S14 | 11 | Homo sapiens gene for H-cadherin, exon 14 and complete cds. | 2.95 | 3.50 | 3.23 |
| hsranbp1 | 11 | H.sapiens mRNA for RanBP1. | 3.98 | 2.57 | 3.27 |
| Hsxprf | 8 | Homo sapiens mRNA for putative transcription factor XPRF. | 3.35 | 3.38 | 3.37 |
| humcd3621 | 11 | Human antigen CD36 (clone 21) mRNA, complete cds. | 3.28 | 3.54 | 3.41 |
| Humsparc | 5 | Human SPARC/osteonectin mRNA, complete cds. | 2.87 | 3.96 | 3.42 |
| Humhcamap3 | 5 | Human gene for hepatitis C-associated microtubular aggregate protein p44, exon 4. | 3.72 | 3.30 | 3.51 |
| Humprofii | 5 | Human profilin II mRNA, complete cds. | 3.07 | 4.07 | 3.57 |
| 5729755 | 2.35 | 4.88 | 3.61 | ||
| Hsrnasmg | 13 | H.sapiens mRNA for Sm protein G. | 2.91 | 4.57 | 3.74 |
| Humpgc | 5 | Human chondroitin sulfate proteoglycan core protein mRNA, 3' end. | 3.67 | 3.91 | 3.79 |
| hstop2a10 | 8 | Homo sapiens topoisomerase II alpha (TOP2A) gene,exons 34 and 35,and complete cds. | 4.02 | 3.76 | 3.89 |
| hsu09559 | 14 | Human Rch1 (RCH1) mRNA, complete cds. | 3.75 | 4.03 | 3.89 |
| humcol1a42 | Human alpha-1 collagen type IV gene, exon 52. | 4.37 | 3.69 | 4.03 | |
| hsinta6r | 11 | Human mRNA for integrin alpha 6. | 3.28 | 5.43 | 4.35 |
| d86983 | 15 | Human mRNA for KIAA0230 gene, partial cds. | 4.71 | 4.84 | 4.78 |
| hsu26555 | Human versican V2 core protein precursor splice-variant mRNA, complete cds. | 6.93 | 5.91 | 6.42 | |
| hspgp95 | 13 | Human mRNA for protein gene product (PGP) 9.5. | 5.66 | 7.54 | 6.60 |
| af052124 | 15 | Homo sapiens clone 23810 osteopontin mRNA, complete cds. | 11.77 | 4.63 | 8.20 |
| Humcolva | Human alpha-2 type V collagen gene, 3' end. | 7.64 | 10.17 | 8.91 | |
| humca1xia | 15 | Human alpha-1 type XI collagen (COL11A1) mRNA, complete cds. | 6.75 | 12.07 | 9.41 |
| Hscalbr11 | Human mRNA for 27-kDa calbindin. | 6.72 | 12.17 | 9.45 | |
| hsu50078 | 8 | Human guanine nucleotide exchange factor p532 mRNA, complete cds. | 12.28 | 17.11 | 14.70 |
| Hsop | 5 | Human mRNA for osteopontin. | 20.23 | 20.22 | 20.23 |
Fcf: function classification average ratio > 3: up regulation average ratio < 0.33: down regulation; R1: the first ratio; R2: the second ratio; AR: R1 + R2 average ratio.
In pericancerous epithelia there were 31 genes found having expression variations more than two times from the control, the up-and down-regulated genes were 27 and 4 respectively. These genes might be divided into 16 groups (Table 3) according to their functions.
Table 3.
Function classification for genes showing expression differences in esophageal carcinoma and pericancerous epithelium that were > 2 or < 0.5 fold from the control tissue
| Gene function classification |
Carcinoma/normal |
Pericancerous/normal |
|||||
| N | > 2 | < 0.5 | N | > 2 | < 0.5 | ||
| 1 | Proto-oncogenes and tumor suppression genes | 2 | 1 | 1 | 0 | 0 | 0 |
| 2 | Cell signals and transducing proteins | 3 | 2 | 1 | 3 | 3 | 0 |
| 3 | Cell cycle proteins | 6 | 5 | 1 | 0 | 0 | 0 |
| 4 | Extra-pressure reaction proteins | 1 | 0 | 1 | 0 | 0 | 0 |
| 5 | Cell regulatory proteins | 7 | 7 | 0 | 3 | 3 | 0 |
| 6 | Cell apoptosis related proteins | 1 | 1 | 0 | 1 | 0 | 1 |
| 7 | DNA synthesis, repair and recombinant proteins | 3 | 3 | 0 | 0 | 0 | 0 |
| 8 | DNA binding, transcription and its factor | 6 | 4 | 2 | 1 | 1 | 0 |
| 9 | Cell receptors | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Cell surface antigen and adhesion proteins | 10 | 8 | 2 | 4 | 4 | 0 |
| 11 | Ion-channel and transporters | 24 | 14 | 10 | 2 | 2 | 0 |
| 12 | Metabolism-related proteins | 21 | 10 | 11 | 6 | 4 | 2 |
| 13 | Protein synthesis-related genes | 14 | 7 | 7 | 2 | 1 | 1 |
| 14 | Development-related genes | 1 | 1 | 0 | 2 | 2 | 0 |
| 15 | Other genes | 27 | 18 | 9 | 7 | 7 | 0 |
| 16 | New genes | 9 | 4 | 5 | 0 | 0 | 0 |
| Total | 135 | 85 | 50 | 31 | 27 | 4 | |
There were 13 genes appeared simultaneously in both pericancerous epithelium and esophageal carcinoma, while another 18 genes were found in pericancerous epithelium only (Table 4).
Table 4.
Genes showing expression difference between the esophageal carcinoma and pericancerous epithelium
| Genbank-ID | Fcf | Gene name | PR | TR |
| Hscalbr | 11 | Human mRNA for 27-kDa calbindin. | 2.91 | 9.45 |
| af052124 | 15 | Homo sapiens clone 23810 osteopontin mRNA, complete cds. | 5.34 | 8.20 |
| Hsinta6r | 11 | Human mRNA for integrin alpha 6. | 2.93 | 4.35 |
| Humprofii | 5 | Human profilin II mRNA, complete cds. | 2.50 | 3.57 |
| Humhcamap3 | 5 | Human gene for hepatitis C-associated microtubular aggregate protein p44, exon 4. | 2.79 | 3.51 |
| hum927a | 10 | Human interferon-inducible protein 9-27 mRNA, complete cds. | 2.43 | 2.92 |
| Hstrr | 10 | Human mRNA for transferrin receptor. | 3.76 | 3.09 |
| hsu50078 | 8 | Human guanine nucleotide exchange factor p532 mRNA, complete cds. | 18.7 | 14.7 |
| Humtfrr | 10 | Human transferrin receptor mRNA, complete cds. | 3.51 | 2.81 |
| hsu30255 | 12 | Human phosphogluconate dehydrogenase(hPGDH)gene, complete cds. | 0.48 | 0.29 |
| hsu05684 | 2 | Homo sapiens dihydrodiol dehydrogenase mRNA, complete cds. | 3.75 | 2.31 |
| Hsop | 5 | Human mRNA for osteopontin. | 37.1 | 20.2 |
| Hspgp95 | 13 | Human mRNA for protein gene product (PGP) 9.5. | 19.4 | 6.60 |
| hsu46571 | 13 | Human tetratricopeptide repeat protein (tpr2) mRNA, complete cds. | 0.41 | |
| Humrsc390 | 6 | Human mRNA for KIAA0018gene, complete cds. | 0.44 | |
| hcox4gn | 12 | Homo sapiens hypothetical protein (COX4AL) gene, partial cds, and cytochromec oxidase subunit IV precursor (COX4) gene, complete cds. | 0.47 | |
| d79997 | Human mRNA for KIAA0175 gene, complete cds. | 2.05 | ||
| ab01755s10 | 15 | Homo sapiens IGSF4 gene, exon 10 and complete cds. | 2.06 | |
| Hsnov | 15 | H.sapiens mRNA for novel gene in Xq28 region. | 2.09 | |
| hsifi56r | Human mRNA for 56-KDa protein induced by interferon. | 2.1 | ||
| af054182 | 12 | Homo sapiens mitochondrial processing peptidase beta-subunit mRNA, complete cds. | 2.11 | |
| af131818 | 15 | Homo sapiens clone 25237mRNA sequence. | 2.19 | |
| af035301 | 2 | Homo sapiens clone 23876 neuronal olfactomedin-related ER localized protein mRNA, partial cds. | 2.28 | |
| Humorfi | 14 | Homo sapiens (clone S240ii117/zap112) mRNA, complete cds. | 2.26 | |
| af038660 | 12 | Homo sapiens chromosome 1p33-p34 beta-1,4-galactosyltransferase mRNA, complete cds. | 2.32 | |
| d88687 | 12 | Homo sapiens mRNA for KM-102-derived reductase-like factor,complete cds. | 2.51 | |
| hsu05598 | 12 | Human dihydrodiol dehydrogenase mRNA, complete cds. | 3.07 | |
| Humalcam | 10 | Homo sapiens CD6 ligand (ALCAM) mRNA, complete cds. | 3.07 | |
| hsu79299 | 2 | Human neuronal olfactomedin-related ER localized protein mRNA,partial cds. | 3.33 | |
| ab003476 | 14 | Homo sapiens mRNA for gravin, complete cds. 3.65 | ||
| af100757 | 15 | Homo sapiens COP9 complex subunit 4 mRNA, complete cds. 5.96 |
Fcf: function classification; TR: esophageal carcinoma average ratio; PR: pericancerous epithelium average ratio.
DISCUSSION
Because of the occult characteristic esophageal carcinoma, one of the most common malignant tumors in China, is difficult to be diagnosed for a size of < 2 cm tumor mass by using CT and ultrasound. At present, almost most of the clinical cases are in the late stage, with a five years survival rate of about 30% only. It is therefore necessary to explore new and efficacious diagnostic method to detect esophageal carcinoma at the early stage.
The carcinogenesis is a process caused by abnormal expression of tumor-associated genes or inactivation of tumor suppression genes or both. Clarifying the gene expression differences between the malignant and normal tissue is therefore the key procedure for the cancer control study. With the advances of molecular biological techniques, gene chip has been used to detect gene expression difference in various specimens by parallel analysis on a large scale[2-29]. Some papers have also been published about the studying human esophageal carcinoma by using gene chips[30-35]. However, a investigation on the difference of gene expression profiles between esophageal carcinoma and its paricancerous epithelium by gene chip has not been reported yet.
In the present study the gene chip technique was employed to analyze the difference of gene expression patterns in esophageal carcinoma and its paricancerous epithelium as well as their normal control. The results showed that: (1) There were 135 genes with expression levels marked as fluorescence intensity of more than 2 times or less that 0.5 times in esophageal carcinoma compared with the normal esophageal epithelium. It suggested these genes might well be related to the occurrence and development of the esophageal carcinoma.
Xu et al[2] reported that the protein tyrosine phosphatase (PTPase) the high and low metastatic human ovarian cancer cell lines were down-regulated with a varied degree (0.64 and 0.27 respectively). PTPase was associated with the cell signaling control, energy metabolism, proliferation and the promotion of MHC-I antigen expression, mediated by numerous hormones (such as epidermal growth factor, insulin, insulin-like growth factor 1 and so on). The down-regulated PTPase would decrease the antigen expression on the cell surface, and result in the malignant cell escaping from the immune surveillance. In the present study, the enzyme was also down-regulated (0.29) in the esophageal carcinoma.
We found expression levels that of a variety of adhesion protein genes such as collagen protein type IV (8.9), integrin (4.45), calbindin protein (9.45) were increased, which coincided with Yanagawa et al[16], Mori et al[24], Hippo et al[25] and Kan et al[33] reported papers, inclusive of colorectal, gastric and esophageal carcinomas. The increased adhesion protein expression might associate with the invasiveness and metastasis of cancer cells.
It has been demonstrated that in proliferous cells the activity of topoisomerase II (TopoII) was rapidly increased from S phase to the end of G2/M stage, which meant that the enzyme might relate to the malignant transformation of the tumor cells. Varis et al[22] and Hu et al[34] reported that TopoII expression levels was obviously up-regulated in human gastric cancer and esophageal squamous cell carcinoma. In our study the TopoII expression was up- regulated as well (3.89).
Cell cycle-protein B1 was also found up-regulated in esophageal carcinoma as Hu et al[34] have reported, which might indicate that the malignant cells were in an abnormal proliferative state.
(2) There were 31 genes with expression levels marked as fluorescence intensity of more than 2 times or less that 0.5 times in pericancerous epithelium compared with normal control. Although an abnormal appearance of pericancerous epithelium was not found in the pathological examination, the gene expression however indicated that there were thirteen genes expressed both in esophageal carcinoma and in pericancerous epithelium. Among these, the expression levels of 6 up-regulated genes in pericancerous epithelium were less than that of the corresponding genes in esophageal carcinoma and as for the other 6 up-regulated and 1 down-regulated genes, the expression levels in the former was higher than that in the latter. There were 18 genes appeared only in pericancerous epithelium, which suggested that these genes were probable related to the promotion and progression of carcinogenesis at the early stage of esophageal carcinoma.
The application of gene chip technique was a revolution of research method in life science. Our experiment illustrated that the detection of gene expression difference between malignant and normal tissue by gene chip might provide a new direction for diagnosis, therapy and prevention of human esophageal carcinoma.
Footnotes
Supported by Zhejiang Medical and Health Science Foundation No. 2002A023
Edited by Zhu L
References
- 1.Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, Wu Z, Stephenson MT, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- 2.Xu S, Mou H, Lü G, Zhu C, Yang Z, Gao Y, Lou H, Liu X, Cheng Y, Yang W. Gene expression profile differences in high and low metastatic human ovarian cancer cell lines by gene chip. Chin Med J (Engl) 2002;115:36–41. [PubMed] [Google Scholar]
- 3.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 4.Furey TS, Cristianini N, Duffy N, Bednarski DW, Schummer M, Haussler D. Support vector machine classification and validation of cancer tissue samples using microarray expression data. Bioinformatics. 2000;16:906–914. doi: 10.1093/bioinformatics/16.10.906. [DOI] [PubMed] [Google Scholar]
- 5.Ono K, Tanaka T, Tsunoda T, Kitahara O, Kihara C, Okamoto A, Ochiai K, Takagi T, Nakamura Y. Identification by cDNA microarray of genes involved in ovarian carcinogenesis. Cancer Res. 2000;60:5007–5011. [PubMed] [Google Scholar]
- 6.Zou TT, Selaru FM, Xu Y, Shustova V, Yin J, Mori Y, Shibata D, Sato F, Wang S, Olaru A, et al. Application of cDNA microarrays to generate a molecular taxonomy capable of distinguishing between colon cancer and normal colon. Oncogene. 2002;21:4855–4862. doi: 10.1038/sj.onc.1205613. [DOI] [PubMed] [Google Scholar]
- 7.Lin YM, Furukawa Y, Tsunoda T, Yue CT, Yang KC, Nakamura Y. Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas. Oncogene. 2002;21:4120–4128. doi: 10.1038/sj.onc.1205518. [DOI] [PubMed] [Google Scholar]
- 8.Sepulveda AR, Tao H, Carloni E, Sepulveda J, Graham DY, Peterson LE. Screening of gene expression profiles in gastric epithelial cells induced by Helicobacter pylori using microarray analysis. Aliment Pharmacol Ther. 2002;16 Suppl 2:145–157. doi: 10.1046/j.1365-2036.16.s2.4.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Gwadry FG, Reinhold WC, Miller LD, Smith LH, Scherf U, Liu ET, Kohn KW, Pommier Y, Weinstein JN. Transcriptional regulation of mitotic genes by camptothecin-induced DNA damage: microarray analysis of dose- and time-dependent effects. Cancer Res. 2002;62:1688–1695. [PubMed] [Google Scholar]
- 10.Iizaka M, Furukawa Y, Tsunoda T, Akashi H, Ogawa M, Nakamura Y. Expression profile analysis of colon cancer cells in response to sulindac or aspirin. Biochem Biophys Res Commun. 2002;292:498–512. doi: 10.1006/bbrc.2002.6648. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DV, Rocke DM. Tumor classification by partial least squares using microarray gene expression data. Bioinformatics. 2002;18:39–50. doi: 10.1093/bioinformatics/18.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Nakeff A, Sahay N, Pisano M, Subramanian B. Painting with a molecular brush: genomic/proteomic interfacing to define the drug action profile of novel solid-tumor selective anticancer agents. Cytometry. 2002;47:72–79. [PubMed] [Google Scholar]
- 13.Takahashi Y, Nagata T, Ishii Y, Ikarashi M, Ishikawa K, Asai S. Up-regulation of vitamin D3 up-regulated protein 1 gene in response to 5-fluorouracil in colon carcinoma SW620. Oncol Rep. 2002;9:75–79. [PubMed] [Google Scholar]
- 14.Hegde P, Qi R, Gaspard R, Abernathy K, Dharap S, Earle-Hughes J, Gay C, Nwokekeh NU, Chen T, Saeed AI, et al. Identification of tumor markers in models of human colorectal cancer using a 19,200-element complementary DNA microarray. Cancer Res. 2001;61:7792–7797. [PubMed] [Google Scholar]
- 15.Fujita M, Furukawa Y, Tsunoda T, Tanaka T, Ogawa M, Nakamura Y. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the beta-catenin/T-cell factor complex, in colorectal carcinomas. Cancer Res. 2001;61:7722–7726. [PubMed] [Google Scholar]
- 16.Yanagawa R, Furukawa Y, Tsunoda T, Kitahara O, Kameyama M, Murata K, Ishikawa O, Nakamura Y. Genome-wide screening of genes showing altered expression in liver metastases of human colorectal cancers by cDNA microarray. Neoplasia. 2001;3:395–401. doi: 10.1038/sj.neo.7900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinheiro NA, Caballero OL, Soares F, Reis LF, Simpson AJ. Significant overexpression of oligophrenin-1 in colorectal tumors detected by cDNA microarray analysis. Cancer Lett. 2001;172:67–73. doi: 10.1016/s0304-3835(01)00625-5. [DOI] [PubMed] [Google Scholar]
- 18.Takemasa I, Higuchi H, Yamamoto H, Sekimoto M, Tomita N, Nakamori S, Matoba R, Monden M, Matsubara K. Construction of preferential cDNA microarray specialized for human colorectal carcinoma: molecular sketch of colorectal cancer. Biochem Biophys Res Commun. 2001;285:1244–1249. doi: 10.1006/bbrc.2001.5277. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem. 2001;276:29681–29687. doi: 10.1074/jbc.M103779200. [DOI] [PubMed] [Google Scholar]
- 20.Kitahara O, Furukawa Y, Tanaka T, Kihara C, Ono K, Yanagawa R, Nita ME, Takagi T, Nakamura Y, Tsunoda T. Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Res. 2001;61:3544–3549. [PubMed] [Google Scholar]
- 21.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 22.Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, Frierson H, Powell SM, Knuutila S, Kallioniemi A, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–2629. [PubMed] [Google Scholar]
- 23.Selaru FM, Xu Y, Yin J, Zou T, Liu TC, Mori Y, Abraham JM, Sato F, Wang S, Twigg C, et al. Artificial neural networks distinguish among subtypes of neoplastic colorectal lesions. Gastroenterology. 2002;122:606–613. doi: 10.1053/gast.2002.31904. [DOI] [PubMed] [Google Scholar]
- 24.Mori M, Mimori K, Yoshikawa Y, Shibuta K, Utsunomiya T, Sadanaga N, Tanaka F, Matsuyama A, Inoue H, Sugimachi K. Analysis of the gene-expression profile regarding the progression of human gastric carcinoma. Surgery. 2002;131:S39–S47. doi: 10.1067/msy.2002.119292. [DOI] [PubMed] [Google Scholar]
- 25.Hippo Y, Taniguchi H, Tsutsumi S, Machida N, Chong JM, Fukayama M, Kodama T, Aburatani H. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002;62:233–240. [PubMed] [Google Scholar]
- 26.Maeda S, Otsuka M, Hirata Y, Mitsuno Y, Yoshida H, Shiratori Y, Masuho Y, Muramatsu M, Seki N, Omata M. cDNA microarray analysis of Helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem Biophys Res Commun. 2001;284:443–449. doi: 10.1006/bbrc.2001.5006. [DOI] [PubMed] [Google Scholar]
- 27.Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, Trent JM, Dalton WS, Chin KV. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000;60:4161–4166. [PubMed] [Google Scholar]
- 28.Sgroi DC, Teng S, Robinson G, LeVangie R, Hudson JR, Elkahloun AG. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res. 1999;59:5656–5661. [PubMed] [Google Scholar]
- 29.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, Jeffrey SS, Botstein D, Brown PO. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Selaru FM, Yin J, Zou TT, Shustova V, Mori Y, Sato F, Liu TC, Olaru A, Wang S, et al. Artificial neural networks and gene filtering distinguish between global gene expression profiles of Barrett's esophagus and esophageal cancer. Cancer Res. 2002;62:3493–3497. [PubMed] [Google Scholar]
- 31.Selaru FM, Zou T, Xu Y, Shustova V, Yin J, Mori Y, Sato F, Wang S, Olaru A, Shibata D, et al. Global gene expression profiling in Barrett's esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene. 2002;21:475–478. doi: 10.1038/sj.onc.1205111. [DOI] [PubMed] [Google Scholar]
- 32.Kihara C, Tsunoda T, Tanaka T, Yamana H, Furukawa Y, Ono K, Kitahara O, Zembutsu H, Yanagawa R, Hirata K, et al. Prediction of sensitivity of esophageal tumors to adjuvant chemotherapy by cDNA microarray analysis of gene-expression profiles. Cancer Res. 2001;61:6474–6479. [PubMed] [Google Scholar]
- 33.Kan T, Shimada Y, Sato F, Maeda M, Kawabe A, Kaganoi J, Itami A, Yamasaki S, Imamura M. Gene expression profiling in human esophageal cancers using cDNA microarray. Biochem Biophys Res Commun. 2001;286:792–801. doi: 10.1006/bbrc.2001.5400. [DOI] [PubMed] [Google Scholar]
- 34.Hu YC, Lam KY, Law S, Wong J, Srivastava G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res. 2001;7:2213–2221. [PubMed] [Google Scholar]
- 35.Lu J, Liu Z, Xiong M, Wang Q, Wang X, Yang G, Zhao L, Qiu Z, Zhou C, Wu M. Gene expression profile changes in initiation and progression of squamous cell carcinoma of esophagus. Int J Cancer. 2001;91:288–294. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1063>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


