Abstract
AIM: To evaluate the preliminary effects of comprehensive prevention of gastric cancer in Zhuanghe County epidemiologically.
METHODS: Stratified sampling and cluster sampling were applied to define the intervention group and the control group. The prospective cohort study was used for evaluating the effect of preventing gastric cancer. The relative risk (RR) and attributable risk percent (AR%) of intervention on gastric cancer death were calculated. Potential years of life lost (PLYY) of the disease was analyzed, and the RR and AR% of PYLL were calculated. Survival analysis was applied among the screened patients.
RESULTS: In the first 4 years after intervening, the relative risk (RR) of intervention on death was 0.5059 (95%CI: 0.3462-0.7392, P < 0.05) with significance statistically. AR% of the intervention on death was 49.41%. The RR of intervention on cumulative PYLL was 0.6778 (95%CI: 0.5604-0.8198, P < 0.05) with statistic significance. AR% of the intervention on cumulative PYLL was 30.32%. The four-year survival rate of the screened patients was 0.6751 (95%CI: 0.5298-0.9047).
CONCLUSION: The initiative intervention results showed that the intervention approach used in the trial was effective, it reduced mortality and increased survival rate, and alleviated the adverse effect of gastric cancer on the health and life of screened population.
INTRODUCTION
Gastric cancer is one of the most common malignant tumor, its prevalence in 1999 was 138.60 × 10-5 according to the report of WHO[1], however, its prevalence (300.87 × 10-5) and mortality rate (29.31 × 10-5) are even higher in China[1]. The incidence of gastric cancer has been declining globally during the recent decades, but not in China[2]. It was believed that the main reasons of the declining were prevention and diagnosis, but not therapy[3]. Most studies show that the incidence of gastric cancer is related to dietary factors, infection of helicobacter pylori(Hp), etc.[4-9]. If these relative factors were eliminated or decreased, the incidence and mortality of gastric cancer would be lowered., thus gastric cancer could be prevented[10-13]. So it is very important to find an effective comprehensive preventive method for gastric cancer.
Zhuanghe is a county in Liaoning Province with total population of approximate 900000, locates along the seaside of the Huanghai Sea. The previous survey showed that the incidence of gastric cancer in this county was higher than the average level of other areas. In 1994, the mortality rate was 49.55 × 10-5 in male, and 22.23 × 10-5 in female The population in Zhuanghe County has been surveyed in census and studied for 18 years by the Stomach Investigation Group of China Medical University because of higher incidence and mortality of gastric cancer. A series of comprehensive preventive approaches have been applied since 1997, including primary and secondary prevention. The goal was to explore a series of comprehensive preventive approaches to reduce the incidence and the mortality of gastric cancer. The initiative effect of intervention approaches by epidemiological study was evaluated in this paper.
MATERIALS AND METHODS
Definition of sample
Stratified sampling and cluster sampling were applied to define the intervention group and the control group. Each village in this county was stratified into two groups according to location geographically close seaside or not, then the sampled villages were defined by cluster sampling in the two groups, and the number of the sampled villages in each group was proportional to its location stratification. All individuals of the sampled villages were used as the samples.
Sixteen villages were sampled as the intervention group, other 14 villages were used as the control based on location, economic level, and proportion of population in each intervention village. 63133 persons from 30 villages, 16870 in the intervention group, and 14900 in the control group were observed, respectively. The age and sex were proportional in each group.
Intervention approaches
The design of the intervention was as the following (Figure 1).
Figure 1.
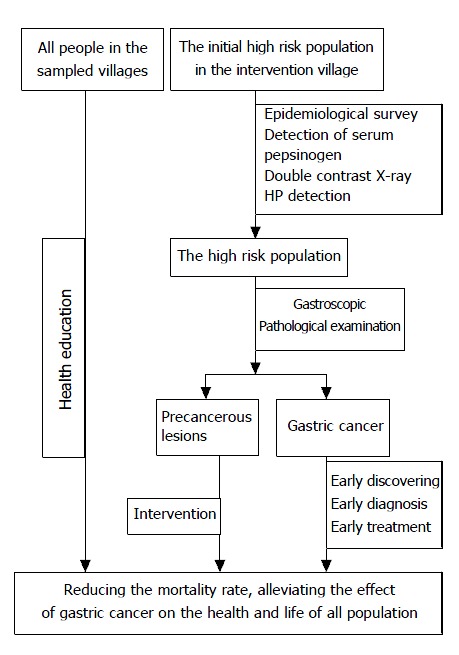
The intervention trial sketch.
Common approaches in intervention and control group Knowledge of prevention and treatment of gastric cancer, especially dietary habits was provided for all residents through broadcasting, video, brochures and face-to-face conversation.
Approaches in intervention groups Initially 3033 persons were selected as suspected high risk population from intervention villages according to the epidemiological survey and clinical symptoms. Persons who had the family history of gastric cancer, and/or were over 35 years old and had history of gastric illness, or had obvious symptoms of gastric illness were grouped as suspected high risk population, from which l781 persons were further detected and grouped as high risk population of gastric cancer[16-18], and were treated with antibiotics, Chinese herb medicine, and nutritional therapy, based on X-ray, HP detected, and gastroscopic and pathological examination[19-21]. Patients with gastric cancer screened from the high risk population were treated promptly.
The whole process of screening and treatment was performed under strict quality control. Twenty five percent of intervened individuals were randomly selected and checked for the compliance to medication by interviewing with individuals and their family and measuring the metabolites of medicine in urine, results showed that the compliance rate was 96.6%. The screening and surveying were initiated in 1997 and had been processed successfully since then.
Analysis of data
The prospective cohort study was applied to observe the samples. The cause of all death including gastric cancer from 1996 to 2000 were documented in both intervention group and control groups. The screened patients with gastric cancer were observed and reported annually.
The relative risk (RR) and attributable risk percent (AR%) of intervention on death of gastric cancer, potential years of life lost (PLYY) of the disease, and the RR and AR% of PYLL were calculated. The survival rate of all the screened patients with gastric cancer was evaluated.
The maximum expectancy method was adopted to calculate PYLL[22].
PYLL = ∑aidi, ai = L-x-0.5
L: the upper limit age, evaluated at 70.
x: the middle value of age interval.
ai: the residual years from age interval (i-i+1) to the maximum expect age (L).
d: the death number of age interval (i-i+1) from the disease.
PYLL = (PYLL/N)*1000‰.
N: the number of total observed population or person-years. Life-table method and product-limit method[23] were applied to analyze the survival rate of all the screened patients with gastric cancer. S(t) = II (1-di/ni)
ni: the adjusted population at the beginning of the period i.
di: the death number of the period i.
RESULT AND ANALYSIS
General
The composition of the sample is shown in Table 1.
Table 1.
Composition of the samples
| Male | Female | Total | |
| Intervention group | 26922 | 26256 | 53178 |
| Control group | 23840 | 23924 | 47788 |
| Total | 50762 | 50204 | 100966 |
During the first screening in 1997, 32 patients with gastric cancer were diagnosed, in which 18 patients were early gastric cancer, and 14 were advanced gastric cancer. The incidence of cancer in the screened group was 1.80% (32/1781). In addition, 1306 persons were detected having other gastric diseases. The screening rate of all gastric diseases was 75.13% (1338/1781), therefore the screening method was very effective. From 1998 to 1999, 12 patients with gastric cancer were detected, in which 7 patients were early gastric cancer, and 5 were advanced gastric cancer.
Mortality of gastric cancer
The mortality rate of gastric cancer in Zhuanghe from 1996 to 2000 was between 45.21 × 10-5-63.29 × 10 -5, the annual rate was 53.24 × 10-5 (person·year)-1, whose 95%CI is 44.57 × 10-5-61.91 × 10-5, which is higher than the average rate in China (29.31 × 10-5). The rate in male was 75.11 × 10-5 (95%CI: 60.60 × 10-5-89.61 × 10-5), and in female was 30.08 × 10-5 (95%CI: 20.98 × 10-5-39.18 × 10-5). The death from gastric cancer was 8.25% of all death from any reasons, and 38.94% of all carcinoma deaths, which were higher than that of the national average level (P < 0.05)[24-26].
The annual mortality rate of gastric cancer in Zhuanghe was shown in Figure 2. The alterations of mortality from 1996 to 2000 were not statistically significant.
Figure 2.
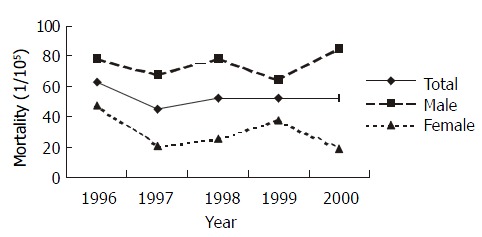
Mortality of gastric cancer in Zhuanghe County from 1996 to 2000.
The annual mortality rates of gastric cancer in intervention groups were shown in Figure 3. The rates in the period from 1997 to 2000 were lower than that in 1996 with no significance by Poison test[27] (P > 0.05 except that in male in 2000. In contrast, the average mortality rate (34.97 × 10-5 ) in intervention groups during post-intervention period (1997-2000) was lower significantly than that (59.31 × 10-5) in pre-intervention period (1996) (one-sided test, χ2 = 2.930, P < 0.05). However, no statistical decrease of mortality was found in control groups (Figure 4).
Figure 3.
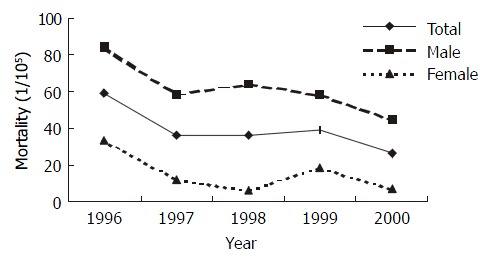
Mortality of gastric cancer in intervention groups from 1996 to 2000.
Figure 4.
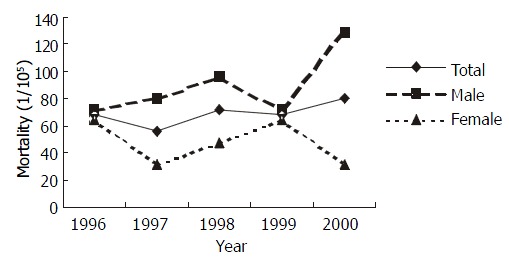
Mortality of gastric cancer in control groups from 1996 to 2000.
The tendency of descending in mortality of gastric cancer was observed (Figure 3). The linear correlation analysis is shown in Table 2. No obvious alteration in the mortality of gastric cancer in control group from 1996 to 2000 was observed.
Table 2.
Linear correlation in gender of gastric cancer mortal-ity in intervention groups
| Group | r | F | P |
| Male | 0.8688 | 9.2369 | 0.0559 |
| Female | 0.6690 | 2.4308 | 0.2169 |
| Total | 0.8148 | 5.9256 | 0.0930 |
The difference of the mortality rate of gastric cancer between the intervention group and the control group in 1996 was not significant (χ2 = 0.0283, P > 0.05). The annual average rates between the intervention group and the control group from 1997 to 2000 differed significantly (χ2 = 5.873, P < 0.05) (Figure 5).
Figure 5.
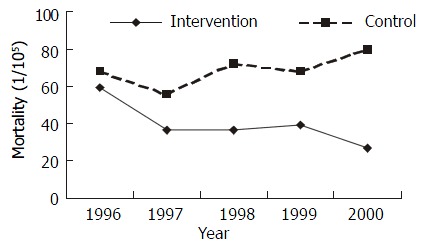
Mortality rate in intervention and control groups from 1996 to 2000.
Relative risk analysis
One hundred and ten patients in both groups had died from gastric cancer since the beginning the trial in 1997. The cumulative mortality rate (CMR) was 34.97 × 10- 5 in intervention villages, and 69.13 × 10-5 in control villages. The relative risk (RR) of intervention was 0.5059 (95%CI: 0.3462-0.7392, P < 0.05) with statistic significance. The attributable risk percent of the intervention was 49.41%, which indicated that intervention reduced gastric cancer death by 49.41% of all population. All these data showed that the intervention approaches can reduce the death of gastric cancer (Table 3).
Table 3.
Cumulative mortality rate and relative risk
| Total person-year | Death No | CMR (10-5) | RR | RR 95%CI | AR% | |
| Control | 99816 | 69 | 69.13 | |||
| Intervention | 117234 | 41 | 34.97 | 0.5059 | 0.3462-0.7392 | 49.41 |
Potential years of life lost in gastric cancer
From 1996 to 2000, the potential years of life lost (PYLL) of gastric cancer were 731 person-years, and the PYLL was 3.07‰, which meant the lost years of 1000 people from gastric cancer were 3.07 every year. In the intervention group, the PYLL was 300 person-years, and the PYLL was 2.89‰. The PYLL of the control group was 431 person-years, and the PYLL was 3.94‰ (Figure 6). The PYLL in intervention group in 1996 and 1998 was not significantly different from that in control group (χ2 = 1.3948, 0.2074, P > 0.05), and the differences in 1997, 1999 and 2000 were statistically significant (χ2 = 5.0603, 73.2124, 11.4119, P < 0.05). Difference of the total PYLL from 1997 to 1999 between the intervention group and the control group was significant (χ2 = 16.1527, P < 0.01).
Figure 6.
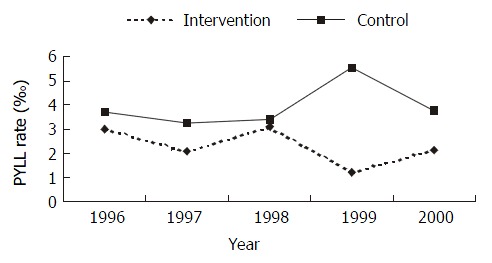
PYLL in intervention group and control group from 1996 to 2000.
The RR of intervention to cumulative PYLL was 0.6778 (95%CI: 0.5604-0.8198, P < 0.05), which was statistically significant. The AR% of the intervention to cumulative PYLL was 30.32%, which showed that the intervention decreased PYLL of gastric cancer by 30.32% of all population(Table 4).
Table 4.
PYLL and its RR
| Total person-year | PYLL | PYLL ( ‰ ) | RR | RR 95%CI | AR% | |
| Control | 87757 | 272 | 3.10 | |||
| Intervention | 103191 | 223 | 2.16 | 0.6778 | 0.5604-0.8198 | 30.32 |
Survival analysis
During the 4-year observation after intervening, 32 patients with gastric cancer were detected by screening, of which 9 patients died. The annual survival rates were shown in Table 5.
Table 5.
Annual survival rate and its 95%CI
| Year | Survival rate | Standard error | 95%CI of survival rate |
| 1- | 0.8305 | 0.0691 | 0.6951, |
| 2- | 0.7173 | 0.0852 | 0.5503, |
| 3- | 0.7173 | 0.0852 | 0.5503, |
| 4- | 0.6751 | 0.0956 | 0.5298, |
The one-year and three-year survival rate of the screened patients with cancer was 0.8305 (95%CI: 0.6951-0.9659), and 0.7173 (95%CI: 0.5503-0.8842), respectively, which are higher than those in Changle County of Fujian Province[28]. In this study, all screened patients were not operated due to some reasons; otherwise, the survival rate would be higher.
DISCUSSION
All intervention approaches were based on the following studies: (1) Many epidemiological and histological studies showed gastric cancer is related with infection of Helicobacter pylori, witch cause excessive hyperplasia and reduction of apoptosis of gastric epithelium. These cellular dynamic changes lead to injury of DNA and the generation of gastric cancer cell clone[29-33]. (2) Garlic oil can inhibit malignant proliferation of cancer cells, and induce their differentiation. The mechanism is likely to adjust the expression of oncogene. Garlic oil can restrain and reverse experimental gastric cancer and precancerous lesions[34,35]. (3) Surveys showed that cancer is correlated with precancerous lesions. Not all precancerous lesions will develop to cancers, but the generation of cancers may be prevented if the precancerous lesions are cared and cured early[36,37].
It was reported that gastric cancer was related with high-salt diet and preserved-food diet[14,15,38,39]. These dietary habits are extremely common in Zhuanghe County, and may be the main cause of gastric cancer[40]. Accordingly, health education for applying and maintaining right dietary habits was stressed in intervention and control groups.
The analysis of the mortality and PYLLs was focused on all the population instead of a single patient in evaluating the initiative effects of comprehensive preventive approaches. The intervention can reduce the mortality rate of patients with gastric cancer and alleviate the effect of gastric cancer on the health and life of all population. The analysis of PYLL endues the death with weight according to the age of the death, and age standardized mortality rate is applied, so this method reflects the “early death” and the “deferred death”. At the initial period of prevention, the decrease of the mortality may not be obvious, but the time on the mortality may be deferred, that is, the survival time of the patients may be prolonged due to early discovering, early diagnosis, and early treatment. It is more suitable to analyze and evaluate the initial effect of comprehensive prevention and treatment of gastric cancer than mortality analysis. Analysis of survival is based on patients with gastric cancer rather than the whole population, and thus it reflects whether the approaches have prolonged the life of patients with gastric cancer.
The results of the survey show that although local mortality rate of gastric cancer in Zhuanghe County is still higher than that of national level in China, the initial outcome of the intervention indicates that the intervention approaches used in the trial are effective, as these approaches have prolonged the screened patients’ life, reduced the mortality rate, and alleviated the adverse effect of gastric cancer on the health and life of all population screened. Because effect of chemical intervention effect is behind of the approaches, the initial outcome showed in this article is mostly due to secondary prevention, that is early discovering, early diagnosis and early treatment.
Footnotes
Supported by National Ninth Five-year Study Program for Taking Key Scientific Problems, No.96-906-01-04, and National Tenth Five-year Study Program for Taking Key Scientific Problems, No. 2001BA703B06(B)
Edited by Ren SY
References
- 1.The World Health Report 1999 of WHO. Available from: http://www.who.int/whr/1999/en/report.htm.
- 2.Li LM. Epidemiology. 4th ed. Beijing: People's Medical Publish-ing House. 2000:86–89. [Google Scholar]
- 3.Vutuc C, Waldhoer T, Haidinger G, Ahmad F, Micksche M. The burden of cancer in Austria. Eur J Cancer Prev. 1999;8:49–55. doi: 10.1097/00008469-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Inoue M, Tajima K, Kobayashi S, Suzuki T, Matsuura A, Nakamura T, Shirai M, Nakamura S, Inuzuka K, Tominaga S. Protective factor against progression from atrophic gastritis to gastric cancer--data from a cohort study in Japan. Int J Cancer. 1996;66:309–314. doi: 10.1002/(SICI)1097-0215(19960503)66:3<309::AID-IJC7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 7.Chyou PH, Nomura AM, Hankin JH, Stemmermann GN. A case-cohort study of diet and stomach cancer. Cancer Res. 1990;50:7501–7504. [PubMed] [Google Scholar]
- 8.Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24:33–41. doi: 10.1093/ije/24.1.33. [DOI] [PubMed] [Google Scholar]
- 9.Trédaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72:565–573. doi: 10.1002/(sici)1097-0215(19970807)72:4<565::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Harris A. Treatment of Helicobacter pylori. World J Gastroenterol. 2001;7:303–307. doi: 10.3748/wjg.v7.i3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DX, Fang DC, Li W, Du QX, Liu WW. A study on rela-tionship between infection of Helicobacter pylori and inactivation of antioncogenes in cancer and pre-cancerous lesion. Shijie Huaren Xiaohua Zazhi. 2001;9:984–987. [Google Scholar]
- 12.Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792–796. doi: 10.3748/wjg.v8.i5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lao SX, Chen GX. The traditional Chinese medicine study of pre-cancerous lesions of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:1117–1120. [Google Scholar]
- 14.Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, Kameya A, Kano T. A comparative case-control analysis of stomach cancer and atrophic gastritis. Cancer Res. 1990;50:6559–6564. [PubMed] [Google Scholar]
- 15.Beno I, Sigmundová V. [Nitrates and nitrites in gastric juice in chronic gastritis] Bratisl Lek Listy. 1993;94:531–535. [PubMed] [Google Scholar]
- 16.Sun LP, Yuan Y. Determinations of plasma pepsinogen levels and its application to diagnosis and treatment of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:1174–1176. [Google Scholar]
- 17.Hou P, Tu ZX, Xu GM, Gong YF, Ji XH, Li ZS. Helicobacter pylori vacA genotypes and cagA status and their relationship to associated diseases. World J Gastroenterol. 2000;6:605–607. doi: 10.3748/wjg.v6.i4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao XD, Liu WZ. Treatment to infection of Helicobacter pylor. Shijie Huaren Xiaohua Zazhi. 1999;7:3–4. [Google Scholar]
- 19.Wang W, Xia T, Zhang ZH. Effects of Yiweicongji Herbs on chronic atrophic gastritis and precancerous conditions. Shijie Huaren Xiaohua Zazhi. 1999;7:541–542. [Google Scholar]
- 20.Yao YL, Zhang WD. Relationship between Helicobacter pylor and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:1045–1049. [Google Scholar]
- 21.Shan ZW. Present situation of study on infection of Helicobacter pylor and expectation of its treament of the traditional Chinese medicine. Shijie Huaren Xiaohua Zazhi. 1998;6:553–554. [Google Scholar]
- 22.Ram F, Dhar M. A modified procedure for calculating person years of life lost. Janasamkhya. 1992;10:1–12. [PubMed] [Google Scholar]
- 23.Zhao ZT. Research Method and Application of Epidemiology. 1st ed. Beijing: Science Publishing house. 2000:543–545. [Google Scholar]
- 24.Health Statistical Information Center. The Annual Data of Na-tional Health in 1996. Beijing: Ministry of health China. 1997:308–310. [Google Scholar]
- 25.Health Statistical Information Center. The Annual Data of Na- tional Health in 1997. Beijing: Ministry of health China. 1998:308–310. [Google Scholar]
- 26.Health Statistical Information Center. The Annual Data of Na-tional Health in 1998. Beijing: Ministry of health China. 1999:308–310. [Google Scholar]
- 27.Jin PH. Medical Statistics. 1st ed. Shanghai: Publishing House of Shanghai Midecal University. 1993:349, 203. [Google Scholar]
- 28.Chen JG. [An epidemiological study on features of the distribution of cancer of some common sites] Zhonghua Liuxingbingxue Zazhi. 1986;7:193–199. [PubMed] [Google Scholar]
- 29.McNamara D, O'Morain C. Helicobacter pylori and gastric cancer. Ital J Gastroenterol Hepatol. 1998;30 Suppl 3:S294–S298. [PubMed] [Google Scholar]
- 30.Sud R, Wells D, Talbot IC, Delhanty JD. Genetic alterations in gastric cancers from British patients. Cancer Genet Cytogenet. 2001;126:111–119. doi: 10.1016/s0165-4608(00)00397-6. [DOI] [PubMed] [Google Scholar]
- 31.Chung YJ, Park SW, Song JM, Lee KY, Seo EJ, Choi SW, Rhyu MG. Evidence of genetic progression in human gastric carcinomas with microsatellite instability. Oncogene. 1997;15:1719–1726. doi: 10.1038/sj.onc.1201343. [DOI] [PubMed] [Google Scholar]
- 32.Xia HX, Fan XG, Talley NJ. Clarithromycin resistance in Helicobacter pylori and its clinical relevance. World J Gastroenterol. 1999;5:263–266. doi: 10.3748/wjg.v5.i3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- 34.Li XG, Xie JY, Lu YY. Suppressive action of garlic oil on growth and differentiation of human gastric cancer cell line BGC-823. Shijie Huaren Xiaohua Zazhi. 1998;6:10–12. [Google Scholar]
- 35.Su Q, Luo ZY, Teng H, Yin WD, Li YQ, He XE. Effect of garlic and garlic-green tea mixture on serum lipids in MNNG-induced experimental gastric cancer and precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1998;6:112–113. [PubMed] [Google Scholar]
- 36.Bajtai A, Hidvégi J. The role of gastric mucosal dysplasia in the development of gastric carcinoma. Pathol Oncol Res. 1998;4:297–300. doi: 10.1007/BF02905220. [DOI] [PubMed] [Google Scholar]
- 37.You WC, Zhang L, Gail MH, Li JY, Chang YS, Blot WJ, Zhao CL, Liu WD, Li HQ, Ma JL, et al. Precancerous lesions in two counties of China with contrasting gastric cancer risk. Int J Epidemiol. 1998;27:945–948. doi: 10.1093/ije/27.6.945. [DOI] [PubMed] [Google Scholar]
- 38.Tominaga K, Koyama Y, Sasagawa M, Hiroki M, Nagai M. A case-control study of stomach cancer and its genesis in relation to alcohol consumption, smoking, and familial cancer history. Jpn J Cancer Res. 1991;82:974–979. doi: 10.1111/j.1349-7006.1991.tb01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisani P, Oliver WE, Parkin DM, Alvarez N, Vivas J. Case-control study of gastric cancer screening in Venezuela. Br J Cancer. 1994;69:1102–1105. doi: 10.1038/bjc.1994.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, Gong W, Xu RT, Wang XJ, Gao H, Dong M, Wu HQ, Wang L, Wang MX, Song XJ, et al. Census Report of gastric cancer in Zhuanghe County, a high-risk area of gastric cancer. Shijie Huaren Xiaohua Zazhi. 1998;6(Supp1 7):478. [Google Scholar]


