Abstract
AIM: To detect the loss of heterozygosity (LOH) frequency of microsatellite sites D9s171, D9s1604 of p16 gene and expression of hMSH2 mRNA in various differentiated types of gastric cancer, adjacent cancer tissues and normal gastric mucosa.
METHODS: LOH was detected by polymerase chain reaction (PCR)-denaturing polyacrylamide gel electrophoresis-silver staining. The expression of hMSH2 mRNA was examined with in situ hybridization.
RESULTS: The frequency rate of LOH was significantly higher in gastric cancers than that in adjacent cancer tissues (P = 0.032). No significant difference was noted among various differentiated types and various clinical stages of gastric cancers. The significantly reduced expression of hMSH2 mRNA positive signal cells exhibited in gastric cancers, in comparison with that in the adjacent cancer tissues and normal gastric mucosa, respectively (P = 0.001). No significant difference was noted among various clinical stages of gastric cancers (P > 0.05). The difference of positive signal cells in poorly differentiated cancers and those in well and moderately differentiated cancers were significant (P < 0.001).
CONCLUSION: The frequencies of LOH in two microsatellite sites, D9s171 and D9s1604, in p16 genome were associated with development of gastric cancer and no significant correlation was demonstrated between the LOH frequency and the cell differentiated types of tumor cells or clinical stages. There was a positive relationship between the expression of hMSH2 mRNA and the differentiated types of gastric cancer.
INTRODUCTION
Microsatellite instability (MI) occurs frequently adjacent to the loci of tumor suppressor genes[1]. The defects of mismatch repair (MMR) gene are closely related to the occurrence of MI and abnormality of genes[2,3]. Expression of p16 gene was significantly reduced in gastric cancer[4-12] and was associated with the progression and metastasis[13,14]. The relationship between the MI of p16 gene in the gastric cancer and adjacent cancer tissue and the abnormal expression of MMR gene has rarely been reported. In this paper, two microsatellite loci, D9s171 and D9s1604 located at the upstream of p16 gene, were selected to study the loss of heterozygosity (LOH) of 9p21-22 region in gastric cancer tissues. The expression of hMSH2 mRNA in gastric cancer, adjacent cancer and normal gastric tissues was detected by in situ hybridization with hMSH2 oligonucleotide probe.
MATERIALS AND METHODS
Specimens
All the specimens were collected from the First and Second Affiliated Hospital of Medical College of Zhengzhou University and the People Hospital of Henan Province.
Specimens used to extract DNA: Specimens of gastric cancer tissue, adjacent cancer tissue and normal gastric mucosa were from each of 20 patients with gastric cancer. Of 20 patients, there were 4 cases with well differentiated and 16 moderately and poorly differentiated gastric cancer tissue. All specimens were used for isolation of DNA.
Specimens used in situ hybridization: gastric cancer specimens in 27 cases (including 20 cases gastric cancer specimens, with no history of radio- or chemotherapy preoperatively), adjacent cancer tissue specimens in 10 cases and normal gastric tissue specimens in 19 cases were used in situ hybridization. All the specimens were diagnosed pathologically (well differentiated in 5 cases, moderately 9 and poorly differentiated cancer 13). According to the PTNM of International Alliance of Anticancer in 1987, the specimens were divided into 4 clinical stages, the number in stage I, II, III and IV was 5, 10, 9 and 3, respectively.
Detection of microsatellite instability
The tissue DNA was extracted by routine phenol-chloroform method. The primers were synthesized by Shanghai Cell Biology Research Institute of China Scientific Institute and purified with PAGE. The sequence of primer was as follows, D9s171: 5’AGCTAAGTGAACCTCATCTCTGTCT3’, 5’ACCCTAGCACTGATGGTATAGTCT3’, and the length of amplified fragment was 159-177bp; D9s1604: 5’CCTGGGTCTCCAATTTGTCA3’, 5’AGCACATGACACTGTGTGTG3’, and the length of amplified fragment was 172-196bp. The annealing temperature of PCR was 60 °C and 50 °C, respectively. The PCR products were electrophoresized on 80 g/L denatured polyacrylamide gel under constant voltage of 30 V/cm. The gel was stained with silver staining after electrophoresis.
In situ hybridization
Digoxigenin-labeled hMSH2 oligonucleotide probe and BCIP/ NBT staining system were used to demonstrate the expression of hMSN2 mRNA. Control consisted of specimens pretreated with 0.05 g/L RNase A at 37 °C for 30 min, and specimens hybridized with hybridization buffer without probe.
Analysis of results
Compared with that of normal gastric mucosa removed from the same case, if the band of the identical allele disappeared or its intensity reduced over 50%, the result was defined as LOH positive. Specimens in situ hybridization observed under microscope, the cells with cytoplasm containing bluish violet granules were determined as positive signal cells. Five fields in each specimen was checked randomly.
Statistic analysis
Data of electrophoretic specimens were analyzed using Fisher’s exact test of probabilities with SPSS 10.0 statistic software. Correlative analysis were decided by using paired χ2 test for numerical samples and P < 0.05 was considered as significant difference. In situ hybridization specimens were treated using one way analysis of variance and P < 0.05 was considered as different significantly.
RESULTS
Results of LOH at D9s171 and D9s 1604 of p16 gene in gastric cancer and adjacent cancer tissues (Figures 1 and 2)
Figure 1.
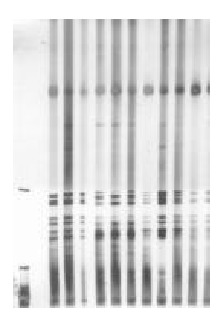
LOH of D9s171 in gastric cancer. Left 1: Marker Left 6: LOH( + ).
Figure 2.
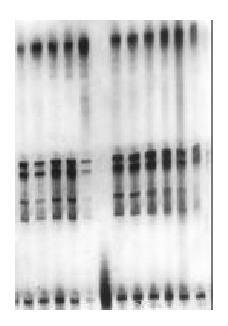
LOH of D9s1604 in gastric cancer. Left 6: LOH( + ).
The number of LOH at D9s171 and D9s1604 in cancer tissues in 20 cases was 3 and 10, respectively, and that in adjacent cancer tissues was 2 and 4, respectively. There was no significant difference between the ratio of LOH at the two microsatellite loci. However, the combined ratio of LOH at the two microsatellite loci in gastric cancer tissues was obviously higher than that in adjacent cancer tissue (P < 0.05) (Table 1).
Table 1.
LOH at D9s171 and D9s 1604 in gastric cancer and adjacent cancer tissue
| LOH(+) | LOH(-) | Total | |
| Gastric cancer tissue | 13 | 7 | 20 |
| Adjacent cancer tissue | 6 | 14 | 20 |
| Total | 19 | 21 | 40 |
aP < 0.05 vs adjacent cancer tissue.
Relationship between the ratio of LOH and the differentiated type and clinical stage of gastric cancer
The difference of incidence of LOH at D9s1604 in well differentiated adenocarcinoma (1/4) and that in moderately and poorly differentiated cancer (10/16) was not significant (P > 0.05). The proportion of LOH in early stage and progressive stage of cancer was 50% (4/8) and 58.2 (7/12), respectively.
Relationship between the LOH occurred at D9s171 and at D9s1604 in gastric cancer and adjacent cancer tissues
The incidence of LOH at D9s171 and D9s1604 were showed in Table 2. Analysis by paired χ2 test for numerical sample showed that an intrinsic relation exhibits between them.
Table 2.
Relation between LOH occurred at D9s171 and at D9s1604 in gastric cancer
| D9s1604 |
D9s171 |
Total | |
| + | - | ||
| + | 4 | 10 | 14 |
| - | 1 | 25 | 26 |
| Total | 5 | 35 | 40 |
+: LOH (+); -: LOH (-); aP < 0.05 vs LOH (-).
Expression of hMSH2 mRNA in normal gastric mucosa, gastric cancer and adjacent cancer tissues
The in situ hybridization positive signals of hMSH2 mRNA appeared as bluish violet granules distributed in the cytoplasm. No positive signals were found in nucleus. There were round or irregular granules in positive cells in normal gastric tissues. The number of positive signal cells increased from the superficial to deep layer of mucosa. A few positive signal cells scattered in the submucosa and no positive signal cells in muscular layer (Figure 3). Expression of hMSH2 mRNA in gastric cancer and adjacent cancer tissues was significantly decreased than that in normal gastric tissues (Table 3). The positive signal cells mainly scattered in the deep layer of mucosa. No positive signal cells were found in the submucosa and muscular layer (Figures 4 and 5).
Figure 3.
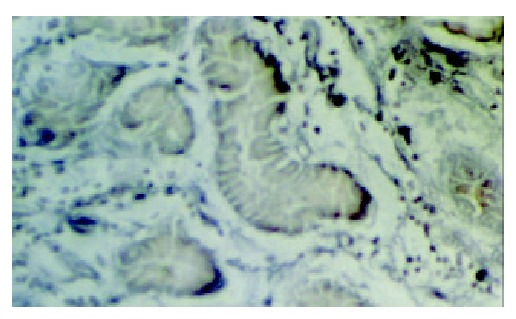
hMSH2 in normal gastric mucosa ( × 1000).
Table 3.
hMSH2 mRNA in the normal gastric mucosa, gastric cancer and adjacent cancer tissue (x ± s) (In situ hybridization)
| n | No. of positive cases (ratio) | No. of positive cells in each scope | |
| Normal gastric mucosa | 19 | 13(68.4%) | 175.8 ± 26.4a |
| Adjacent cancer tissue | 10 | 6(60%) | 99.7 ± 16.8b |
| Gastric cancer tissue | 27 | 20(74.1%) | 42.1 ± 25.9c |
P < 0.01 vs adjacent cancer tissue;
P < 0.01 vs gastric cancer tissue.
Figure 4.
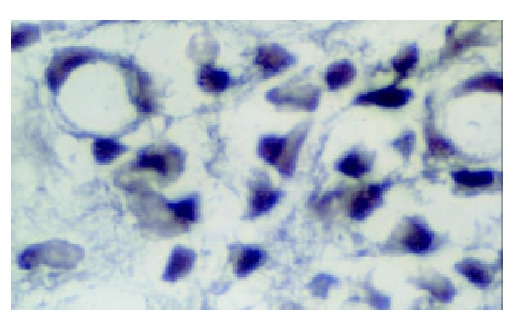
hMSH2 in adjacent gastric cancer tissue ( × 1000).
Figure 5.
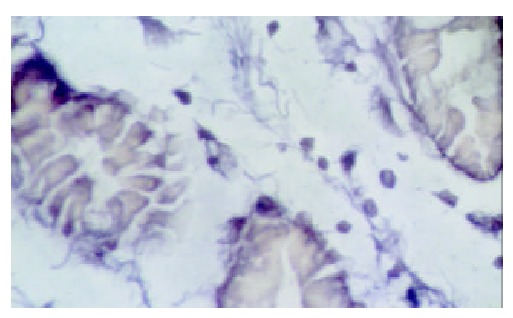
hMSH2 in gastric cancer tissue ( × 1000).
Expression of hMSH2 mRNA in various clinical stage of gastric cancer
Compared with that in the normal gastric tissue, the expression of hMSH2 mRNA in gastric cancer tissues was reduced significantly. However, there was no obvious difference in the number of positive cells among the various clinical stages of gastric cancer (P > 0.05) (Table 4).
Table 4.
hMSH2 mRNA in various clinical stage gastric cancer (In situ hybridization)
| Clinical stage | n | No. of positive cases | No. of positive cells | F value | P value |
| I | 5 | 4 | 62.8 ± 25.4 | 2.495 | 0.097 |
| II | 10 | 8 | 46.3 ± 24.4 | ||
| III | 9 | 6 | 32.3 ± 22.3 | ||
| IV | 3 | 2 | 13.5 ± 3.5 |
Expression of hMSH2 mRNA in various differentiated types of gastric cancer
Compared with that in the normal gastric tissue, the expression of hMSH2 mRNA in gastric cancer tissue was decreased significantly (P > 0.05). The number of positive signal cells differed among various differentiated types of gastric cancer. In poorly differentiated cancer tissue, the positive signal cells scattered in the middle and lower parts of mucosa, and the number of positive signal cells was smallest (Figure 6). There was a significant difference between the number of positive signal cells in the poorly differentiated gastric cancer tissue and that in the moderately and well differentiated gastric cancer tissue (Figures 7 and 8).
Figure 6.
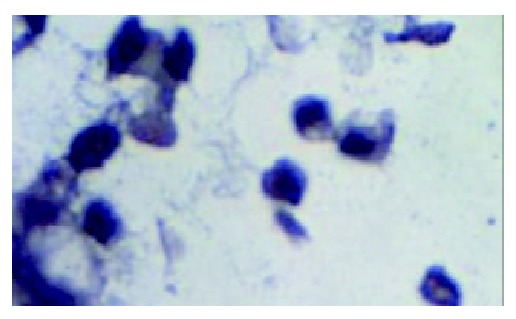
hMSH2 in poorly differentiated gastric cancer ( × 1000).
Figure 7.
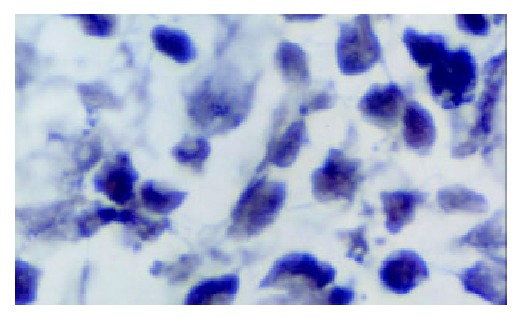
hMSH2 in moderately differentiated gastric cancer ( × 1000).
Figure 8.
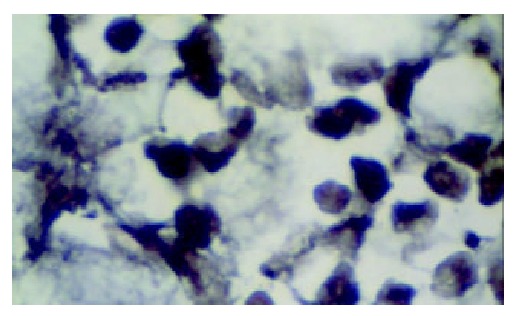
hMSH2 in well differentiated gastric cancer ( × 1000).
Negative reaction was showed in situ hybridization assay in control specimen.
DISCUSSION
Microsatellite instability of p16 gene
Microsatellite DNA is a genome-wide simple repeat sequence. Its normal length is shorter than 350bp, and the number of repeating is less than 60. The number of repeat unit of MI varied with individuals or tissues even in same body[15]. MI varied in different kinds of cancer[16-19]. In the gastric cancer tissues, MI was a frequent event, and the average frequency of MI was reported in different papers to be 33.9%, 32.1% and 25%, respectively[20-22]. The results showed that the LOH frequency of D9s171 and D9s1604 microsatellite loci, located at upstream of p16 gene, were 15% and 50%, respectively. The LOH frequency in well differentiated gastric cancer tissue was lower than that in the moderately and poorly differentiated gastric cancer tissue without significant difference (P > 0.05). The relation of MI and the differentiation degree of gastric cancer has been not known clearly[23,24].
It was showed that MI occurred at early stage of malignancy and gradually caused the formation of cancer[25,26]. The results in this study showed that the LOH of microsatellite loci exhibited 50% frequency at the early stage of gastric cancer, which suggested that the alteration of these loci might activate specific oncogenes and deactivate tumor suppressor genes, therefore, cause the development and progress of cancer. The alteration of microsatellite DNA normally appears as LOH. If LOH occurs frequently at the same locus of one chromosome in the tumor, the site of occurring LOH usually is the location of tumor suppressor gene[27]. Through the analysis of the loci adjacent to p16 gene, small losses ( < 200bp) of p16 gene could be found in many kinds of cancer, which might be one of deactivation mechanisms of p16 gene. In this paper, two microsatellite loci were selected to demonstrate MI, D9s171 located at the site between the upstream of p16 gene and an adjacent tumor suppressor gene p15, D9s1604 located at the site between the exon 1α and exon 1β of p16 gene. LOH was detected at both D9s171 and D9s1604, and correlation between the occurrence of MI at the D9s171 and at the D9s1604 was existed, which suggested that occurrence of MI at the two loci might be related molecular event.
Expression of hMSH2 mRNA
Mismatch repair gene superfamily belongs to housekeeping genes, and is able to correct unmatched or mismatched bases in the process of DNA replication and DNA damage repairing, and control the accuracy of replication and recombination. Up to date, 6 human mismatch repair genes have been found, including 3 homologous of bacterial MutS (hMSH2, hMSH6 and hMSH3) and 3 homologous of bacterial MutL (hMSH1, hPMS1 and hPMS2). Loss of hMSH2 protein existed in the colonic and other cancer[28-33] and genetic alterations in hMSH2 was observed in gastric cancer cell line[34]. Loss of hMSH2 protein in the cancer tissue indicated that hMSH2 peptide or its coding mRNA was at an instable state. The number of the positive signal cells of hMSH2 mRNA in normal gastric mucosa, in adjacent cancer tissue, and in gastric cancer tissue was 175.8 ± 26.4, 99.7 ± 16.8 and 42.1 ± 25.9, respectively. The number of positive signal cells was decreased significantly in adjacent cancer and gastric cancer tissue (P < 0.01) No significant difference was found in the number of the positive signal cells of hMSH2 mRNA at various clinical stages in gastric cancer. A correlation might exist between the instability of gene expression and the development of gastric cancer. Tumor development is a multi-step process of somatic cell mutation and colonial amplification. With the hMSH2 (or other mismatch repair genes) mutation and the cell proliferation, the instability of genome occurred, and then the mutator acted selectively at the mutated site, caused enlargement of genome instability in deepness and wideness, the accumulation of oncogene mutation was accelerated and caused the formation of tumor finally[35].
Expression of hMSH2 protein in 115 bladder cancers was studied with immunohistochemistry[36] and showed that low expression of hMSH2 protein exhibited in 25% cases and complete loss in 2 cases. A closely correlation existed between the decrease of hMSH2 mRNA and the recurrence of poorly differentiated cancer. The results in this work showed that expression of hMSH2 mRNA significantly decreased in gastric cancer tissues, especially in moderately and poorly differentiated cancer (P < 0.05). The results suggested that low expression of hMSH2 mRNA in poorly differentiated cancer might be related to the metastasis and prognosis of cancer. The lower the gastric cancer was differentiated, the more unstable the gene expression was. As the ratio of DNA mismatch increased, the instability of genome enhanced, tumor became more invasive and the prognosis got worse.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39170440
Edited by Ren SY
References
- 1.Yamamoto H, Sawai H, Perucho M. Frameshift somatic mutations in gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res. 1997;57:4420–4426. [PubMed] [Google Scholar]
- 2.Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090–1095. [PubMed] [Google Scholar]
- 3.Kang YJ, Wang LN, Zhang ZK. Microsatellite instability and DNA mismatch repair system. Shijie Huaren Xiaohua Zazhi. 2000;8:1139–1140. [Google Scholar]
- 4.Wang B, Shi LC, Zhang WB, Xiao CM, Wu JF, Dong YM. Expres-sion of tumor suppressor gene p16 in gastric cancer and precancerouslesions. Shijie Huaren Xiaohua Zazhi. 2001;9:39–42. [Google Scholar]
- 5.Zhou Y, Gao SS, Li YX, Fan ZM, Zhao X, Qi YJ, Wei JP, Zou JX, Liu G, Jiao LH, et al. Tumor suppressor gene p16 and Rb expression in gastric cardia precancerous lesions from subjects at a high incidence area in northern China. World J Gastroenterol. 2002;8:423–425. doi: 10.3748/wjg.v8.i3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515–521. doi: 10.3748/wjg.v7.i4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei TY, Wei MX, Yang SM. Expression of cyclin D1 P16 and Rb protein in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:234–235. [Google Scholar]
- 8.Zhu ZY, Tian X, Wang X, Yang XL. Mutation of p16 and APC gene in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1418–1419. [Google Scholar]
- 9.Yang SM, Yang LS, Li L, Deng LY, Wang CY, Yuan XB, Shen XD. Methylation of MTS1/P16 gene and expression of P16 pro-tein in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1427–1429. [Google Scholar]
- 10.Zhao Y, Zhang XY, Shi XJ, Hu PZ, Zhang CS, Ma FC. Expression of P16, P53 and proliferating cell nuclear antigen in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:246–248. [Google Scholar]
- 11.Li GX, Li GQ, Zhao CZ, Xu GL. Relationship between telomerase hTRT and the expression of tumor suppressor gene p53 and p16. Shijie Huaren Xiaohua Zazhi. 2002;10:591–593. [Google Scholar]
- 12.Jiang YX, Zhao MY, Geng M, Chao YC, Wang XY. Expression of P16, cerB-2 protein in gastric tumor. Shijie Huaren Xiaohua Zazhi. 2002;10:1050–1051. [Google Scholar]
- 13.Yang ZL, Li YG, Huang YF, Wang QW. Expression of cyclin D1, CDK4, P16 and Rbin gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:362–363. [Google Scholar]
- 14.Wang GT. Progression in the study on gastric precancerouslesions and its reversion. Shijie Huaren Xiaohua Zazhi. 2000;8:1–4. [Google Scholar]
- 15.Ji XL. Microsatellite instability: New point in gene study. Shijie Huaren Xiaohua Zazhi. 1999;7:372–374. [Google Scholar]
- 16.Spanakis NE, Gorgoulis V, Mariatos G, Zacharatos P, Kotsinas A, Garinis G, Trigidou R, Karameris A, Tsimara-Papastamatiou H, Kouloukousa M, et al. Aberrant p16 expression is correlated with hemizygous deletions at the 9p21-22 chromosome region in non-small cell lung carcinomas. Anticancer Res. 1999;19:1893–1899. [PubMed] [Google Scholar]
- 17.Niederacher D, Yan HY, An HX, Bender HG, Beckmann MW. CDKN2A gene inactivation in epithelial sporadic ovarian cancer. Br J Cancer. 1999;80:1920–1926. doi: 10.1038/sj.bjc.6690621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Zhang ZF, Reuter VE, Cordon-Cardo C. Chromosome 3 allelic losses and microsatellite alterations in transitional cell carcinoma of the urinary bladder. Am J Pathol. 1996;149:229–235. [PMC free article] [PubMed] [Google Scholar]
- 19.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Ke Y, Ning T, Feng L, Lu G, Liu W, E Z. [Studies of microsatellite instability in Chinese gastric cancer tissues] Zhonghua Yixue Yichuanxue Zazhi. 1998;15:155–157. [PubMed] [Google Scholar]
- 21.Fang DC, Zhou XD, Luo YH, Wang DX, Lu R, Yang SM, Liu WW. Microsatellite instability and the loss of heterologous of tumor suppressor gene in gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:479–481. [Google Scholar]
- 22.Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787–791. doi: 10.3748/wjg.v8.i5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han HJ, Yanagisawa A, Kato Y, Park JG, Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993;53:5087–5089. [PubMed] [Google Scholar]
- 24.Lin JT, Wu MS, Shun CT, Lee WJ, Wang JT, Wang TH, Sheu JC. Microsatellite instability in gastric carcinoma with special references to histopathology and cancer stages. Eur J Cancer. 1995;31A:1879–1882. doi: 10.1016/0959-8049(95)00349-n. [DOI] [PubMed] [Google Scholar]
- 25.Wirtz HC, Müller W, Noguchi T, Scheven M, Rüschoff J, Hommel G, Gabbert HE. Prognostic value and clinicopathological profile of microsatellite instability in gastric cancer. Clin Cancer Res. 1998;4:1749–1754. [PubMed] [Google Scholar]
- 26.Kim JJ, Baek MJ, Kim L, Kim NG, Lee YC, Song SY, Noh SH, Kim H. Accumulated frameshift mutations at coding nucleotide repeats during the progression of gastric carcinoma with microsatellite instability. Lab Invest. 1999;79:1113–1120. [PubMed] [Google Scholar]
- 27.Weissenbach J, Gyapay G, Dib C, Vignal A, Morissette J, Millasseau P, Vaysseix G, Lathrop M. A second-generation linkage map of the human genome. Nature. 1992;359:794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- 28.Jass JR, Iino H, Ruszkiewicz A, Painter D, Solomon MJ, Koorey DJ, Cohn D, Furlong KL, Walsh MD, Palazzo J, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43–49. doi: 10.1136/gut.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Børresen AL, Lothe RA, Meling GI, Lystad S, Morrison P, Lipford J, Kane MF, Rognum TO, Kolodner RD. Somatic mutations in the hMSH2 gene in microsatellite unstable colorectal carcinomas. Hum Mol Genet. 1995;4:2065–2072. doi: 10.1093/hmg/4.11.2065. [DOI] [PubMed] [Google Scholar]
- 30.Bock N, Meden H, Regenbrecht M, Jünemann B, Wangerin J, Marx D. Expression of the mismatch repair protein hMSH2 in carcinoma in situ and invasive cancer of the breast. Anticancer Res. 2000;20:119–124. [PubMed] [Google Scholar]
- 31.Leach FS, Hsieh JT, Molberg K, Saboorian MH, McConnell JD, Sagalowsky AI. Expression of the human mismatch repair gene hMSH2: a potential marker for urothelial malignancy. Cancer. 2000;88:2333–2341. [PubMed] [Google Scholar]
- 32.Parc YR, Halling KC, Burgart LJ, McDonnell SK, Schaid DJ, Thibodeau SN, Halling AC. Microsatellite instability and hMLH1/hMSH2 expression in young endometrial carcinoma patients: associations with family history and histopathology. Int J Cancer. 2000;86:60–66. doi: 10.1002/(sici)1097-0215(20000401)86:1<60::aid-ijc9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Chen HC, Bhattacharyya N, Wang L, Recupero AJ, Klein EA, Harter ML, Banerjee S. Defective DNA repair genes in a primary culture of human renal cell carcinoma. J Cancer Res Clin Oncol. 2000;126:185–190. doi: 10.1007/s004320050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin KH, Park JG. Microsatellite instability is associated with genetic alteration but not with low levels of expression of the human mismatch repair proteins hMSH2 and hMLH1. Eur J Cancer. 2000;36:925–931. doi: 10.1016/s0959-8049(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 35.Perucho M. Microsatellite instability: the mutator that mutates the other mutator. Nat Med. 1996;2:630–631. doi: 10.1038/nm0696-630. [DOI] [PubMed] [Google Scholar]
- 36.Jin TX, Furihata M, Yamasaki I, Kamada M, Liang SB, Ohtsuki Y, Shuin T. Human mismatch repair gene (hMSH2) product expression in relation to recurrence of transitional cell carcinoma of the urinary bladder. Cancer. 1999;85:478–484. [PubMed] [Google Scholar]


