Abstract
AIM: Oxidative stress participates in the cell carcinogenesis by inducing DNA mutations. Our aim was to assess whether ascorbic acid, an antioxidant, could have a role in preventing ROS (Reactive Oxygen Species) generation in experimental gastric carcinoma in a rat model.
METHODS: Experimental gastric cancer was induced in twelve Wistar male rats (weighting 250-350 g) by profound duodeno-gastric reflux throught split gastrojenunostomy. The rats were allocated to the following groups: Group I (n = 6) was the control; Group II (n = 6) which was mantained with daily intake of tape water with Vitamin C (30 mg/Kg). After 6 or 12 months, samples of gastric tumor or non tumor mucosa were taken from the anastomosis of both groups. Oxidative stress was measured by superoxide quantification through lucigenin-amplified chemiluminescence base and by staining with Nitrobluetetrazolium. The histopathologic confirmation of adenocarcinoma was made by eosin-hemathoxilin method.
RESULTS: The intestinal type of gastric adenocarcinoma was microscopically identified in all animals of group I whereas only 3 rats of group II showed an adenocarcinoma without macroscopic evidence of them. The cancers were located in the anastomosis in all cases. Basal luminescence from tumor gastric tissue generated 38.4 ± 6.8 count per minute/mg/x106 (mean ± SD) and 14.9 ± 4.0 count per minute/mg/x106, respectively, in group I and II animals (P < 0.05). The Nitrobluetetrazolium method showed intense staining in tumor tissues but not in non neoplasic mucosa.
CONCLUSION: Experimental gastric tumors seem to produce more reactive oxygen species than non neoplasic gastric tissue. The reduction of oxidative stress and gastric tumor incidence in rats were induced by the intake of ascorbic acid. Therefore, it may have a role in the prevention of gastric carcinoma.
INTRODUCTION
The pathogenesis of human gastric cancer is a multistep and multifactorial process[1]. The complex of cellular and molecular changes can be mediated by a diversity of endogenous and enviromental agents that include free radicals, H. pylori infection [2,3] bile reflux, intake of diets high salt and low consuption of fruits and vegetables[1]. Free radicals or reactive oxygen species (ROS) are low molecular weight metabolites reactive enough to damage essential biological molecules. The inability of the cell to scavenge ROS, overproduced by failure of the antioxidant systems, induced lesions in macromolecules such as DNA, proteins and lipids of cytosolic membranes. The release of ROS inside the nuclear membranes of the cell can damage the DNA, and there it induces mutations[8,9] which is one of bases of the carcinogenesis[4]. There are several mechanisms that could explain the relationships between the cell oxidative stress and the growth of tumors, such as proto-oncogene expression, generation of genotoxic products like 8-hydroxynonenal and convertion of procarcinogens to carcinogens[4].
Epidemiologic studies has shown that vitamin C dietary intake decreases the risk for gastric tumor development[5,6]. Both antioxidant property and the ability to react with nitrite make vitamin C a putative candidate agent in the prevention of of N-nitroso compound generation[5,7,8]. There were a number reports on the role of the ROS as the first step in cancer induction, but there were few on ROS generation in tumor tissue and on the possible properties of vitamin C in protecting the cell against tumor transformation using an intragastric bile reflux rat model. The objectives of this work were to determine what is the oxidative stress involvement in experimental gastric carcinogenesis induced by intragastric bile reflux and what are the effects of the ascorbic acid on ROS generation in this model.
MATERIALS AND METHODS
The animal experiments were carried out in accordance to the Institute of Experimental Animals of School of Medicine, University of São Paulo Guidelines for Care and Use of Laboratory Animals.
Study groups
Wistar male rats (weighting 250-350 g) were submitted to the surgical procedure and were randomilly allocated into two groups: Group I (n = 6) was the control animals; Group II (n = 6) was animals with daily oral administration (7 days per week) of Vitamin C (30 mg/Kg body weight) tape water solution. The total amount of vitamin C for 4 animals, in each cage, was dissolved in the total minimal volume of water intake that was previously measured for each animal. All rats were mantained in light and dark alternatively each phase for 12 h.
Surgical procedures
Experimental gastric cancer was induced by profound duodeno-gastric reflux in twelve Wistar rats underwent split gastrojejunostomy. The jejunum was divided at 5 cm distant from duodeno-jejunal angle. The afferent loop was anatomosed to the gastric body whereas the efferent jejunal loop was anastomosed in pre-pyloric antrum. The animals were killed at 6th month after the surgical procedure according with the appearence of weigth loss, intestinal obstruction, ascites, anemia and visible abdominal mass. When these signs did not appear, the animals were killed at the 12th month after the operation. Just after killing, three fragments of 5 mm2 each, were taken from the gastric tumor and from the gastric mucosa without tumor, at the same place on the anastomosis.
Lucigenin-amplified chemiluminescence assays
Each fragment was immersed in Krebs-HEPES buffer composition in mmol/L: NaCl 118.3; KCl 4.69; CaCl2 1.87; MgSO4 1.20; KH2PO4 1.03; NaHCO3 25.0; Glucose 11.1; Na-HEPES 20.0) at 37 °C, strictly maintained at pH 7.40, for at least 15 min. The gastric fragments were rapidly transfered to a counter vial, under light protection, and immersed in 2.0 mL of a solution of Krebs-HEPES buffer and 0.10 mol/L lucigenin (Sigma Chemicals). This lucigenin concentration was chosen because, instead of higher concentration ranges, it has been shown to reflect superoxide generation by tissues. Each fragment was counted for 10 min in a luminometer (Berthold Multi Biolumat). Vials containing the buffer and lucigenin alone were counted and these blank values were subtracted from the signals obtained from the tissue fragments. The counts were normalized for the dry weight of each fragment. The results were expressed as counts per min per mg. In some experiments the gastric fragments were hold for 45 min in a solution of superoxide dismutase (100 kU/L) before and during the counts. NADH (0.1 mol/L) or NADPH (0.1 mol/L) were added to the counter vials in some experiments.
Histochemistry with nitrobluetrazolium
Nitrobluetetrazolium (NBT) is a yellow dye that, under double reduction, generates insoluble bluish/black diformazan precipitates that are visible by light microscopy. NBT can be reduced directly by superoxide radicals, but it can also work as an electron acceptor during diaphorase enzyme activity. The gastric fragments (tumoral or not) were prepared as above, and immersed in a solution containing NBT (1.25 mg/mL), bovine serum albumin (17 mg/mL), NADH (0.1 mmol/L) and NADPH (0.1 mmol/L) for 30 minutes. The fragments were, then, fixed in 4% buffered formalin, pH = 7.40, for 24 hours. Afterwards, these specimens were then, embedded in paraffin, cut into 10 micra-thick slices and examined by light microscopy, without additional staining.
Hystology
The fragments previously fixed in 100 mL/L formalin, were dehydrated though increasing concentration of alcohol solutions, soaked in paraffin, cut into 5 mm -thick slices, stained with hematoxilin-eosin (HE) and examined by light microscopy.
Statistical analysis
The data are expressed as mean ± SEM. The values of differences between groups were compared using the Student test. P critical value of 0.05 was choosen to identified significant different means.
RESULTS
Each of the six rats of Group I at 6th month after surgery had two advanced gastric tumor (Figure 1A), each one on the side of gastric mucosa of both afferent and efferent anastomosis. The rats of Group II had no signs of gastric tumor. However, in three of them, an early well differenciated intestinal adenocarcinoma of the anastomosis was microscopically detected (Figures 1B). The stromal tissue of these tumors had plenty inflammatory cells. The three other rats had epithelial inflammation and intestinal metaplasia in the same place of the macroscopic tumors in the another three animals. The well differentiated patterns of tumors were similar in both groups.
Figure 1.
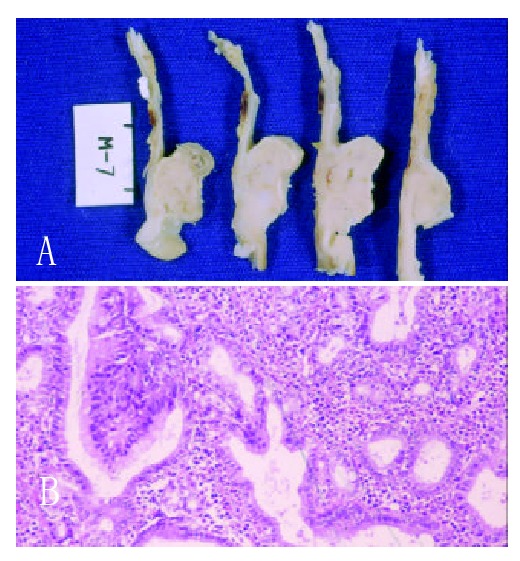
A. Microscopic appearance of the gastric tumor (Note the tubular pattern with variability of glomerular size, rudimentary papillary and nuclear atipia); B. Macroscopic appearance.
The values of basal luminescence were higher in Group I (38.4 ± 6.8 count per minute/per mg/ × 106) than in Group II (14.9 ± 4.0) (P < 0.05). The addition of NADPH to the substract increased the luminescence in the non visible tumor tissue of the rats of Group II more than tumor tissues of Group I. Addition of NADH, however, increased the luminescence production by tumors and non tumorous mucosa. The luminescence generation after addition of superoxide dismutase (SOD) was reduced in about 80% and 30% respectively, is the tumors and in non tumor gastric mucosa (Figure 2). Histochemical features produced by NBT showed insoluble bluish/black diformazan precipitates, visible with light microscopy in tumor tissue but not in the non tumoral tissues.
Figure 2.
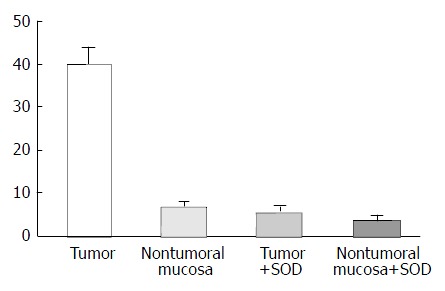
Lucigenin amplified-luminescence (count per minute × 106). Basal Luminescence in the tumor and non tumoral mucosa. The addition of SOD (100 kU/L) to the substrate de-creases the amplified-luminescence in visible tumoral mucosa and non tumoral gastric mucosa of rats.
DISCUSSION
The choice of this experimental design was based on our previous studies which demonstrated that the appearence of gastric cancer was evident in these models at least after the 6th month of the surgical procedure. Gastric carcinogenesis is a multifactorial event. Endogenous and environmental stimuli are involved like H. pylori infection and gastric accumulation of N-nitroso compounds[9-11]. Indeed there are evidences that oxidative stress participates in the carcinogenic cell transformation induced by DNA mutations[1,4]. Studies on populations at high risk to gastrointestinal cancer have shown that dietary antioxidants are able to reduce the prevalence rates of cancer and, therefore, seem to protect them against the carcinogenesis in the stomach[12,13]. In our rat model of profound duodeno-gastric reflux induced gastric adenocarcinomas within a 6-12 month period after the surgical procedure without antioxidant therapy. On the contrary, in animals with daily intake of vitamin C during the postoperative period, no obvious signs of gastric tumor were detected after 12 months. Though, in three of them, microscopic early gastric adenocarcinoma was found. Interestingly, the other three rats of this group remained without gastric carcinoma for as long as twelve months after the potential carcinogenetic surgical procedure. So, vitamin C seems to have a breaking role on the carcinogenetic process in the gastric mucosa under chronic and intense duodenal-gastric reflux. Probably this action might be due to the its antioxidant properties and the ability to react with nitrites, preventing the formation of gastric N-nitroso compounds.
The lucigenin-amplified luminescence method showed that the generation of ROS was higher in gastric neoplasic than in non neoplasic tissue. Macroscopic tumor in the rats of group I produced more luminescence than gastric tissue in the rats of group II without any visible tumor. These features suggest that increased ROS activity participates in gastric carcinogenesis during the development to the most advanced stages of the cancer. Indeed, the presence of ROS in non tumorous mucosa during the presence of cancer in the anastomosis suggests that is not possible to preclude the primary effect of the reflux that might induce cancer during the early stage of carcinogenetic process. The use of an antioxidant agent, such as vitamin C, can reduce the ROS generation and, thus tumor cell transformation. Recent studies have suggested that the gastric concentration of ascorbic acid is decreased in H.pylori infected patients[2,3], especially, in patients who had intestinal metaplasia and serious and extensive gastritis. This fact could be associated with the gastric carcinogenic mechanism. On the other hand, intragastric bile reflux seems to induce intestinal metaplasia in humans, but only in the presence of H.pylori gastritis[14]. In our previous studies using rats without H.pylori infection, metaplasia had preceeded the appearance of gastric adenocarcinoma. In the present study a relationship between intragastric bile reflux and oxidative stress could be observed. The use of vitamin C reduced the ROS generation that might have had a role in the tumoral cell transformation. The addition of NADPH induces superoxide generation by action of NADPH oxidase produced by activated neutrophils present in inflammatory tissues. The ROS generation in group I seems to be produced by the tumor and not by the inflammatory activity because: (1) there was no increase of luminescence when it was added to phorbol myristate acetate and (2) the luminescent response to NADH addition was higher than NADPH addition. However, in group II, the addition of NADPH increased the luminescent activity more than it did after the NADH addition. These observations are relevant, because, in gastric tissue with visible tumors, superoxide generation might be related to gastric adenocarcinoma but not to the inflammatory process. Notwithstanding, when the gastric tissue without visible tumors was exposed to NADPH, there was an increase in the luminescence measures, in spite of the inflammation present in the tissue samples. A relationship was also established between histochemical data and superoxide generation. Moreover, the addition of a superoxide blocker (superoxide dismutase) to the substrate of tumor tissues caused a 80% reduction of the luminescence generation.
As previously reported, the present study confirms the relationship between gastric superoxide anion generation and gastric carcinogenesis of the intestinal type, in rats. Vitamin C also seems to have a proctective role in the cell mutagenic factors by a retarding action upon the growth of gastric tumors. This putative protection may be explained by the reduction of oxidative stress in gastric tissue. Consequently, vitamin C could be used in further therapeutic approaches.
Footnotes
Edited by Xu JY
References
- 1.Correa P. A New Paradigm For Human Gastric Carcinogenesis. J Clin Gastroenterol. 2000;30:381–385. doi: 10.1097/00004836-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Nair S, Norkus EP, Hertan H, Pitchumoni CS. Micronutrient antioxidants in gastric mucosa and serum in patients with gastritis and gastric ulcer: does Helicobacter pylori infection affect the mucosal levels. J Clin Gastroenterol. 2000;30:381–385. doi: 10.1097/00004836-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Reed PI. Vitamin C, Helicobacter pylori infection and gastric carcinogenesis. Int J Vitam Nutr Res. 1999;69:220–227. doi: 10.1024/0300-9831.69.3.220. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut. 1998;43:322–326. doi: 10.1136/gut.43.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–356. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo JC, Bravo LE, Zarama G, Realpe JL. Review article: Antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther. 1998;12 Suppl 1:73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsubono Y, Tsugane S, Gey KF. Plasma antioxidant vitamins and carotenoids in five Japanese populations with varied mortality from gastric cancer. Nutr Cancer. 1999;34:56–61. doi: 10.1207/S15327914NC340108. [DOI] [PubMed] [Google Scholar]
- 8.Mowat C, Carswell A, Wirz A, McColl KE. Omeprazole and dietary nitrate independently affect levels of vitamin C and nitrite in gastric juice. Gastroenterology. 1999;116:813–822. doi: 10.1016/s0016-5085(99)70064-8. [DOI] [PubMed] [Google Scholar]
- 9.Dabrowska-Ufniarz E, Dzieniszewski J, Jarosz M, Wartanowicz M. Vitamin C concentration in gastric juice in patients with precancerous lesions of the stomach and gastric cancer. Med Sci Monit. 2002;8:CR96–C103. [PubMed] [Google Scholar]
- 10.You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607–1612. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2:88–94. doi: 10.1016/S1470-2045(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZW, Abdullahi M, Farthing MJ. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut. 2002;50:165–169. doi: 10.1136/gut.50.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs EJ, Connell CJ, McCullough ML, Chao A, Jonas CR, Rodriguez C, Calle EE, Thun MJ. Vitamin C, vitamin E, and multivitamin supplement use and stomach cancer mortality in the Cancer Prevention Study II cohort. Cancer Epidemiol Biomarkers Prev. 2002;11:35–41. [PubMed] [Google Scholar]
- 14.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 15.Dixon MF. Prospects for intervention in gastric carcinogenesis: reversibility of gastric atrophy and intestinal metaplasia. Gut. 2001;49:2–4. doi: 10.1136/gut.49.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]


