Abstract
AIM: To study the effects of transmitters ET, AgII, PGI2, CGRP and GG on experimental rat hepatic fibrosis and the antifibrogenic effects of IL-10.
METHODS: One hundred SD rats were randomly divided into 3 groups: control group (N): intraperitoneal injection with saline 2 mL·kg-1 twice a week; the fibrogenesis group (C): intraperitoneal injection with 50% CCl4 2 mL·kg-1 twice a week; IL-10 treated group (E): besides same dosage of CCl4 given, intraperitoneal injection with IL-10 4 μg·kg-1 from the third week. In the fifth, the seventh and the ninth week, rats in three groups were selected randomly to collect plasma and liver tissues. The levels of ET, AgII, PGI2, CGRP and GG were assayed by radioimmunoassay (RIA). The liver fibrosis was observed with silver staining.
RESULTS: The hepatic fibrosis was developed with the increase of the injection frequency of CCl4. The ET, AgII, PGI2, CGRP and GG levels in serum of group N were 71.84 ± 60.2 ng·L-1, 76.21 ± 33.3 ng·L-1, 313.03 ± 101.71 ng·L-1, 61.97 ± 21.4 ng·L-1 and 33.62 ± 14.37 ng·L-1, respectively; the levels of them in serum of group C were 523.30 ± 129.3 ng·L-1, 127.24 ± 50.0 ng·L-1, 648.91 ± 357.29 ng·L- 1, 127.15 ± 62.0 ng·L-1 and 85.26 ± 51.83 ng·L- 1, respectively; the levels of them in serum of group E were 452.52 ± 99.5 ng·L-1, 90.60 ± 44.7 ng·L-1, 475.57 ± 179.70 ng·L-1, 102.2 ± 29.7 ng·L-1 and 38.05 ± 19.94 ng·L-1, respectively. The histological examination showed that the degrees of the rats liver fibrosis in group E were lower than those in group C.
CONCLUSION: The transmitters ET, AgII, PGI2, CGRP and GG play a significant role in the rat hepatic fibrosis induced by CCl4. IL-10 has the antagonistic action on these transmitters and can relieve the degree of the liver fibrosis.
INTRODUCTION
Hepatic fibrosis is a disease which is characterized by an increase of type I and type III collagens, proteoglycans fibroneetin and hyaluronic acid in extracellular matrix (ECM) deposition[1-9]. It is an inevitable phase during the formation of liver cirrhosis, which is an irreversible stage of several liver pathological changes[10-12]. So it is important for how to prevent and cure hepatic fibrosis, i.e. antifibrogenetic treatment. Transmitters play an important role in the portal hypertention which is associated with the fibrosis[13,14]. In our study, the transmitters endothelin (ET), angiotensin II(Ag II), prostacyclin (PGI2), calcitonin-gene related peptide (CGRP) and glucagon (GG) were selected to explore their effects on hepatic fibrosis induced by CCl4 and the antifibrogenesis effect of interleukin-10 (IL-10) was explored as well.
MATERIALS AND METHODS
Animals
One hundred clean SD rats weighing 140-180 g were randomly divided into 3 groups. The control group (group N) included 24 rats; the fibrogenesis group (group C) included 40 rats and the IL-10 treated group (group E) included 36 rats, respectively. All the rats were breeding in the routine condition (room temperature 22 ± 2 °C, humidity 55% ± 5%, lighting 12hrs per day, to drink tap water and eat in any time when they needed, animal food was provided by BK company in shanghai.).
Establishment of the fibrosis model
Rats in group N were injected intraperitoneally with saline 2 mL·kg-1 twice a week. Rats in group C and group E were injected intraperitoneally with 50% CCl4 2 mL·kg-1 twice a week[15]. From the third week, rats in group E were injected intraperitoneally with IL-10 4 ug·kg-1 (dissolved in saline)[16] 20 minutes before they were injected with CCl4. All injections were performed in Monday and Thursday, rats’ body weight was recorded before the injection. In the fifth week, 3 rats in group C and 2 rats in group E died, in the seventh week, total 8 rats in group C and 4 rats in group E died, in the ninth week, total 10 rats in group C, 6 rats in group E and 3 rats in group N died. In 5,7,9 weeks, 10 rats of group C and E and 7 rats in the control group were selected randomly to collect their plasma and liver tissue samples.
Assessment of samples
The blood samples were added into the tubes with 30 μL 10% EDTA and 40 μL trasylol in ice bath, the tubes were centrifuged at 3000 rpm for 10 minutes at 4 °C, then the plasma was frozen for the assessment. The plasma levels of ET, Ag II, PGI2, CGRP and GG were assayed by radioimmunoassay (RIA, kits provided by EastAsia Immune-technology Institute, Beijing). Each plasma sample was taken 100 μL into the tube, then 200 μL buffer and 100 μL antiserum were added into each sample, they were agitated and incubated for 24 hour at 4 °C; then 100 μL 125I-marked serum was added, agitated and incubated for 24 hour at 4 °C; also 500 μL precipitation was added, after incubation for 20 min at room temperature, the tubes were centrifuged at 3500 rpm for 25 min at 4 °C, the upper layer was carefully removed, the cpm account was measuredusing γ radioimmunocounter. The blank control and the standard control was measured respectively at the same time. The liver tissue was made of paraffin section with silver staining.
Statistical analysis
All data were expressed as -x ± s, t test was used for comparison between groups.
RESULTS
Plasma levels of ET, AgII, 6-K-PGF1α , CGRP and GG
The plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG in group C were higher than those in the control (P < 0.05). After the intervention of IL-10, the levels of them were decreased, and had no difference with group N (P > 0.05). Furthermore, their levels were increased with the development of hepatic fibrosis.
Table 1 and Figure 1 showed that after the treatment of CCl4, the plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG were increased, their levels were significantly higher than those in the normal controls (P < 0.05). After treated with IL-10, their levels were obviously decreased, and there was no significant difference with those in the normal controls. It was showed that when the effective treatment was applied in the fibrosis rats, the levels of these transmitters showed the descending trend. It suggested that the levels of those transmitters were increased in liver fibrosis and they might play important pathogenic roles during the development of liver fibrosis.
Table 1.
Plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG in fibrosis and normal rats (ng·L-1)
| n | ET | AgII | 6-K-PGF1α | CGRP | GG | |
| N | 21 | 71.84 ± 60.2 | 76.21 ± 33.3 | 313.03 ± 101.71 | 61.97 ± 21.4 | 33.62 ± 14.37 |
| Ca | 30 | 523.30 ± 129.3 | 127.24 ± 50.0 | 648.91 ± 357.29 | 127.15 ± 62.0 | 85.26 ± 51.83 |
| Eb | 30 | 452.52 ± 99.5 | 90.60 ± 44.7 | 475.57 ± 179.70 | 102.2 ± 29.7 | 38.05 ± 19.94 |
P < 0.05 vs group N,
P < 0.05 vs group N.
Figure 1.
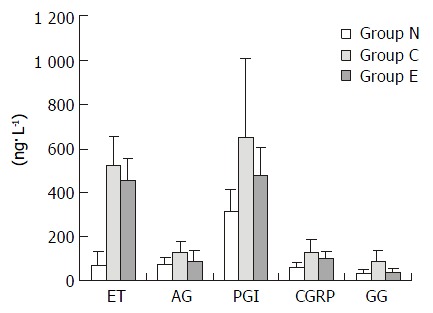
Plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG in fibrosis and normal rats.
Table 2 and Figure 2 showed that the levels of ET, AgII, 6-K-PGF1α, CGRP and GG were gradually increased and associated with the increase of CCl4-treated frequency, especially in the ninth week (P < 0.05). It suggested that there was close relation between the levels of the transmitters and the degrees of liver fibrosis.
Table 2.
Plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG in fibrosis rats (ng·L -1)
| Week | n | ET | AgII | 6-K-PGF1α | CGRP | GG |
| No.5 | 10 | 421.48 ± 52.3 | 105.73 ± 36.3 | 323.15 ± 76.2 | 88.68 ± 23.2 | 54.48 ± 18.9 |
| No.7 | 10 | 489.80 ± 87.7 | 131.42 ± 18.9 | 684.98 ± 214.0 | 118.14 ± 24.3 | 55.77 ± 19.2 |
| No.9 | 10 | 658.61 ± 102.3 | 144.58 ± 72.2 | 1081.61 ± 294.3 | 174.65 ± 87.7 | 141.66 ± 50.8 |
Figure 2.
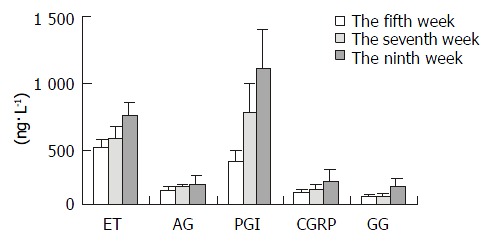
Plasma levels of ET, AgII, 6-K-PGF1α, CGRP and GG in fibrosis rats.
Pathological assay
The histological feature showed that liver of control rats had no appreciable alterations (Figure 3). The degree of liver fibrosis in group C was up-going with the increas of the treatment frequency of CCl4. In the fifth week, few reticular fiber deposited in the periportal tissue space. In the seventh week, the reticular fiber extended with hepatic plate but the full delimitation was not formed, while in the ninth week the integrity fibrous septum was developed in the interlobular septum, sometimes psedulobular could be seen (Figure 4, Figure 5, Figure 6). The degrees of inflammation of hepatocytes were decreased evidently in the seventh week after the treatment of IL-10, in the ninth week, the reticular fiber in the interlobular septum was limited remarkably, no psedulobular could be seen (Figure 7).
Figure 3.
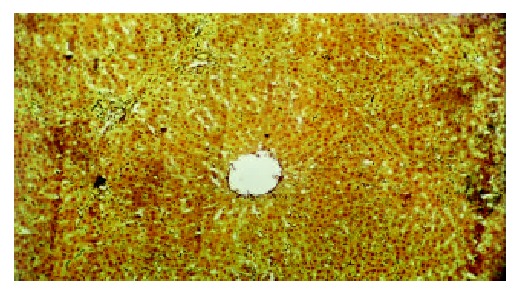
The liver of normal rat (silver staining, ×100).
Figure 4.

The liver of the rat in group C (the fifth week, silver staining, ×100).
Figure 5.
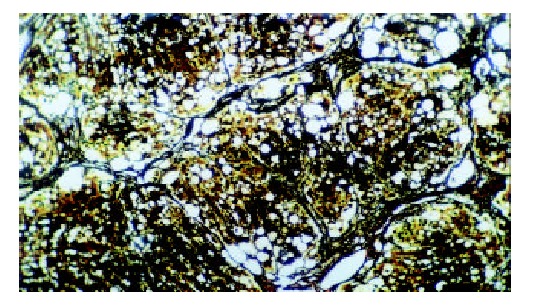
The liver of the rat in group C (the seventh week, silver staining, ×100).
Figure 6.
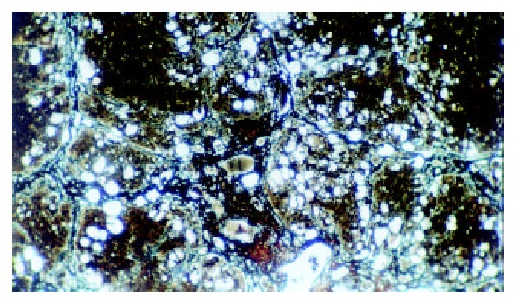
The liver of the rat in group C (the ninth week, silver staining, ×100).
Figure 7.
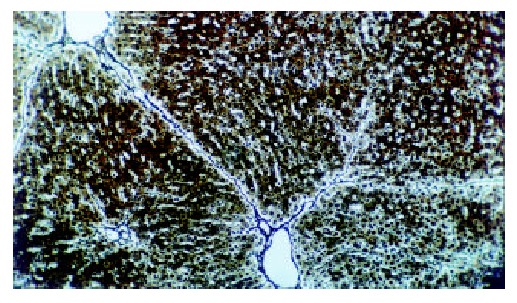
The liver of the rat in group E (the ninth week, silver staining, ×100).
DISCUSSION
Endothelins are a family of polypeptides consisting of 21-amino acids[17-19]. ET-1 is initially noted for its powerful vasoconstrictor properties[20-24]. It is markedly overexpressed in different cellular elements in cirrhotic liver tissue, and particularly in sinusoidal endothelial cells and hepatic stellate cells (HSCs) in their activated phenotype located in the sinusoids of the regenerating nodules and at the edges of fibrous septa[25]. It plays an important role in the regulation of hepatic vascular tone. They elicit biological responses via the ETA and ETB receptors. ET-1 induces contraction, proliferation, and collagen synthesis of HSCs in vitro, which may be mediated via the ETA receptors[26]. ET-1 is able to increase [Ca2+] i in a dose-dependent fashion in HSCs, which results from both intracellular release of Ca2+ and extracellular Ca2+ influx via a dihydropyridine-insensitive pathway. ET-1-induced contractility of HSCs is maintained through all stages of activation and is independent of the absolute number of ETA-binding sites if a threshold level of expression is maintained. It has been shown that ET-1 could act as a cell growth promoter via the ETA receptor to promote the proliferation of smooth muscle cell. Also, ET-1 is able to elicit MAPK (mitogen-activated protein kinase) activity in human HSCs with time-course and dose-response kinetics similar to those reported in mesangial cells through the ETA receptor. Recent studies have shown that the ETR antagonist modifies the development of portal hypertension in carbon tertrachloride treated rats[27,28]. Some studies suggest that ET has two effects on HSC[29]. ET can inhibit the contraction and collagen synthesis in cells that have more ETB receptors than ETA receptors; it indicates that ET could restrict the development of liver fibrosis. The difference is linked to the active, contractile HSC phenotype. The cellular sites of action of AgII within the hepatic vasculature are incompletely defined; recent studies have shown that HSCs may be a potential cell target for the AgII actions in the hepatic vasculature[30]. Two different types of AgII receptors have been described. The AT1 receptors are present in most mesenchymal cells and mediate most of the biological effects of AgII. The AT2 receptors are mainly found in fetal cells, but their physiological role is not completely understood. AgII receptors (AT1 subtype) exist in many cells, including the human HSC[31], the activated HSCs may be an important target of the AgII in the hepatic vasculature[32]. The binding of AgII to AT1 receptor induces contraction and proliferation[33,34]. AgII causes a marked increase in [Ca2+] i and cell contraction, which largely depends on the entrance of Ca2+ through L-type Ca2+ channels. In recent years, much attention has been focused on the growth-promoting effects of AgII and it has been found that AgII is also a mitogenic factor for activated HSCs through an MAPK- dependent pathway. So we could hypothesis that AgII plays a role in the proliferation of HSCs and in the progression of liver fibrosis. The inflammation may be the initial fibrogenic event. PGI2, a potent vasodilator produced by the splanchnic endothelium, would account for much of the observed hyperemia[35]. Cyclooxygenase blockade reverses the splanchnic hyperemia[36]. The mechanism for the increase of portal PGI2 remains unknown. Some have suggested of increase that blood pressure alone will increase the production of PGI2. Theoretically, damage to any type of liver cell membrane can serve as a source of AA metabolites that initiate fibrosis. In the intact liver, the most probable target cells are the nonparenchymal cells such as endothelial cells. The inflammation may be the initial fibrogenic event. The inflammation involving the release of arachidonic acid (AA) from phospholipids by activation of phospholipae A2 in damaged cell membranes and formation of bioactive AA metabolites (prostaglandins, thromboxane A2 and leukotrienes) by way of 5’lipoxygenase pathway is one of the earliest biochemical events in hepatic fibrosis. The concentration of 6-keto-PGF1α, the stable metabolite of PGI2, represents the plasma level of PGI2. The enhanced production of 6-keto-PGF1α increases the TGF-β1 gene expression by way of enhancing degranulation of platelets and inflammatory cells which are rich source of the fibrotic cytokine TGF-β1[37]. As we all know, TGF-β1 can promote the synthesis and deposition of ECM and inhibit the degradation of ECM[38,39]. CGRP is a highly potent vasodilator and is widely distributed in nerve fibers with relation to vascular structures[40]. The circulating CGRP is elevated in liver cirrhosis[41,42], but little information is known about CGRP in these patients[43]. Some authors have reported that CGRP could inhibit the lipid peroxidation on the liver, which antagonists the effects of ET[44]. So it is a protector in the liver fibrosis. Whether the CGRP has effects on the activation of HSC and the synthesis of collagen is not clarified. GG is a stress hormone whose release is stimulated by catecholamines, cortisol, and growth hormone[45]. GG plays an important role in the formation of portal hypertension[46]. The present studies show that plasma GG levels are elevated in cirrhotic patients with portal hypertension. It is also clearly demonstrated that plasma GG levels is increased with the progression of cirrhosis. In addition, positive correlations has been found between plasma GG levels and Pugh’s score or liver functions. In our study the increase of GG was associated with the failure of GG’s degradation in liver and the hyperexcretion of pancreas. IL-10 is a potent anti-inflammatory cytokine that inhibits the synthesis of pro-inflammatory cytokines by T helper type 1 cells. It is produced locally in the liver and acts in an autocrine or paracrine way. IL-10 can inhibit a range of macrophage effecter functions, including nitric oxide and reactive oxygen intermediate production, MHC class II antigen expression, and eicosanoid synthesis. IL-10 can down-regulate expression of adhesion molecules, ICAM-1 and B7, on human monocytes, and also the nuclear transcription factor, nuclear factor κB. It is able to inhibit chemokine synthesis in T cells, neutrophils, and fibroblasts . Moreover, proinflammatory cytokines synthesis by a wide range of cells, particularly monocytes and macrophages, is profoundly inhibited by IL-10[47]. Previous reports indicated that IL-10 had a role in the remodeling of the extracellular matrix[48]. In vitro, IL-10 down regulates collagen type I while up regulates metalloproteinase gene expression. It also has antifibrogenic properties by down regulating profibrogenic cytokines, like TGF-β1 and TNF-α[47,49]. Nelson et al[16] had treated 24 patients with chronic hepatitis C with IL-10, they found that IL-10 normalized serum ALT levels, decreased hepatic inflammation, reduced liver fibrosis and was well tolerated in patients.
After that treatment of IL-10, all of the transmitters decreased. Therefore, transmitters play important roles in rat hepatic fibrosis induced by CCl4. IL-10 decreases the levels of these transmitters so it has antifibrogenesis effect.
Footnotes
Supported by Natural Science Foundation of Fujian Province, No. C96042
Edited by Xu XQ
References
- 1.Nie QH, Cheng YQ, Xie YM, Zhou YX, Bai XG, Cao YZ. Methodologic research on TIMP-1, TIMP-2 detection as a new diagnostic index for hepatic fibrosis and its significance. World J Gastroenterol. 2002;8:282–287. doi: 10.3748/wjg.v8.i2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363–369. doi: 10.3748/wjg.v7.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng HL, Cai WM, Liu RH. Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol. 2001;7:42–48. doi: 10.3748/wjg.v7.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun DL, Sun SQ, Li TZ, Lu XL. Serologic study on extracellular matrix metabolism in patients with viral liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1999;7:55–56. [Google Scholar]
- 5.Chen PS, Zhai WR, Zhang YE, Zhang JS. The effects of hypoxia on hepatic stellate cell generate collagen and matrix metalloproteinase. Shijie Huaren Xiaohua Zazhi. 2000;8:586–587. [Google Scholar]
- 6.Liu SR, Gu HD, Li DG, Lu HM. A comparative study of fat stor-ing cells and hepatocytes in collagen synthesis and collagen gene expression. Xin Xiaohua Bingxue Zazhi. 1997;15:761–762. [Google Scholar]
- 7.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 8.Jiang HQ, Zhang XL. Mechanism of liver fiborsis. Shijie Huaren Xiaohua Zazhi. 2000;8:687–689. [Google Scholar]
- 9.Wang YJ, Sun ZQ, Quan QZ, Yu JJ. Fat-storing cells and liver fibrosis. Chin J New Gastroenterol. 1996;2:58–60. [Google Scholar]
- 10.Missale G, Ferrari C, Fiaccadori F. [Cytokine mediators in acute inflammation and chronic course of viral hepatitis] Ann Ital Med Int. 1995;10:14–18. [PubMed] [Google Scholar]
- 11.Wang YJ, Sun ZQ. The cytology and molecular biology investi-gate advance in liver fibrosis. Xin Xiaohua Bingxue Zazhi. 1994;2:244–246. [Google Scholar]
- 12.Wang FS, Wu ZZ. Current situation in studies of gene therapy for liver cirrhosis and liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:371–373. [Google Scholar]
- 13.Zhang LJ, Wang XZ. Liquid substance and portal hypertension. Shijie Huaren Xiaohua Zazhi. 2000;8:1280–1281. [Google Scholar]
- 14.Zhang LJ, Wang XZ, Huang YH, Chen ZX. The effects of CGRP, AgII and ET on the liver fibrosis rats. Shijie Huaren Xiaohua Zazhi. 2001;9:457–459. [Google Scholar]
- 15.Takahara T, Kojima T, Miyabayashi C, Inoue K, Sasaki H, Muragaki Y, Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988;59:509–521. [PubMed] [Google Scholar]
- 16.Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655–660. doi: 10.1016/s0016-5085(00)70134-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Ren XL. Endothelin, nitric oxide and liver cirrhosis. Chin J New Gastroenterol. 1996;4:40–41. [Google Scholar]
- 18.Cheng RC, Jin XL. The changes of plasma endothelin level in the patient with discompensation liver cirrhosis. Xin Xiaohua Bingxue Zazhi. 1995;3:110–111. [Google Scholar]
- 19.Li XR, Wu JS, He ZS, Ma QJ. The contents of endothelin in portal vein and peripheral blood of patients with portal hypertension of liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1998;6:827. [Google Scholar]
- 20.Liu F, Li JX, Li CM, Leng XS. Plasma endothelin in patients with endotoxemia and dynamic comparison between vasoconstrictor and vasodilator in cirrhotic patients. World J Gastroenterol. 2001;7:126–127. doi: 10.3748/wjg.v7.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298–300. doi: 10.3748/wjg.v6.i2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZY, Ren XL, Yao XX. Effects of endothelin and nitric ox-ide in hemodynamics disturbance of cirrhosis. Shijie Huaren Xiaohua Zazhi. 1998;6:588–590. [Google Scholar]
- 23.Chen S, Liu B, Cai XM, Gu CH. Clinical significance of changes of endothelin and nitric oxide levels in peripheral blood of pa-tients with severe hepatitis. Shijie Huaren Xiaohua Zazhi. 1999;7:122–124. [Google Scholar]
- 24.Chen YK. The significance of changes of endothelin in patients with severe hepatitis. Shijie Huaren Xiaohua Zazhi. 1998;6:157. [Google Scholar]
- 25.Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 26.Reinehr RM, Kubitz R, Peters-Regehr T, Bode JG, Häussinger D. Activation of rat hepatic stellate cells in culture is associated with increased sensitivity to endothelin 1. Hepatology. 1998;28:1566–1577. doi: 10.1002/hep.510280617. [DOI] [PubMed] [Google Scholar]
- 27.Sogni P, Moreau R, Gomola A, Gadano A, Cailmail S, Calmus Y, Clozel M, Lebrec D. Beneficial hemodynamic effects of bosentan, a mixed ET(A) and ET(B) receptor antagonist, in portal hypertensive rats. Hepatology. 1998;28:655–659. doi: 10.1002/hep.510280308. [DOI] [PubMed] [Google Scholar]
- 28.Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology. 2000;118:1169–1178. doi: 10.1016/s0016-5085(00)70370-2. [DOI] [PubMed] [Google Scholar]
- 29.Rockey D. Endothelin in hepatic fibrosis--friend or foe. Hepatology. 1996;23:1698–1700. doi: 10.1053/jhep.1996.v23.ajhep0231698. [DOI] [PubMed] [Google Scholar]
- 30.Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology. 1999;29:334–339. doi: 10.1002/hep.510290203. [DOI] [PubMed] [Google Scholar]
- 31.Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, Cheng JL, Xu QF. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: effects of RAS inhibitors on hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:824–828. doi: 10.3748/wjg.v6.i6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:540–545. doi: 10.3748/wjg.v6.i4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–1156. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 34.Görbig MN, Ginès P, Bataller R, Nicolás JM, Garcia-Ramallo E, Tobías E, Titos E, Rey MJ, Clària J, Arroyo V, et al. Atrial natriuretic peptide antagonizes endothelin-induced calcium increase and cell contraction in cultured human hepatic stellate cells. Hepatology. 1999;30:501–509. doi: 10.1002/hep.510300201. [DOI] [PubMed] [Google Scholar]
- 35.García-Pagán JC, Bosch J, Rodés J. The role of vasoactive mediators in portal hypertension. Semin Gastrointest Dis. 1995;6:140–147. [PubMed] [Google Scholar]
- 36.Yue QL, Zhang XK, Zhang XR. The changes of contents of nitrix oxide and prostaglandine in gastric mucosa and plasma of rats with portal hypertensive gastropathy. Shijie Huaren Xiaohua Zazhi. 1999;7:547. [Google Scholar]
- 37.Geraci JP, Mariano MS. Radiation hepatology of the rat: association of the production of prostacyclin with radiation-induced hepatic fibrosis. Radiat Res. 1996;145:93–97. [PubMed] [Google Scholar]
- 38.De Bleser PJ, Niki T, Rogiers V, Geerts A. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 1997;26:886–893. doi: 10.1016/s0168-8278(97)80257-7. [DOI] [PubMed] [Google Scholar]
- 39.Sun ZQ, Wang YJ. The regulate effect of soluble cytokines on liver fibrosis. Xin Xiaohua Bingxue Zazhi. 1994;2:163–164. [Google Scholar]
- 40.Schifter S. Expression of the calcitonin gene family in medullary thyroid carcinoma. Peptides. 1997;18:307–317. doi: 10.1016/s0196-9781(96)00169-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Wen QS, Huang YX, Zhong YX, Chu YQ, Wang QL. The effects of calcitonin gene-related peptide on portal vein pres-sure of rats with liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1998;6:933. [Google Scholar]
- 42.Liu CQ, Pu J, Li ZX, Liu XF, Zhao YT. The changes of plasma peptides in the patients with liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1999;7:1089. [Google Scholar]
- 43.Henriksen JH, Schifter S, Møller S, Bendtsen F. Increased circulating calcitonin in cirrhosis. Relation to severity of disease and calcitonin gene-related peptide. Metabolism. 2000;49:47–52. doi: 10.1016/s0026-0495(00)90663-2. [DOI] [PubMed] [Google Scholar]
- 44.Møller S, Bendtsen F, Schifter S, Henriksen JH. Relation of calcitonin gene-related peptide to systemic vasodilatation and central hypovolaemia in cirrhosis. Scand J Gastroenterol. 1996;31:928–933. doi: 10.3109/00365529609052004. [DOI] [PubMed] [Google Scholar]
- 45.Johnson TJ, Quigley EM, Adrian TE, Jin G, Rikkers LF. Glucagon, stress, and portal hypertension. Plasma glucagon levels and portal hypertension in relation to anesthesia and surgical stress. Dig Dis Sci. 1995;40:1816–1823. doi: 10.1007/BF02212707. [DOI] [PubMed] [Google Scholar]
- 46.Greco AV, Crucitti F, Ghirlanda G, Manna R, Altomonte L, Rebuzzi AG, Bertoli A. Insulin and glucagon concentrations in portal and peripheral veins in patients with hepatic cirrhosis. Diabetologia. 1979;17:23–28. doi: 10.1007/BF01222973. [DOI] [PubMed] [Google Scholar]
- 47.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–159. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 48.Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597–1606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 49.Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A, et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607–1615. doi: 10.1002/hep.510280621. [DOI] [PubMed] [Google Scholar]


