Abstract
AIM: To observe the preventive effects of combined therapy of AMD (allantoin, metronidazolem and dexamethasone in combination) on intra-abdominal adhesion in dogs.
METHODS: 20 dogs of both sexes were used in this study. After laparotomy under anesthesia, 2 cm section of cecal end was clamped and ligated, then 1 cm cecum section was cut and another 1 cm was kept. The cecum stump was closed with purse-string suture. Both parietal and visceral peritonea were stripped for an area of about 3 × 4 cm2. Before the skin closure, the animals were divided into two groups randomly. The abdominal cavities in Group AMD (n = 10) were rinsed by 200 mL of AMD solution, and with 50 mL left, whereas the control (n = 10) received the equal volume of normal saline. After 7 d, the degree of intra-abdominal adhesions was evaluated by using the score method of ultrasonography and traditional dissection.
RESULTS: Compared with the control, both the ultrasonography and traditional dissection scores in Group AMD were significantly decreased that marked as 2.0 ± 1.25 vs 3.3 ± 0.82 and 1.91 ± 0.83 vs 3.3 ± 0.82 respectively (P < 0.01).
CONCLUSION: The combined therapy of AMD is an effective way to prevent intra-abdominal adhesion, and ultrasonography is an useful tool to diagnose intra-abdominal adhesion.
INTRODUCTION
Intra-abdominal adhesions are almost inevitable to some extent after major abdominal surgery. Weibel and Majno reviewed 289 subjects at post-mortem who had had previous laparotomies, and 67% of the patients showed adhesions; after multiple operations, the incidence rose to 93%[1]. However, there has been little advances in the treatment and prevention of this complication in recent years. Numerous attempts with agents and surgical techniques often obtain conflicting results[2,3]. The cause may be mainly, at least partly, due to multi-factor in the adhesion etiology and multi-pathway in the adhesion mechanism[4-6], which made the adhesions difficult to be prevented by using a single drug or certain measures. Besides, the evaluation of intra-abdominal adhesion was based classically on traditional dissection method, which was impossible to be applied to the clinical settings. In this study, we employed a new reproducible animal model of intra-abdominal adhesion cased by multi-factors, and a new evaluation method by ultrasonography to assess the effects of combined AM D ( allantoin , metronidazolem and dexamethasone in combination) therapy.
MATERIALS AND METHODS
Materials
Allantoin (Alt) powder with the purity of 99.6% was obtained from Jiangsu Huanghai Pharmaceutical Factory. Metronidazole (Met) powder was from Tianjing Hebei Pharmaceutical Factory with the purity of 99.85%. Dexamethasone (Dex) powder was purchased from Roussel Uclaf Co and the purity is 99.6%. They were dissolved and mixed with 5% GS, the ratio of Alt: Met:Dex is 50:32:1. The HEWLETT-PACKARD Sonos-2000 Color Ultrasonic Doppler Method Diagnostic Equipment was employed in this study, using a real-time sonolayer SSA-270Aultrasound scanner (Toshiba, Tokyo, Japan) and a 3.5 MHz sector transducer.
Experimental animal
20 adult healthy dogs of both sexes, weighing from 7 to 10 kg were purchased from the Animal Center of Wannan Medical College.
Animal models
The experiment was carried out in clean but not sterile condition. Under 3% sodium pentobarbital anesthesia (1 mL/kg iv), following shaving and skin disinfecting, the laparotomy was performed through a 5 cm, vertical, midline incision. 2 cm section from the cecal end was clamped and ligated, 1 cm of the section was cut, and the other 1 cm from the ligated site was kept. The cecum stump was closed with the purse-string suture. Then a 3 × 4 cm2 patch of parietal peritoneum corresponding to the cecal was carefully stripped. In addition, both sides of peritoneum along the abdomen incision were scraped for an area of about 3 × 4 cm2. Before skin closure of abdomen, the animals were randomly divided into 2 groups. The abdominal cavity in Group AMD were rinsed by 200 mL of AMD solution and with 50 mL left, whereas the control received the equal volume of normal saline. All the animals were fasted for 8 hours after operation.
Adhesion assessment by ultrasonography
At the seventh day after operation, all the dogs were reanesthesiaed. Selecting the skin point corresponding to the appendix, 1000 mL of normal saline was instilled into the abdominal cavity with a 12G needle to improve the acoustic window[7]. The whole abdomen was divided into four areas artificially by a horizon through nave and a vertical line through xiphoid (Figure 1), the number and density of adhesion sites were graded on the basis of ultrasonographic findings (Table 1).
Figure 1.
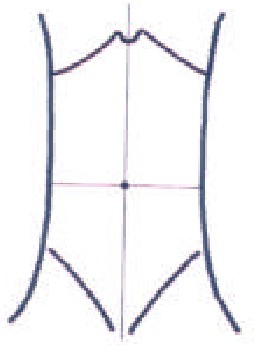
Abridged general view of dog abdomen subregion.
Table 1.
Adhesion assessment by ultrasonography
| Score | Description |
| 1 | Echogenic bands in one area |
| 2 | Echogenic bands in two areas |
| 3 | Echogenic bands in three areas or alveolate echogenic bands in one area |
| 4 | Massive agglutinating adhesion or adhesion between viscera and abdominal wall |
Adhesion assessment by traditional dissection
After ultrasonic examination, the dogs were sacrificed and an autopsy examination was carried out with the attention to the number, density and site of the adhesion formation, which was scored by a modified scale by Swolin[8,9] (Table 2). The highest score for each dog was taken to be further processed.
Table 2.
Adhesion assessment by traditional dissection
| Score | Description |
| 1 | Filmy connections sparated spontaneously |
| 2 | Firm adhesions separated by gravity |
| 3 | Firm adhesions by traction |
| 4 | Dense adhesions requiring sharp dissection |
Statistical analysis
Quantitative results were expressed as mean ± SD. Statistical analysis was performed using Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
One week postoperation animals in control group developed strip or round adhesions (Figure 2), attached either to the closed peritoneal defect or to the midline scar, or connected between the bowels. All the control animals had positive sonographic findings. Transabdominal sonogram clearly showed echogenic bands floating in the abdominal cavity like mice-tails (Figure 3). In more serious subjects, the adhesions were so dense that the sonogram showed alveolate echogenic masses (Figure 4); Some adhesions were formed between the organs in abdominal cavity (Figure 5). All the sonographic positive findings were proved to be the adhesion formation in laparotomy. The adhesion formed in Group AMD was significantly decreased compared with the control group as shown by both ultrasonography and traditional dissection score that marked as 2.0 ± 1.25 vs 3.3 ± 0.82 and 1.91 ± 0.83 vs 3.3 ± 0.82 respectively (P < 0.01)
Figure 2.
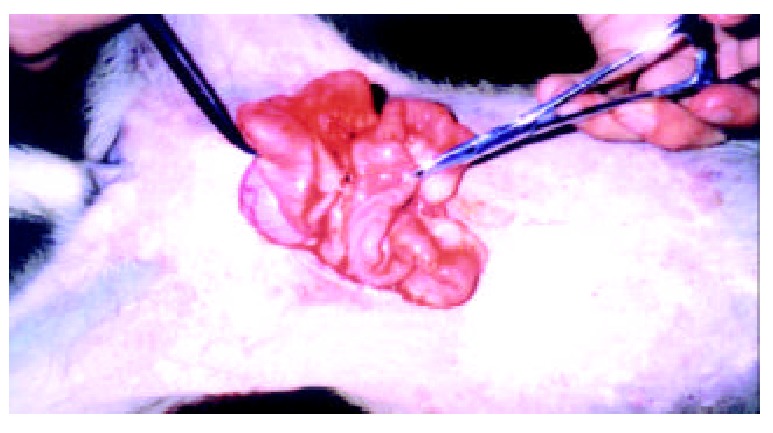
Adhesions between the bowls of animal in control group.
Figure 3.
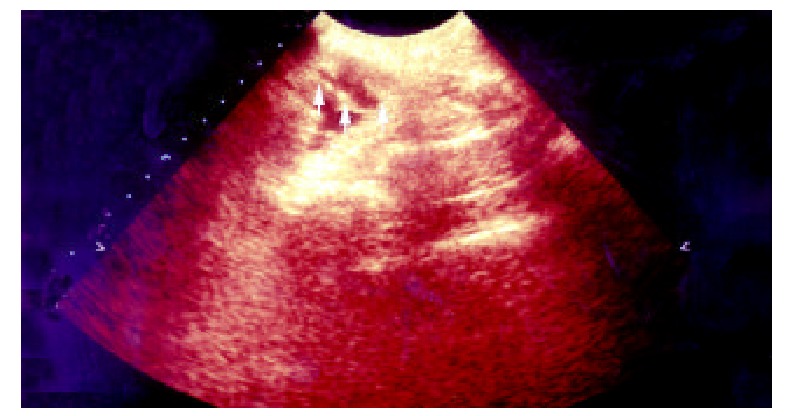
Transabdominal sonogram showed the adhesions between bowls, the adhesion looks like a mouse tail, and the score is 1.
Figure 4.
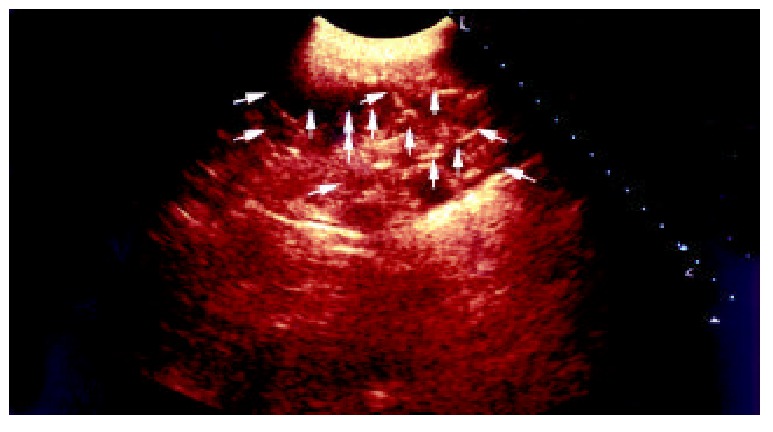
Transabdominal sonogram showed massive agglu-tinating adhesion between bowls, and the score is 3.
Figure 5.
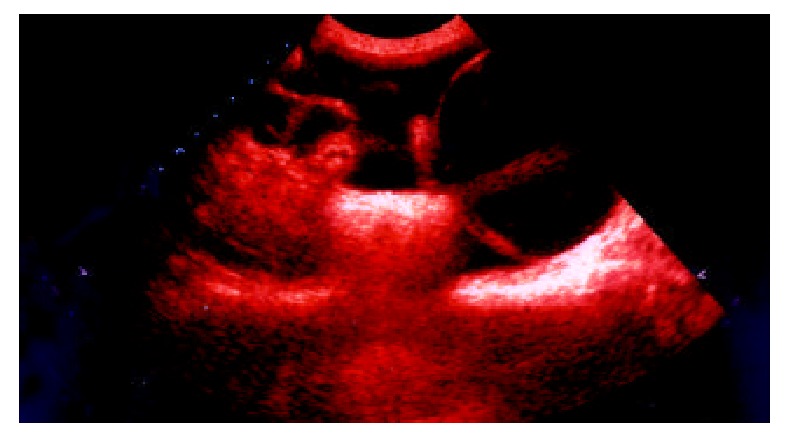
Transabdominal sonogram showed the adhesions between bladder and bowls, and the score is 4.
DISCUSSION
Because of the high incidence of intra-abdominal adhesion formation postoperation, plenty of investigations have been made for the past decades; However, up to the present, satisfactory results have not been achieved yet, which reflected such a fact that there still existed some obstacles waiting to be overcome.
The animal model of intra-abdominal adhesion was such a problem. Traditionally, the model was made mainly on mice, rats and rabbits by a series of America, British investigators in different means[10-12]; however, these small animals were quite different from human beings in phylogenesis, metabolism reactivity and so on. In addition, such model making laid much stress on mechanical trauma, many important factors, such as tissue ischemia, infection, inflammation and exudation[13-16] were severely neglected, which made the experimental results inconsistent with clinical settings. In the present, study a novel dog model of intra-abdominal adhesion caused by multi-factors was employed, which might reflect really the complexity of etiology and the nature of intra-abdominal adhesion formation.
There are a large number of substances used to combat adhesion formation at present[17-26], however, because of the multi-factors in the adhesion etiology and multi-pathways in the adhesion mechanism, the single use one or two of these agents could not turn out satisfactory results. Besides, the majority of these agents have been proven to be too toxic to be used[27-29].
The formation of intra-abdominal adhesion has been attributed to the local depression of plasminogen activator activity (PAA) for more than 3 decades[30-33]. This deficit permits the deposited fibrin on peritoneal surface to form fibrous adhesion. In this study, we used the combined AMD composed of allantoin, metronidazole and dexamethasone, which was proven to play a role of anti-inflammation, anti-bacteria and anti-exudation by prohibiting the fibrin rich exudate into the abdominal cavity and increasing the activity of endogenetic tissue plaminogen activator, to prevent intra-abdominal adhesion postoperation. According to our pilot study, the best proportion of these three drugs was 50:32:1, which made the effectiveness of the combination reinforced, whereas the toxicity didn’t increase[34]. As for the impairment of the combination on wound healing, it was too slight to be noticed, for the intra-abdominal adhesion formed mainly in 6 hours postoperation, after that, the wound healing began to occur while the effect of the combination was gradually disappeared.
Intra-abdominal adhesion failed to be detected by routine ultrasonography. Lee et al[7] identified instilling normal saline into abdominal cavity could diagnose the female pelvic lesions, we employed this method to perform an assessment for the intra-abdominal adhesion. It was demonstrated that this method was visible and accurate, the score of intra-abdominal adhesion was well in agreement with that done by the laparotomy. The most notable finding of sonography in the examination of intra-abdominal adhesion was the mouse-tail appearance, which was believed as the sign of adhesion band. In conclusion, we suggested that the combined AMD might be an effective way to prevent intra-abdominal adhesion, and the ultrasonography an useful tool to diagnose intra-abdominal adhesion, and their applications might be valuable to the clinical settings.
Footnotes
Edited by Zhu L
References
- 1.Lower AM, Hawthorn RJ, Ellis H, O'Brien F, Buchan S, Crowe AM. The impact of adhesions on hospital readmissions over ten years after 8849 open gynaecological operations: an assessment from the Surgical and Clinical Adhesions Research Study. BJOG. 2000;107:855–862. doi: 10.1111/j.1471-0528.2000.tb11083.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi CA, Sterzel A, Braumann C, Halle E, Stösslein R, Krähenbühl L, Müller JM. The impact of conventional and laparoscopic colon resection (CO2 or helium) on intraperitoneal adhesion formation in a rat peritonitis model. Surg Endosc. 2001;15:380–386. doi: 10.1007/s004640000359. [DOI] [PubMed] [Google Scholar]
- 3.Farmer L, Ayoub M, Warejcka D, Southerland S, Freeman A, Solis M. Adhesion formation after intraperitoneal and extraperitoneal implantation of polypropylene mesh. Am Surg. 1998;64:144–146. [PubMed] [Google Scholar]
- 4.Tulandi T. Introduction--prevention of adhesion formation: the journey continues. Hum Reprod Update. 2001;7:545–546. doi: 10.1093/humupd/7.6.545. [DOI] [PubMed] [Google Scholar]
- 5.Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Re-cent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Gastroenterol Suppl. 2000;232:52–59. [PubMed] [Google Scholar]
- 6.Thompson J. Pathogenesis and prevention of adhesion formation. Dig Surg. 1998;15:153–157. doi: 10.1159/000018610. [DOI] [PubMed] [Google Scholar]
- 7.Li GJ, Zhou YC, Wu J. A preliminary application of normal sa-line instilling abdomen cavity in diagnosis of female pelvic lesions. Zhongguo Chaosheng Yixue Zazhi. 2000;16:535–538. [Google Scholar]
- 8.Gül A, Kotan C, Dilek I, Gül T, Taş A, Berktaş M. Effects of methylene blue, indigo carmine solution and autologous erythrocyte suspension on formation of adhesions after injection into rats. J Reprod Fertil. 2000;120:225–229. doi: 10.1530/jrf.0.1200225. [DOI] [PubMed] [Google Scholar]
- 9.Tran HS, Chrzanowski FA, Puc MM, Patel NG, Geldziler B, Malli D, Ramsamooj R, Hewitt CW, DelRossi AJ. An in vivo evaluation of a chondroitin sulfate solution to prevent postoperative intraperitoneal adhesion formation. J Surg Res. 2000;88:78–87. doi: 10.1006/jsre.1999.5714. [DOI] [PubMed] [Google Scholar]
- 10.Yesildaglar N, Koninckx PR. Adhesion formation in intubated rabbits increases with high insufflation pressure during endoscopic surgery. Hum Reprod. 2000;15:687–691. doi: 10.1093/humrep/15.3.687. [DOI] [PubMed] [Google Scholar]
- 11.Mueller PO, Hay WP, Harmon B, Amoroso L. Evaluation of a bioresorbable hyaluronate-carboxymethylcellulose membrane for prevention of experimentally induced abdominal adhesions in horses. Vet Surg. 2000;29:48–53. doi: 10.1111/j.1532-950x.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 12.Toosie K, Gallego K, Stabile BE, Schaber B, French S, de Virgilio C. Fibrin glue reduces intra-abdominal adhesions to synthetic mesh in a rat ventral hernia model. Am Surg. 2000;66:41–45. [PubMed] [Google Scholar]
- 13.Sjösten AC, Ellis H, Edelstam GA. Post-operative consequences of glove powder used pre-operatively in the vagina in the rabbit model. Hum Reprod. 2000;15:1573–1577. doi: 10.1093/humrep/15.7.1573. [DOI] [PubMed] [Google Scholar]
- 14.van den Tol MP, Haverlag R, van Rossen ME, Bonthuis F, Marquet RL, Jeekel J. Glove powder promotes adhesion formation and facilitates tumour cell adhesion and growth. Br J Surg. 2001;88:1258–1263. doi: 10.1046/j.0007-1323.2001.01846.x. [DOI] [PubMed] [Google Scholar]
- 15.Qiu G, Wang C, Smith R, Harrison K, Yin K. Role of IFN-gamma in bacterial containment in a model of intra-abdominal sepsis. Shock. 2001;16:425–429. doi: 10.1097/00024382-200116060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Halverson AL, Barrett WL, Bhanot P, Phillips JE, Iglesias AR, Jacobs LK, Sackier JM. Intraabdominal adhesion formation after preperitoneal dissection in the murine model. Surg Endosc. 1999;13:14–16. doi: 10.1007/s004649900888. [DOI] [PubMed] [Google Scholar]
- 17.Reijnen MM, de Man BM, Hendriks T, Postma VA, Meis JF, van Goor H. Hyaluronic acid-based agents do not affect anastomotic strength in the rat colon, in either the presence or absence of bacterial peritonitis. Br J Surg. 2000;87:1222–1228. doi: 10.1046/j.1365-2168.2000.01506.x. [DOI] [PubMed] [Google Scholar]
- 18.Verco SJ, Peers EM, Brown CB, Rodgers KE, Roda N, diZerega G. Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: pre-clinical studies. Hum Reprod. 2000;15:1764–1772. doi: 10.1093/humrep/15.8.1764. [DOI] [PubMed] [Google Scholar]
- 19.Szabo A, Haj M, Waxsman I, Eitan A. Evaluation of seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. Eur Surg Res. 2000;32:125–128. doi: 10.1159/000008751. [DOI] [PubMed] [Google Scholar]
- 20.Ghellai AM, Stucchi AF, Chegini N, Ma C, Andry CD, Kaseta JM, Burns JW, Skinner KC, Becker JM. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J Gastrointest Surg. 2000;4:316–323. doi: 10.1016/s1091-255x(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 21.Kramer K, Senninger N, Herbst H, Probst W. Effective prevention of adhesions with hyaluronate. Arch Surg. 2002;137:278–282. doi: 10.1001/archsurg.137.3.278. [DOI] [PubMed] [Google Scholar]
- 22.Cubukçu A, Alponat A, Gönüllü NN. Mitomycin-C prevents reformation of intra-abdominal adhesions after adhesiolysis. Surgery. 2002;131:81–84. doi: 10.1067/msy.2002.118316. [DOI] [PubMed] [Google Scholar]
- 23.Reijnen MM, Skrabut EM, Postma VA, Burns JW, van Goor H. Polyanionic polysaccharides reduce intra-abdominal adhesion and abscess formation in a rat peritonitis model. J Surg Res. 2001;101:248–253. doi: 10.1006/jsre.2001.6288. [DOI] [PubMed] [Google Scholar]
- 24.Vela AR, Littleton JC, O'Leary JP. The effects of minidose heparin and low molecular weight heparin on peritonitis in the rat. Am Surg. 1999;65:473–477. [PubMed] [Google Scholar]
- 25.Ozoğul Y, Baykal A, Onat D, Renda N, Sayek I. An experimental study of the effect of aprotinin on intestinal adhesion formation. Am J Surg. 1998;175:137–141. doi: 10.1016/s0002-9610(97)00273-0. [DOI] [PubMed] [Google Scholar]
- 26.Gül A, Kotan C, Dilek I, Gül T, Taş A, Berktaş M. Effects of methylene blue, indigo carmine solution and autologous erythrocyte suspension on formation of adhesions after injection into rats. J Reprod Fertil. 2000;120:225–229. doi: 10.1530/jrf.0.1200225. [DOI] [PubMed] [Google Scholar]
- 27.Trickett JP, Rainsbury RM, Green R. Paradoxical outcome after use of hyaluronate barrier to prevent intra-abdominal adhesions. J R Soc Med. 2001;94:183–184. doi: 10.1177/014107680109400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reijnen MM, de Man BM, Hendriks T, Postma VA, Meis JF, van Goor H. Hyaluronic acid-based agents do not affect anastomotic strength in the rat colon, in either the presence or absence of bacterial peritonitis. Br J Surg. 2000;87:1222–1228. doi: 10.1046/j.1365-2168.2000.01506.x. [DOI] [PubMed] [Google Scholar]
- 29.Haverlag R, van Rossen ME, van den Tol MP, Bonthuis F, Marquet RL, Jeekel J. Hyaluronate-based coating solution for prevention of surgical adhesions has no major effect on adhesion and growth of intraperitoneal tumour cells. Eur J Surg. 1999;165:791–795. doi: 10.1080/11024159950189609. [DOI] [PubMed] [Google Scholar]
- 30.Edelstam G, Lecander I, Larsson B, Astedt B. Fibrinolysis in the peritoneal fluid during adhesions, endometriosis and ongoing pelvic inflammatory disease. Inflammation. 1998;22:341–351. doi: 10.1023/a:1022322814288. [DOI] [PubMed] [Google Scholar]
- 31.Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7:556–566. doi: 10.1093/humupd/7.6.556. [DOI] [PubMed] [Google Scholar]
- 32.Cao TS, Liu RH, Sun XM. [Clinical study of the effect of methylene blue combined with aprotinin on intraperitoneal adhesion] Zhonghua Weichang Waike Zazhi. 2005;8:24–25. [PubMed] [Google Scholar]
- 33.Gimbel ML, Chelius D, Hunt TK, Spencer EM. A novel approach to reducing postoperative intraperitoneal adhesions through the inhibition of insulinlike growth factor I activity. Arch Surg. 2001;136:311–317. doi: 10.1001/archsurg.136.3.311. [DOI] [PubMed] [Google Scholar]
- 34.Zheng QS, Gui CQ, Sun RY, Wang M. A novel animal model of intra-abdominal adhesion and quantitative evaluation with re-lated indices. Zhongguo Linchuang Yaolixue Yu Zhiliaoxue Zazhi. 2000;5:101–104. [Google Scholar]


