Abstract
AIM: To assess the role of thyroid disease as a risk for fractures in Crohn’s patients.
METHODS: A cross-sectional study was conducted from 1998 to 2000. The study group consisted of 210 patients with Crohn’s disease. A group of 206 patients without inflammatory bowel disease served as controls. Primary outcome was thyroid disorder. Secondary outcomes included use of steroids, immunosuppressive medications, surgery and incidence of fracture.
RESULTS: The prevalence of hyperthyroidism was similar in both groups. However, the prevalence of hypothyroidism was lower in Crohn’s patients (3.8% vs 8.2%, P = 0.05). Within the Crohn’s group, the use of immunosuppressive agents (0% vs 11%), steroid usage (12.5% vs 37%), small bowel surgery (12.5% vs 28%) and large bowel surgery (12.5% vs 27%) were lower in the hypothyroid subset as compared to the euthyroid subset. Seven (3.4%) Crohn’s patients suffered fracture, all of whom were euthyroid.
CONCLUSION: Thyroid disorder was not found to be associated with Crohn’s disease and was not found to increase the risk for fractures. Therefore, screening for thyroid disease is not a necessary component in the management of Crohn’s disease.
INTRODUCTION
Recent studies have shown that patients with Crohn’s disease have decreased bone density[1,2] that predisposes to an increased risk of fracture[3,4]. A chronic inflammatory state with the release of inflammatory cytokines, steroid usage and malnutrition is the proposed mechanism. The co-existence of other medical conditions, which are independently associated with osteoporosis, will further increase the risk of fracture in patients with Crohn’s disease. Thyroid disorder is associated with changes in bone metabolism and is an important cause of osteoporosis[5-7]. Thyroid disorder has also been reported in patients with underlying inflammatory bowel disease (IBD)[8-12]. One study showed altered thyroid physiology and anatomy in patients with IBD[9] and suggests that it may be important to investigate the association between thyroid disorders and Crohn’s disease. If patients with Crohn’s disease have a higher prevalence of thyroid disease, then screening and treatment of the thyroid disease may reduce the risk of fractures in the Crohn’s disease population. Secondly, it has been reported that the presence of hyperthyroidism makes the treatment of inflammatory bowel disease more difficult[10,13] and, therefore, correcting the thyroid disorder may have an impact on treatment response.
We compared the prevalence of hypothyroidism and hyperthyroidism in patients with Crohn’s disease and those without Crohn’s disease. To investigate whether co-existing thyroid dysfunction has an impact on the treatment of Crohn’s disease, we compared the use of immunosuppressive medications and surgical procedures for the underlying Crohn’s disease in patients with and without co-existing thyroid dysfunction.
MATERIALS AND METHODS
Study design and subjects
A retrospective cross-sectional study was conducted at this hospital from 1998 to 2000. The study group included 210 patients with Crohn’s disease admitted to the hospital or referred to the endoscopy suite for endoscopic procedures. A control group comprised of 206 consecutive patients who did not have inflammatory bowel disease and who were referred for either screening colonoscopy or esophagogastroduodenoscopy (EGD).
Data collection
Medical records of patients were reviewed to extract information regarding their baseline demographic information, diagnosis and extent of Crohn’s disease, presence of thyroid disease and use of either steroid or immunosuppressive agents. The diagnosis of Crohn’s disease was made either endoscopically or radiographically. A history of other medical diagnoses (e.g. rheumatoid arthritis, chronic obstructive pulmonary disease) where the potential use of steroids or immunosuppressive agents may be a confounding factor was also recorded. Sample size was calculated based on a reference value of 1% of thyroid dysfunction in the general population. To detect the difference of 5% between the two groups with power of 0.8 and a two sided alpha error of 0.05, more than 200 patients were required in both the groups.
Outcome
The primary outcome was the presence of hypothyroidism or hyperthyroidism. Secondary outcomes included the use of immunosuppressive medications and surgery for the Crohn’s disease. Thyroid disorder was considered present if the patient had a prior diagnosis of thyroid disorder and was taking thyroid medication (thyroxine, propylthiouracil, methimazole) or had an abnormal thyroid stimulating hormone (TSH) level.
Statistical analysis
Descriptive statistics were reported as proportions, means ± standard deviation or median and range. Comparison between groups was done using the Pearson chi-square test for categorical variable (or the Fisher exact test if appropriate) and two-sided student t-test for continuous variables (SPSS for Windows, release 10.0, Chicago, IL). P < 0.05 was considered significant.
RESULTS
Demographic and clinical characteristics
There were 210 patients with Crohn’s disease and 206 controls. The two groups were matched for age (49.24 yr. vs 48.58 yr., P = 0.68), sex (88 males/122 females vs 91 males/133 females, P = 0.29), and race (153 whites/1 black/34 others vs 118 whites/ 14 blacks/36 others, P = 0.34). The groups differed in body mass index (24.35 kg/m2 vs 27.46 kg/m2, P = 0.001). (Table 1).
Table 1.
Comparison of baseline characteristics and thyroid status in patients with Crohn’s disease and control group
| Case (n = 210) | Control (n = 206) | P | |
| Age (years) | 49.24 ± 13.71 | 48.58 ± 18.43 | 0.68 |
| Sex (M/F) | 88/122 | 91/113 | 0.29 |
| Body Mass Index (kg/cm2) | 24.35 | 27.46 | 0.001 |
| Race (white/black/others) | 153/1/34 | 118/14/36 | 0.34 |
| Steroid use | 75 (35.7%) | None | _ |
| Immunosuppressive therapy | 22 (10.4%) | None | _ |
| Hypothyroidism | 8 (3.8%) | 17 (8.2%) | 0.05 |
| Hyperthyroidism | 2 (0.01%) | None | 0.25 |
Primary outcome - thyroid dysfunction
In the Crohn’s group there were 8 (3.8%) cases of hypothyroidism and 2 (0.01%) cases of hyperthyroidism. The control population had 17 (8.2%) cases of hypothyroidism and 0 (0%) case of hyperthyroidism. The difference in hypothyroidism showed borderline significance (P = 0.05) while hyperthyroidism was not significant (P = 0.25) (Figure 1).
Figure 1.

Bar graph showing the prevalence of thyroid disor-der in patients with Crohn’s disease and in the control group.
Secondary outcome - use of immunosuppressives and surgery
Patients with Crohn’s disease were divided into those with hypothyroidism and those without any thyroid disorder. There was clinically higher use of immunosuppressive agents (0% vs 11%, P = 0.59), steroid usage (12.5% vs 37%, P = 0.15), small bowel surgery (12.5% vs 28%, P = 0.34) and large bowel surgery (12.5% vs 27%, P = 0.38) in Crohn’s patients with hypothyroidism in comparison to those who were euthyroid. This difference however, was not statistically significant. (Table 2, Figure 2).
Table 2.
Comparison of medical and surgical treatment in pa-tients with hypothyroidism and euthyroidism in Crohn’s disease
| Hypothyroidism (n = 8) | Euthyroid (n = 201) | P | |
| Immunosuppresive medications | None | 22 (11%) | 0.59 |
| Steroid use | 1 (12.5%) | 74 (37%) | 0.15 |
| Small bowel surgery | 1 (12.5%) | 56 (28%) | 0.34 |
| Large bowel surgery | 1 (12.5%) | 54 (27%) | 0.38 |
Figure 2.
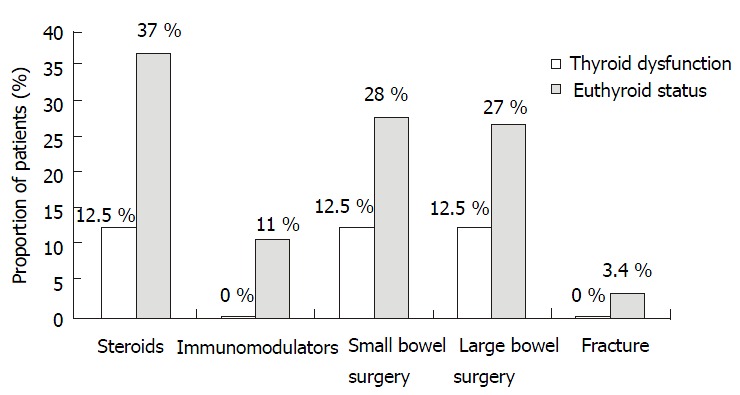
Bar graph showing the usage of steroids, immuno-suppressive agents, small bowel surgery, large bowel surgery and fractures in Crohn’s patients with thyroid disorder and those without thyroid disorder.
DISCUSSION
Crohn’s disease is a chronic illness that is associated with a lower bone mineral density and a higher incidence of fracture[1-4]. This results in increased morbidity and mortality. Thyroid disorder may potentiate the risk of fracture. The purpose of this study was to investigate whether thyroid disorder contributed to this adverse event and if present, whether thyroid disorder has an impact on the management of Crohn’s disease. We did not find a higher prevalence of thyroid disease in our Crohn’s population when compared to a control group. In fact, the prevalence of hypothyroidism was less in the Crohn’s group. Sub-group analysis of the Crohn’s group showed that there was lower usage of steroids, immunosuppressive medications and surgery in those who were also hypothyroid when compared to those who were euthyroid. There were 7 (3.4%) fractures in the Crohn’s population. All of the fractures occurred in those who were euthyroid.
The prophylactic use of a bisphosphonate has been shown to increase the bone mineral density in patients with Crohn’s disease[14]. However, the beneficial effect in lowering the incidence of fracture has not been reported. Due to the lack of an effective prophylactic medication, it becomes important to identify and treat any other co-existing illness that can enhance bone loss and increase the risk of fracture. One common disease that is linked to Crohn’s and has an impact on bone metabolism is thyroid disease[8-12].
In our study, we did not find a difference in the prevalence of thyroid disease between patients with Crohn’s disease and our controls. These findings are consistent with two prior studies[8,12]. Snook et al[8] found a higher prevalence of thyroid disorder in patient with ulcerative colitis, but not Crohn’s disease, when comparing patients with IBD to the general population. Hammer et al[12] also found a higher prevalence of thyroid disorder in the ulcerative colitis subset of their IBD population when compared to the general population. The lack of association of clinical thyroid disorder and Crohn’s disease is contradicted by the objective findings of Jenerot et al[9]. They showed that patients with IBD had a 35% higher thyroid volume and were three times more likely to have an enlarged thyroid. Their finding of thyroid enlargement may be secondary to iodine deficiency, which has been reported in Crohn’s disease and is unrelated to functional derangement of the thyroid[15]. When we subdivided our thyroid disorder into hyperthyroid and hypothyroid, we did not find a difference in the prevalence of hyperthyroid between those with Crohn’s disease and controls. Surprisingly, we found a lower prevalence of hypothyroidism in the Crohn’s group as compared to controls.
Finding no difference in the prevalence of hyperthyroidism between patients with Crohn’s disease and controls has clinical significance. Hyperthyroidism is associated with accelerated bone turnover and shortening of the normal bone remodeling cycle[6,7]. This results in increased bone metabolism and osteoporosis. Since patients with Crohn’s disease do not have a higher prevalence of hyperthyroidism, an elevation of thyroxine levels cannot be the basis for the increased osteoporosis seen in this group of patients. Factors such as chronic inflammation, prolonged steroid usage and malnutrition are the more likely etiological agents for osteoporosis[16]. Thus, evaluating patients with Crohn’s disease for hyperthyroidism should be done on clinical grounds and not as a screening procedure.
Our finding of a lower prevalence of hypothyroidism in our Crohn’s population as compared to our controls is similar to those of Hammer et al[12]. It is possible that since both Crohn’s disease and thyroid disorder have a possible autoimmune etiology, treating Crohn’s disease with corticosteroids and immune modulating agents may prevent the manifestation of an autoimmune thyroid disorder. The difference in prevalence of hypothyroidism may also be the result of a higher than expected prevalence of hypothyroidism in our control group. The prevalence of hypothyroidism in population based studies varies from 0.5% to 5%[17-19], however, we had an 8% prevalence of hypothyroidism in our controls. This higher prevalence of hypothyroidism may be explained by the availability of third generation assays for thyroid stimulating hormone (TSH) and increased frequency of screening for thyroid disorder, leading to detection of sub-clinical disease. There is also the possibility of a selection bias in the control group. The controls included people who underwent screening colonoscopy. Those persons are more likely to be health conscious and, therefore, are more likely to visit a physician; thus, this would result in increased screening for thyroid disease as compared to the general population. In addition, the control group had a higher body mass index (BMI) as compared to the Crohn’s group. BMI can act as a confounding factor as it is related to both hypothyroidism and reflux symptoms. Hypothyroidism is associated with weight gain1[8,19]. Patients who have a higher BMI are more likely to have reflux symptoms[20,21] and thus, are more likely to undergo EGD than the general population. Since our controls included patients having EGD, it is possible that our controls contained a higher percentage of people who are hypothyroid. This may be a theoretical concern as a recent, well design; prospective study[22] did not show an association between obesity and reflux symptoms.
There are concerns that the presence of thyroid disease may have an impact on the management of IBD. Moreover, there are case reports that suggest that thyroid disease needs to be treated in order to successfully treat IBD[10,13]. We subdivided our Crohn’s disease population into those who are euthyroid and those who had thyroid disease. The two groups were compared with regard to steroid usage, use of immune modulating agents, surgery for the management of Crohn’s and rate of fracture. There was a higher rate of steroid usage, use of immune modulating agents, and both small and large bowel surgeries in patients who were euthyroid. For instance, there were seven fractures in the Crohn’s group, all of which occurred in patients who were euthyroid. The differences, though clinically significant, were not statistically significant. This is probably due to a small sample size. A larger prospective, controlled study is needed to evaluate this issue. Hypothyroidism is known to decrease metabolism and slow physiological functions. The presence of hypothyroidism may act as endogenous disease modification in patients with Crohn’s disease.
Our study was limited by retrospective data collection. This makes it difficult to evaluate and compare disease severity index and extent of disease between Crohn’s disease patients who are euthyorid and those who had a thyroid disorder present. It also makes it difficult to establish a temporal relationship between thyroid disease and Crohn’s disease. Our results are based on the assumption that patients who were characterized as euthyroid are actually euthyroid. Symptoms may not suggest thyroid disease when it is mild and are often atypical in the elderly[23-25]. Thus, it is possible that we missed patients with subclinical thyroid disease.
In conclusion, patients with Crohn’s disease do not have a higher prevalence of thyroid disease than the general population and, therefore, routine screening for thyroid disease is not a necessary component in the management of Crohn’s disease. However, it is apparent that thyroid disease has a significant clinical impact on the course of Crohn’s disease, which needs to be studied in a prospective manner.
ACKNOWLEDGEMENTS
The authors thank S.D. Rampertab, M.D. for her critical comments on the manuscript.
Footnotes
Edited by Xu XQ
References
- 1.Silvennoinen JA, Karttunen TJ, Niemelä SE, Manelius JJ, Lehtola JK. A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut. 1995;37:71–76. doi: 10.1136/gut.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreassen H, Rungby J, Dahlerup JF, Mosekilde L. Inflammatory bowel disease and osteoporosis. Scand J Gastroenterol. 1997;32:1247–1255. doi: 10.3109/00365529709028155. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P, Krogh K, Rejnmark L, Laurberg S, Mosekilde L. Fracture risk is increased in Crohn's disease, but not in ulcerative colitis. Gut. 2000;46:176–181. doi: 10.1136/gut.46.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med. 2000;133:795–799. doi: 10.7326/0003-4819-133-10-200011210-00012. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 6.Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19:35–63. [PubMed] [Google Scholar]
- 7.Ross DS. Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid. 1994;4:319–326. doi: 10.1089/thy.1994.4.319. [DOI] [PubMed] [Google Scholar]
- 8.Snook JA, de Silva HJ, Jewell DP. The association of autoimmune disorders with inflammatory bowel disease. Q J Med. 1989;72:835–840. [PubMed] [Google Scholar]
- 9.Järnerot G, Kågedal B, von Schenck H, Truelove SC. The thyroid in ulcerative colitis and Crohn's disease. V. Triiodothyronine. Effect of corticosteroids and influence of severe disease. Acta Med Scand. 1976;199:229–232. doi: 10.1111/j.0954-6820.1976.tb06722.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonapace ES, Srinivasan R. Simultaneous occurrence of inflammatory bowel disease and thyroid disease. Am J Gastroenterol. 2001;96:1925–1926. doi: 10.1111/j.1572-0241.2001.03896.x. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi GP, Marchesini G, Gueli C, Zoli M. Thyroid involvement in patients with active inflammatory bowel diseases. Ital J Gastroenterol. 1995;27:291–295. [PubMed] [Google Scholar]
- 12.Hammer B, Ashurst P, Naish J. Diseases associated with ulcerative colitis and Crohn's disease. Gut. 1968;9:17–21. doi: 10.1136/gut.9.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modebe O. Autoimmune thyroid disease with ulcerative colitis. Postgrad Med J. 1986;62:475–476. doi: 10.1136/pgmj.62.728.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn's disease. Gastroenterology. 2000;119:639–646. doi: 10.1053/gast.2000.16518. [DOI] [PubMed] [Google Scholar]
- 15.Järnerot G. The thyroid in ulcerative colitis and Crohn's disease. I. Thyroid radioiodide uptake and urinary iodine excretion. Acta Med Scand. 1975;197:77–81. doi: 10.1111/j.0954-6820.1975.tb04881.x. [DOI] [PubMed] [Google Scholar]
- 16.Andreassen H, Rungby J, Dahlerup JF, Mosekilde L. Inflammatory bowel disease and osteoporosis. Scand J Gastroenterol. 1997;32:1247–1255. doi: 10.3109/00365529709028155. [DOI] [PubMed] [Google Scholar]
- 17.Sawin CT, Castelli WP, Hershman JM, McNamara P, Bacharach P. The aging thyroid. Thyroid deficiency in the Framingham Study. Arch Intern Med. 1985;145:1386–1388. [PubMed] [Google Scholar]
- 18.Dale J, Daykin J, Holder R, Sheppard MC, Franklyn JA. Weight gain following treatment of hyperthyroidism. Clin Endocrinol (Oxf) 2001;55:233–239. doi: 10.1046/j.1365-2265.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 19.Pinkney JH, Goodrick SJ, Katz J, Johnson AB, Lightman SL, Coppack SW, Mohamed-Ali V. Leptin and the pituitary-thyroid axis: a comparative study in lean, obese, hypothyroid and hyperthyroid subjects. Clin Endocrinol (Oxf) 1998;49:583–588. doi: 10.1046/j.1365-2265.1998.00573.x. [DOI] [PubMed] [Google Scholar]
- 20.Wajed SA, Streets CG, Bremner CG, DeMeester TR. Elevated body mass disrupts the barrier to gastroesophageal reflux; discussion 1018-1019. Arch Surg. 2001;136:1014–1018. doi: 10.1001/archsurg.136.9.1014. [DOI] [PubMed] [Google Scholar]
- 21.Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94:2840–2844. doi: 10.1111/j.1572-0241.1999.01426.x. [DOI] [PubMed] [Google Scholar]
- 22.Lagergren J, Bergström R, Nyrén O. No relation between body mass and gastro-oesophageal reflux symptoms in a Swedish population based study. Gut. 2000;47:26–29. doi: 10.1136/gut.47.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evered DC, Ormston BJ, Smith PA, Hall R, Bird T. Grades of hypothyroidism. Br Med J. 1973;1:657–662. doi: 10.1136/bmj.1.5854.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenzel KW, Meinhold H, Raffenberg M, Adlkofer F, Schleusener H. Classification of hypothyroidism in evaluating patients after radioiodine therapy by serum cholesterol, T3-uptake. Total T4, FT4-index, total T3, basal TSH and TRH-test. Eur J Clin Invest. 1974;4:141–148. doi: 10.1111/j.1365-2362.1974.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 25.Bahemuka M, Hodkinson HM. Screening for hypothyroidism in elderly inpatients. Br Med J. 1975;2:601–603. doi: 10.1136/bmj.2.5971.601. [DOI] [PMC free article] [PubMed] [Google Scholar]


