Abstract
Cognitive deficits have been observed in patients with multiple sclerosis (MS) because of hippocampal insults. Oxidative stress plays a key role in the pathophysiology of MS. The aim of this study was to evaluate the effects of Crocus sativus L., commonly known as saffron, on learning and memory loss and the induction of oxidative stress in the hippocampus of toxic models of MS. One week after MS induction by intrahippocampal injection of ethidium bromide (EB), animals were treated with two doses of saffron extract (5 and 10 μg/rat) for a week. Learning and spatial memory status was assessed using Morris Water Maze. After termination of behavioral testing days, animals were decapitated and the bilateral hippocampi dissected to measure some of the oxidative stress markers including the level of hippocampi thiobarbituric acid reactive substances and the activity of antioxidant enzymes such as glutathione peroxidase and superoxide dismutase. Treatment with saffron extract ameliorated spatial learning and memory impairment (P<0.05). Total antioxidant reactivity capacity, lipid peroxidation products and antioxidant enzymes activity in the hippocampus homogenates of EB treated group were significantly higher than those of all other groups (P<0.01). Indeed, treatment with a saffron extract for 7 consecutive days significantly restored the antioxidant status to the normal levels (P<0.01). These observations reveal that saffron extract can ameliorate the impairment of learning and memory as well as the disturbances in oxidative stress parameters in the hippocampus of experimental models of MS.
Keywords: Multiple sclerosis, Saffron, Cognitive deficits, Oxidative stress
INTRODUCTION
Axonal myelination in the vertebrate central nervous system is pivotal for saltatory nerve impulses, whereas demyelination can construct serious diseases such as multiple sclerosis (MS) (1). MS is recognized as an autoimmune, inflammatory, demyelinating and neurodegenerative disease of the central nervous system affecting over 2 million people worldwide (2). It is characterized by a progressive clinical decline due to axonal loss from chronic demyelination (3). Nowadays it is widely accepted that cognitive dysfunction occurs in 40-70% of MS patients (4). The most common cognitive deficits are memory dysfunction and spatial perception impairment (5,6). Recent studies, especially using magnetic resonance imaging (MRI) technique, has indicated that structural derangements of both the cerebral cortex and the hippocampus (a principle locus of memory consolidation) take place in patients with MS (7,8).
Although different mechanisms may contribute to the demyelination and neurodegeneration in MS, it became clear in recent years that oxidative stress plays the key role in the process (9,10). The brain is believed to be particularly vulnerable to free radical damage because of the high content of polyunsaturated fatty acids (PUFA), the high utilization of oxygen account and the limited possession of antioxidant defenses in comparison to other organs. Thus it is clear that oxidative stress is involved in the formation and persistence of MS lesions (11). Recently, researchers have turned to experimental autoimmune encephalomyelitis in order to explore the relationship between hippocampal dysfunction and neuropathology during MS (12). Intriguingly, it has been observed that the progressive decline in spatial learning and memory function correlated with oxidative stress in the brain of experimental autoimmune encephalomyelitis models (8).
Toxic demyelination by ethidium bromide (EB) is one of the most commonly used models for analyzing the cellular changes that occur during demyelinating pathologies such as MS (7). Direct injection of EB induces focal demyelinating lesions at the site of injection by selectively damaging oligodendrocytes and astrocytes, and consequently interfering with the demyelination and remyelination processes (7,13). Previous studies have indicated the increase in oxidative stress following EB injection in different areas of the brain (11,14).
Antioxidants as substances which could delay or inhibit oxidative damage even in small quantities compared to an oxidizable substrate can help in disease prevention by effectively scavenging free radicals or inhibiting damage caused by them (15).
The use of antioxidants, such as α-tocopherol, ascorbic acid and β-carotene, which suppress the effects of reactive oxygen species (ROS) is considered a promising approach to neuroprotection (16). Crocus sativus L., commonly known as saffron, has been used for centuries as an herbal remedy for various human disorders such as depression and inflammation. Compounds present in saffron that are considered pharmacologically active and important include volatile agents (e.g., safranal), bitter principles (e.g., picrocrocin) and dye materials (e.g., crocetin and its glycoside, crocin) (17).
These components have free radical scavenging and antioxidant effects as well as learning and memory improving properties (18). Particularly in the traditional Indian medicine, saffron has been used for the treatment of cognitive dysfunctions (19). In the modern pharmacological studies, saffron and its active constituents have illustrated a wide range of activities including antioxidant, anti-cancer, anticonvulsant, anti-inflammatory, antinociceptive, hypolipidemic and anti-atherosclerotic properties (17,20). It is also efficient in enhancing cognitive behavior in adult rodents which have previously been exposed to amnesic agents (e.g., scopolamine or ethanol) (21,22). Furthermore, recent work has revealed that the saffron extract exerts protective effects on spatial memory impairment and oxidative stress induced by chronic stress in rats (18). However, previous studies have not investigated the possibilities that saffron extract may improve learning and memory deficits and oxidative stress in clinical or experimental models of MS. The aim of the present study was to investigate the effect of a 7-day intrahippocampal administration of saffron extract on the learning and memory of EB-demyelinated rats and their oxidative status in the hippocampus.
MATERIALS AND METHODS
Animals
Adult male Wistar rats weighing 200-250 g were housed in standard hygienic plastic cages (four in each) under a 12 h light/dark cycle (lights on at 07:00 a.m.) in a room with controlled temperature (23 ± 2 °C). Food and water were available ad libitum. The experiments were carried out during the light phase of the cycle. All animal procedures were performed according to the National Institutes of Health: Guide for the care and use of laboratory animals. Efforts were made to minimize the number of animals used and their suffering.
Plant material and extraction
The saffron stigma extract used in this study was prepared by Novin Zaferan Co (Mashhad, Iran) in November 2011. An extract of the stigma was prepared as follows. Briefly, 10 g of dried and milled stigmas were macerated for 3 days in 300 ml of 80% ethanol at room temperature. The stigmas were then separated from the solvent, and then the solvent was removed by evaporation at 50-60 °C. The ethanolic extract was chilled in refrigerator until the use (23).
Experimental demyelination with ethidium bromide and treatments
The animals were randomly divided into following groups (8 animals per group): I (control, no treatment); II (sham, saline); III (EB); IV (EB + 5 μg/rat saffron extract) and V (EB + 10 μg/rat saffron extract). For the surgical demyelination procedure, animals were deeply anaesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg) (Alfasan, Holland) and placed on the rat stereotaxic instrument (Stoelting, USA) in the skull-flat position. Hair of the skull surface was shaved and then, an incision was made to expose the rat skull.
Two holes were drilled in the skull according to appropriate coordinates to achieve Cornu ammonis (CA1) of hippocampal formation (3.8 mm dorsal to the bregma, 2.4 mm deep from the dura surface and ± 2.2 mm laterality) (24). Two guide cannulae (21 gauges) were inserted into the holes and fixed using dental cement. After the surgery, dummy inner cannulae were inserted into the guide cannulae and left in place until the injections were made. All animals were allowed to recover for 1 week before starting the microinjections.
Experimental model of MS was induced bilaterally by direct single injection of 3 μl of 0.01% EB (Sigma, Germany) in sterile 0.9% saline (7), in the animals from groups III, IV and V. Animals in experimental groups (IV and V), received 3 μl of 5 μg/rat and 10 μg/rat saffron extract, respectively, for 7 days post lesion (7,25). The animals from groups II and III were injected an equal volume of sterile 0.9% saline. Injections for all groups were made at the rate of 1 μl/min using a 10 μl Hamilton syringe, and the needle was kept in the guide cannulae for an additional 60 s in order to facilitate the diffusion of the drug.
Morris Water Maze testing
Characteristic of learning and spatial memory was evaluated using Morris Water Maze (MWM) for 6 consecutive days following the treatment period. MWM consists of a circular pool (136 cm in diameter and 60 cm in height) that was filled with opaque water (20 ± 1 °C) and a hidden platform which was located in a fixed spot. The walls of the pool and the platform were dyed black to conceal the platform. Four positions around the edge of the tank were arbitrarily designated north (N), south (S), east (E) and west (W) to provide four alternative start positions, and define the division of the pool into four quadrants. The escape platform (10 cm in diameter) was placed at the midpoint of one of the quadrants, 2 cm below water surface. A video camera was mounted directly above the tank to record the rats’ swim paths. The parameters escape latency (time to reach the platform, in seconds), the path length (distance traveled to reach the platform, in centimeters) and swimming speed were analyzed for all 6 days.
The animals were given 60-s training trial session 4 times each day, over 6 consecutive days. For each training trial, the rats were placed in the water facing the pool wall at one of the four starting positions (N, S, E and W, varied from trial to trial in a pseudorandom order) and were given 60 s to reach the platform. Rats were carefully guided by hand to the platform if they failed to locate it within 60 s. Regardless of success or failure to reach the hidden platform; all rats were left on the platform for 15 s during the inter-trial interval and then placed in their heated cage until their next trial on the next day (26).
After the completion of MWM test, one animal from each group was randomly selected and received neutral red in order to infer the location of the cannulae.
The animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg) and then their brains were removed. The samples were kept in formalin (20%) and transferred to the histology laboratory for histological confirmation.
Measurements of oxidative stress markers in the hippocampus
After termination of behavioral testing days, rats were killed by decapitation and the hippocampi were quickly removed and kept at -70 °C until used for preparation of homogenates. At the day of analysis, the hippocampus tissues was homogenized in cold KCl solution (1.15%) to give a 10% homogenous suspension and used for biochemical assays. The level of total protein in supernatants was determined by the Bradford method using bovine serum albumin as the standard (18).
Total antioxidant activity assay
Total antioxidant activity was measured by ferric reducing antioxidant power (FRAP) according to the method of Benzie and Strain (1999) with some modifications. FRAP reagent was prepared freshly by mixing 2.5 ml of 2,4,6-Tripyridyl-s-Triazine (TPTZ) solutions (10 mM, dissolved in 40 mM HCl) and FeCl3 (20 mM) in 25 ml of acetate buffer (300 mM concentration and pH, 3.6), the light blue reagent contains Fe3+–TPTZ that changes to dark blue after interaction with antioxidants, which is explained by the presence of Fe2+–TPTZ in the reagent. These alterations were associated with the absorbance increase as monitored at a wavelength of 593 nm. Ferric reducing antioxidant power (FRAP) values were achieved by standard calibration curve obtained by using different concentrations of FeSO47H2O. FRAP values are expressed as mmol Fe2+/L (27).
Lipid peroxidation assay
Malondialdehyde (MDA) results from degradation of polyunsaturated fatty acids. The production of this substance is used as a biomarker to measure the level of lipid peroxidation. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to form a 1:2 MDA-TBA adduct, which absorbs light at 535 nm. Thus, the quantity of TBARS is proportion to the amount of MDA. Concentration of TBARS was determined according to the method of Mihara and Uchiyama (1978). Briefly, 3 ml of 1% phosphoric acid and 1 ml of 0.6% w/v TBA aqueous solution were added to 0.5 ml of homogenate supernatant and heated for 45 min in a boiling water bath. After cooling, 4 ml n-butanol was added; the mixture was shaken and then centrifuged at 3,000 × g for 10 min. Then the absorbance of the samples was measured at 535 nm. The concentration of TBARS was calculated using the MDA standard curve and is expressed as nmol/mg of protein (18).
Antioxidant enzyme activity assays
Glutathione peroxidase assay
GPx activity was measured in homogenates by the method of Paglia and Valentine (1967). GPx catalyzes the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH), the oxidized glutathione was immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The diminution in absorbance of NADPH was measured at 340 nm (Ransel kit, Randox, UK) (18).
Superoxide dismutase assay
Superoxide dismutase (SOD) activity in the hippocampus homogenates was assayed using a method based on the ability of the enzyme to inhibit the autoxidation of pyrogallol. Briefly, 1 ml of Tris-HCl (45 mM) buffer containing ethylenediaminetetraacetic acid (EDTA) was mixed with 5 μl of homogenate supernatant and was placed in the spectrophotometer. The unit was autozerod at 420 nm and then 50 μl of pyrogallol (0.2 mM) was added to the above solution and the absorbance of samples was quickly measured at 420 nm every 15 s, up to 2 min. The inhibition of pyrogallol autoxidation is proportional to the activity of SOD present in the sample. Enzyme inhibitory capacity is defined as one unit of SOD (28).
Statistical analysis
The values were presented as mean ± standard error of the mean (SEM). Statistical differences were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey test. Statistical significance was accepted at P<0.05 and P<0.01.
RESULTS
Saffron extract ameliorates cognitive impairment in experimental models of MS. As shown in Fig. 1, the swim speed of the animals revealed no differences between groups during the MWM test trial. Each animal swimming speed was recorded in order to control for any differences in the water maze performance. The latency data of the experimental groups during the 6 days of MWM test are illustrated in Fig. 2. The mean latency time in finding the hidden platform decreased during the test trial in all groups. Between-group comparisons indicated that for the animals from group III took longer to find the platform than the other groups on days 3, 5 and 6 (P<0.05).
Fig. 1.
Swimming speed during the test trial by rats demyelinated with ethidium bromide and rats demyelinated and treated with saffron extract in the MWM test. Groups: I; Control, no treatment,; II; Sham, saline, III; EB, IV, EB + 5 μg saffron extract and V; EB + 10 μg saffaron extract. Data points are mean ± SEM (n=8, in each group).
Fig. 2.
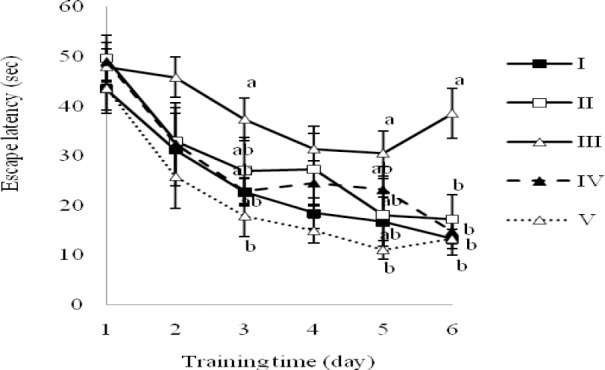
Escape latency to reach hidden platform during the test trial by rats demyelinated with ethidium bromide and treated with saffron extract in the MWM test. Groups: I; Control, no treatment, II, Sham, saline, III; EB,; IV; EB + 5 μg saffron extract and V; EB + 10 μg saffron extract. Data points are mean ± SEM (n=8, in each group). Groups with different letters are statistically different on the same day (P<0.05).
The data revealed a dose-dependent pattern in the saffron-treated groups, because the escape latency of the group V was significantly shorter than those of the group III on day 3, 5 and 6 (P<0.05); while the same parameter for group IV was statistically different from group III, just on the last day (day 6) (P<0.05). The differences between the groups I, II, IV and V were not significant on any day (Fig. 2). Fig. 3 represents the traveled distance to reach to hidden platform during the 6 successive days of training.
Fig. 3.
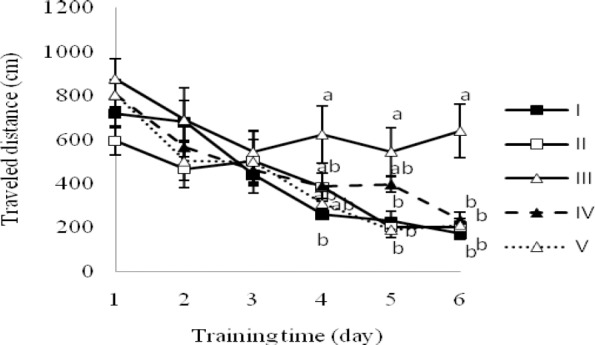
Distance traveled to reach the platform during the test trial by rats demyelinated with ethidium bromide and treated with saffron extract in the MWM test. Groups: I; Control, no treatment, II; Sham, saline, III; EB, IV; EB + 5 μg saffron extract and V; EB + 10 μg saffron extract. Data points are mean ± SEM (n=8, in each group). a and b indicate differences between groups on the same day (P<0.05).
The sham-operated groups (I and II) and the saffron 10 μg/rat group (V) swam the shorter distance to reach the hidden platform in comparison with EB-demyelinated rats (group III) on day 5 and 6 (P< 0.05, Fig. 3). Likewise, the traveled distance to find the platform in saffron-treated groups (IV and V) indicated a dose-dependent pattern owing to statistical-difference between the groups III and IV just on the last day of training (P<0.05) (Fig. 3).
The results indicated a significant increase in FRAP value, TBARS levels and activity of antioxidant enzymes; GPx and SOD in the group demyelinated by EB (group III) compared to normal control rats (groups I and II) (P < 0.01 and P<0.05) (Fig. 4, Fig. 5, Table 1). The ethanolic extract of saffron with doses of 5 μg/rat and 10 μg/rat decreased antioxidant power (FRAP value) and TBARS levels (as an indicator of lipid peroxidation) into normal levels as seen in groups I and II, in EB-demyelinated rats treated with saffron extract (groups IV and V) (P< 0.01) (Fig. 4, Fig. 5). GPx activity declined dose-dependently in saffron-treated groups (IV and V) in comparison with group III (P<0.05, Table 1). Similarly, the activity of SOD was declined in experimental models of MS treated with ethanolic extract of saffron, however, saffron extract treatment could not restore the SOD activity into the normal levels seen in groups I and II (P<0.05, Table 1).
Fig. 4.
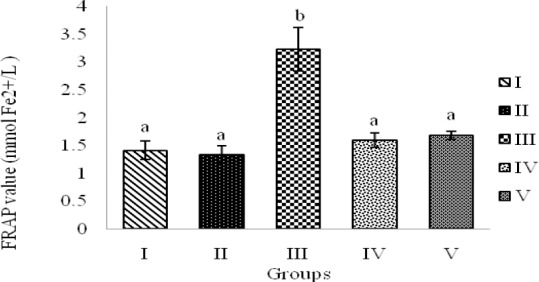
FRAP value in hippocampal homogenate samples of rats demyelinated with ethidium bromide and treated with saffron extract (SE). Groups: I; Control, no treatment, II; Sham, saline, III; EB, IV; EB + 5 μg SE and V; EB + 10 μg SE. Bars represent mean ± SEM (n=8, in each group). a and b indicate statistical differences between groups (P<0.01).
Fig. 5.
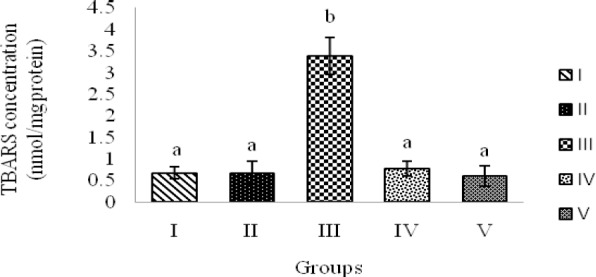
TBARS levels in hippocampal homogenate samples of rats demyelinated with ethidium bromide and treated with saffron extract. Groups: I; Control, no treatment, II; Sham, saline, III; EB, IV; EB + 5 μg saffron extract and V; EB + 10 μg saffron extract. Bars represent mean ± SEM (n=8, in each group). a and b indicate statistical differences between groups (P<0.01).
Table 1.
Antioxidant enzyme activities in rats demyelinated with ethidium bromide and treated with saffron extract.

DISCUSSION
The main findings of the present study are that direct single injection of EB impairs spatial learning and memory and induces oxidative stress. These harmful effects of EB in experimental models of MS can be alleviated by saffron extract treatment for 7 consecutive days, suggesting that this herbal remedy has potential therapeutic applications protecting against the detrimental effects of toxic damage on cognitive functions via modulation of oxidative stress markers.
EB induces focal demyelination by selectively damaging glial cells, which include oligodendrocytes (CNS myelin forming cells) and astrocytes (29). Several studies have proposed that demyelinating insults occur in the CNS gray matter of MS patients. Hippocampal formation is known as one of the important gray matters which are reported to be affected by MS (30). Using this model, our group found that direct single injection of a 0.01% EB solution into the CA1 of hippocampal formation impairs hippocampal-dependent spatial learning and memory performance (Fig. 2 and Fig. 3, group III). The finding that microinjection of EB into the hippocampal formation debilitates spatial learning and memory is in agreement with other studies indicating similar deficits in spatial learning and memory performance in autoimmune models of MS (6,8,31). Although we found that direct injection of EB impairs spatial learning and memory, it is required to construe whether altered acquisition reflects derangement of learning or memory. In our experiment, the escape latency time, the traveled distances to reach the hidden platform and the speed of the animals during trials were used to assess acquisition of the water maze task. Analyses of swimming velocity to reach the hidden platform revealed no differences between experimental groups (Fig. 1), disproving any non-specific effects of EB microinjection on spatial acquisition and memory. These results demonstrate that the impairing effects of gliotoxin microinjection on spatial learning and memory are not due to any non-specific fluctuations in gross motor activity or motivational state.
It would seem the impairing effects of EB microinjection on learning and memory are mainly mediated via increasing of oxidative stress, which causes irreparable damage to the brain and the neurons (32,33). As explained in the introduction, brain cells are at particular risk of being damaged by free radicals because the brain has a high oxygen turnover, and central nervous system neuronal membranes are rich in PUFA that are potential targets for lipid peroxidation. In the other words, ROS can react with PUFA to form lipid peroxides, and the accumulation of the end products of lipid peroxidation due to oxidative stress may contribute to cognitive deficits (18). Experimental demyelination also altered some oxidative stress markers. In the present study, our findings demonstrate that local injection of EB into the rat hippocampus resulted in the increased oxidative burden (Fig. 4, Fig. 5, Table 1, group III). These results corroborate the findings of other researchers showed that EB as one of the important gliotoxins induces oxidative stress (11,14,29); however, our outcomes are not completely in line with the existing data in the literature. These findings nonetheless confirm the idea that direct single injection of EB into the hippocampal formation affects spatial learning and memory via oxidative stress and the subsequent neural injury. Although different mechanisms may contribute to demyelination and neurodegeneration in MS, it recently became clear that mitochondrial injury and subsequent energy failure are major factors driving tissue injury. Accordingly, it seems mitochondrial alterations secondary to oxidative damage are likely to lead to an enhanced endogenous production of ROS. This selfamplifying process may contribute to neurodegeneration in MS (10), which led to subsequent cognitive deficits.
The findings of the present study revealed that microinjection of saffron extract with two different doses significantly improved spatial memory deficits in experimental models of MS (Fig. 2 and Fig. 3, groups IV and V). The data showed that in comparison with group III, saffron extract could significantly reduce the escape latency time and the traveled distance in finding the hidden platform. No significant difference was observed in the swim speed between experimental groups (Fig. 1). It meant that decreasing the escape latency was not due to the effect of saffron extract on swimming speed. These data indicates that the saffron extract did not have any effect on motor ability, either. Therefore, the present study demonstrates that the impairment of learning acquisition induced by EB microinjection in toxic models of MS is ameliorated by saffron extract in a dose-dependent manner. These findings are in agreement with previous studies showing saffron extract ameliorate impairments of learning and memory in a variety of tasks and conditions (18,34,35). This established effect may be due to the antioxidative properties of saffron. Saffron contains many carotenoids, i.e., crocin, crocetin, safranal and other antioxidant compounds with powerful antioxidant effects which may protect CNS neurons from oxidative damage via preserving the cell redox status and energy metabolism (36,37). Saffron extract modulates oxidative stress markers in experimental models of MS. The results of present study show that saffron extract significantly modulates the levels of oxidative markers in the hippocampus (Fig. 4, Fig. 5, Table 1, groups IV and V). As mentioned above, the brain tissue is highly vulnerable to oxidative stress because of its oxidative damage potential (10,18). We found that increased oxidative burden induced by local injection of EB causes lipid oxidation in the hippocampus, and this effect was blocked by saffron extract treatment. This finding is in line with recent studies showing a decrement of lipid peroxidation products by saffron extract in some pathological conditions (18,38,39). We also found a significant elevation in total antioxidant power in the animals demyelinated with EB, which was restored into the normal levels by saffron extract treatment (Fig. 4). Although these results are not in line with the existing data in the literature (18,19,40), the finding reveals the antioxidant effects of this herbal remedy in experimental models of MS.
Organisms have some antioxidant defense enzymes to assert against oxidative damage. These antioxidant defense enzymes comprise SOD and GPx. In agreement with a recent study (18), we demonstrated a significant increase in the activities of these enzymes in the hippocampus following induction of oxidative burden (Table 1, group III). The results of the present study can be commented in terms of a very important mechanism: Oxidative stress activates a specific stress response, an adaptive mechanism aimed to protect cells against ROS mediated toxicity and to maintain tissue redox balance. This stress response includes enhanced protein expression of endogenous antioxidant enzymes.
Antioxidant enzymes can selectively detoxify various types of ROS. In fact, recent studies clearly show that several antioxidant enzymes are significantly increased in inflammatory and demyelinated lesions of MS, including SOD (10,41). On the other hand, augmentation of hippocampal GPx and SOD activities following microinjection of EB suggests that elevated oxidative burden induce increased production of reactive oxygen species which accompanied with a compensatory adaption of the tested free-radical scavenging enzymes. We have indicated that seven-day treatment with a saffron extract reduced the activities of these enzymes (Table 1, groups IV and V). It has been illustrated that saffron extract is a potent antioxidant which can scavenge free radicals, especially superoxide anions, and thereby may protect cells from oxidative stress (35,42). Indeed, saffron extract leads to depletions of ROS, which in turn declines the need for antioxidant enzymes. Another possibility is that saffron extract directly modulates synthesis of these enzymes (18). Saffron extract is known to scavenge free radicals (37) and has been demonstrated to exert protective actions in different pathological models, including anti-inflammatory (43), antidepressant (44), anticonvulsant (45) and antinociceptive activities (46). As well as saffron extract and its active constituents, crocin and safranal, have an overall protective effect against chronic cerebral hypoperfusion and muscle ischemia-reperfusion in a rat model (35,47).
The outcomes of present study represent that microinjection of saffron extract for 7 days in toxic models of MS alleviated the oxidative damage induced by EB and significantly restored the mentioned biochemical parameters near normalcy. Moreover, our findings that saffron extract can alleviate the oxidative stress and cognitive deficits induced by single direct injection of EB in experimental models of MS provide evidence of the possible therapeutic effects of saffron extract in neurodegenerative disorders, which have been identified by oxidative stress as basic pathophysiology and cognitive impairment as a major clinical complication.
CONCLUSION
In conclusion, the present work demonstrates that saffron extract can ameliorate EB-induced deficits in spatial learning and memory and oxidative stress in the hippocampus. Thus, this substance should be useful as a new pharmacological entity for studying the mechanism of oxidative stress-induced cognitive impairment and for alleviating cognitive deficits. In fact, these results suggest that saffron extract as potent antioxidant combats oxidative stress in neurons and could be useful in the therapy of brain neurodegenerative disorders such as multiple sclerosis.
ACKNOWLEDGMENTS
Financial support of the Research Foundation of Tabriz University is highly appreciated.
REFERENCES
- 1.Mozafari S, Sherafat MA, Javan M, Mirnajafi-zadeh J, Tiraihi T. Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res. 2010;1351:50–56. doi: 10.1016/j.brainres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Kouhsar SS, Karami M, Tafreshi AP, Roghani M, Jalali Nadoushan MR. Microinjection of L-arginine into corpus callosum cause reduction in myelin concentration and neuroinflammation. Brain Res. 2011;1392:93–100. doi: 10.1016/j.brainres.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Patel JR, Klein RS. Mediators of oligodendrocyte differentiation during remyelination. FEBS Lett. 2011;585:3730–3737. doi: 10.1016/j.febslet.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartori E, Edan G. Assessment of cognitive dysfunction in multiple sclerosis. J Neurol Sci. 2006;245:169–175. doi: 10.1016/j.jns.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Glanz BI, Healy BC, Hviid LE, Chitnis T, Weiner HL. Cognitive deterioration in patients with early multiple sclerosis: a 5-year study. J Neurol Neurosurg Psychiatry. 2012;83:38–43. doi: 10.1136/jnnp.2010.237834. [DOI] [PubMed] [Google Scholar]
- 6.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA 1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30:289–299. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PloS One. 2012;7:e35476–e35484. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acar A, Cevik MU, Evliyaoglu O, Uzar E, Tamam Y, Arıkanoglu A, et al. Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol Belg. 2012;112:275–280. doi: 10.1007/s13760-012-0059-4. [DOI] [PubMed] [Google Scholar]
- 10.Van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med. 2008;45:1729–1737. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Salam OM, Khadrawy YA, Mohammed NA. Neuroprotective effect of nitric oxide donor isosorbide-dinitrate against oxidative stress induced by ethidium bromide in rat brain. EXCLI J. 2012;11:125–141. [PMC free article] [PubMed] [Google Scholar]
- 12.Ziehn MO, Avedisian AA, Dervin SM, Umeda EA, O’Dell TJ, Voskuhl RR. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci. 2012;32:12312–12324. doi: 10.1523/JNEUROSCI.2796-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzanti CM, Spanevello R, Ahmed M, Schmatz R, Mazzanti A, Salbego FZ, et al. Cyclosporine A inhibits acetylcholinesterase activity in rats experimentally demyelinated with ethidium bromide. Int J Dev Neurosci. 2007;25:259–264. doi: 10.1016/j.ijdevneu.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Salam OM, Khadrawy YA, Salem NA, Sleem AA. Oxidative stress in a model of toxic demyelination in rat brain: the effect of piracetam and vinpocetine. Neurochem Res. 2011;36:1062–1072. doi: 10.1007/s11064-011-0450-1. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Poduval TB, Tilak JC, Devasagayam TPA. A modified, economic, sensitive method for measuring total antioxidant capacities of human plasma and natural compounds using Indian saffron (Crocus sativus) Clin Chim Acta. 2005;352:155–163. doi: 10.1016/j.cccn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of a-tocopherol. Neurosci Lett. 2004;362:61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 17.Fukui H, Toyoshima K, Komaki R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus) Phytomedicine. 2011;18:726–730. doi: 10.1016/j.phymed.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Bandegi AR, Motamedi F, Haghighi S, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Nam KN, Park Y, Jung H, Lee JY, Min BD, Park S, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura M, Saito H, Abe K, Shoyama Y. Ethanol extract of Crocus sativus L. Antagonizes the inhibitory action of ethanol on hippocampal long-term potentiation in vivo. Phytother Res. 1995;9:100–104. [Google Scholar]
- 23.Ramadan A, Soliman G, Mahmoud SS, Nofal SM, Abdel-Rahman RF. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J Saudi Chem Soc. 2012;16:13–21. [Google Scholar]
- 24.Paxinos G, Watson C. 6th ed. New York: Academic press; 2006. The rat brain in stereotaxic coordinates; pp. 52–67. [Google Scholar]
- 25.Hooshmandi Z, Rohani AH, Eidi A, Fatahi Z, Golmanesh L, Sahraei H. Reduction of metabolic and behavioral signs of acute stress in male Wistar rats by saffron water extract and its constituent safranal. Pharm Biol. 2011;49:947–954. doi: 10.3109/13880209.2011.558103. [DOI] [PubMed] [Google Scholar]
- 26.Deng XH, Ai WM, Lei DL, Luo XG, Yan XX, Li Z. Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning–memory deficit in rats. Neuroscience. 2012;209:161–170. doi: 10.1016/j.neuroscience.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Dehghan G, Khoshkam Z. Tin (II)-quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012;131:422–426. [Google Scholar]
- 28.Gao R, Yuan Z, Zhao Z, Gao X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectroch Bioener. 1998;45:41–45. [Google Scholar]
- 29.Spanevello R, Mazzanti CM, Schmatz R, Bagatini M, Stefanello N, Correa M, et al. Effect of vitamin E on ectonucleotidase activities in synaptosomes and platelets and parameters of oxidative stress in rats experimentally demyelinated. Brain Res Bull. 2009;80:45–51. doi: 10.1016/j.brainresbull.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Geurts JJ, Bö L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 31.Nizri E, Irony-Tur-Sinai M, Faranesh N, Lavon I, Lavi E, Weinstock M, et al. Suppression of neuroinflammation and immunomodulation by the acetylcholinesterase inhibitor rivastigmine. J Neuroimmunol. 2008;203:12–22. doi: 10.1016/j.jneuroim.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Tasset I, Agüera E, Sánchez-López F, Feijóo M, Giraldo AI, Cruz AH, et al. Peripheral oxidative stress in relapsing–remitting multiple sclerosis. Clin Biochem. 2012;45:440–444. doi: 10.1016/j.clinbiochem.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dashti-r MH, Zeinali F, Anvari M, Hosseini SM. Saffron (Crocus sativus L.) extract prevents and improves D-galactose and NaNO2 induced memory impairment in mice. EXCLI J. 2012;11:328–337. [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 36.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 37.Del-Angel DS, Martínez NLH, Cruz MEG, Urrutia EC, Riverón-Negrete L, Abdullaev F. Saffron extract ameliorates oxidative damage and mitochondrial dysfunction in the rat brain. Acta Hort (ISHS) 2007;739:359–366. [Google Scholar]
- 38.El-Beshbishy HA, Hassan MH, Aly HA, Doghish AS, Alghaithy AAA. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf. 2012;83:47–54. doi: 10.1016/j.ecoenv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Das I, Das S, Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: a histopathological study. Acta Histochem. 2010;112:317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Shati A, Elsaid F, Hafez E. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011;175:66–74. doi: 10.1016/j.neuroscience.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Schreibelt G, Van Horssen J, Van Rossum S, Dijkstra CD, Drukarch B, de Vries HE. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev. 2007;56:322–330. doi: 10.1016/j.brainresrev.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–152. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Vosooghi S, Mahmoudabady M, Neamati A, Aghababa H. Preventive effects of hydroalcoholic extract of saffron on hematological parameters of experimental asthmatic rats. Avicenna J Phytomed. 2013;3:279–287. [PMC free article] [PubMed] [Google Scholar]
- 44.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Irn Med. 2002;5:44–47. [Google Scholar]
- 46.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83:888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Hosseinzadeh H, Modaghegh MH, Saffari Z. rocus sativus L. (saffron) extract ad its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]


