Abstract
Angiogenesis, formation of new blood vessels, play an important role in some diseases such as cancer and its metastasis. Using angiogenesis inhibitors, therefore, is one of the ways for cancer treatment and prevention of metastasis. Medicinal plants have been shown to play a major role in the treatment of a variety of cancers. In this direction, cytotoxic and angiogenic effects of oleo gum resin extracts of Rhus coriaria, Pistacia vera and Pistacia khinjuk from Anacardiaceae family were studied. For IC50 values, cytotoxic effects of the plant extracts were evaluated at different concentrations (1, 10, 20, 40, 80,100 μg/ml) against human umbilical vein endothelial normal cell (HUVEC) and Y79 cell lines using 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. In vitro tube formation on matrigel base was used to evaluate angiogenic effects in the presence of increasing concentrations (50, 100, 250 μg/ml) of the extracts. Vascular endothelium growth factor was used as angiogenesis stimulator. Gas chromatography results showed that α-pinene and β-pinene were the major essential oils constituents of all plant extracts. According to the MTT assay results, the R. coriaria resin extract was more cytotoxic than those of P. vera and P. khinjuk extracts (IC50, 9.1 ± 1.6 vs 9.8 ± 2.1 and 12.0 ± 1.9, respectively; P<0.05). Cytotoxic effects of all extracts against Y79 cell line was significantly higher than those of HUVEC used as a normal cell line (P<0.05). Tube formation assay also showed that extract of R. coriaria resin inhibited angiogenesis more significantly than other tested extracts (P<0.05). It could be concluded that R. coriaria resin extract possess cytotoxic effect and antiangiogenesis against cancer cells and as an anticancer natural product has a good potential for future studies.
Keywords: Angiogenesis, MTT assay, Rhus coriaria, Pistacia khinjuk, Pistacia vera
INTRODUCTION
Angiogenesis is defined as formation of new blood vessels in the presence of biochemical stimulator such as vascular endothelium growth factor (VEGF) and other angiogenic inducers. It can play an important role in wound healing, ocular disease embryonic improvement or in fatal disease like cancer and its metastasis.
Therefore one of the strategies to stop or treat cancers is prevention of angiogenesis by antiangiogenic agents. Recently, some antiangiogenic drugs including thalidomide, avastin, and endostatin have been approved by Food and Drug Administration (FDA) to use in chemotherapy (1,2).
Medicinal plants have been considered as a valuable source to discover new chemotherapeutic agent including camptothecin (cytotoxicity effect), paclitaxel (mitotic inhibitor), combretastatin (inhibitor of tubulin polymerization) and vinblastine (anti microtubular agent) (3,4).
Pistacia and Rhus genera from anacardiaceae family are growing wild in central parts of Iran. In Iranian folk medicine, Pistacia vera L. (Vera) and Pistacia khinjuk Stoks. (Khinjuk) are used for the treatment of eczema, throat infection, renal stone, and asthma.
Distinguished Persian scientists like Avicenna, and Rhazes were familiar with these kinds of herbal treatments and classified them for the treating of different kinds of wounds such as abrasion, punctured, cut, perforated, chronic, purulent, and septic wounds (3,4,5,6,7,8).
In addition, some Pistacia species have shown various biological activities including anti atherogenic, antioxidant, antifungal and antiinflammatory (9,10). Also, recent research on some plants of this family such as P. lentiscus have demonstrated cytotoxic and antiangiogenic effects (11). P. atlantica resin also has angiogenesis effects in wound healing in low dose and opposite effect in high dose. R. verniciflua as another species of this family also showed cytotoxic effect (12,13).
R. coriaria (Persian name: Sumac) in this family, has been traditionally introduced in the treatment of ocular disease. Famous persian scientists like Avicenna and Rhazes used sumac oleoresin for the treatment of fibrovascular proliferation in the eye's conjunctiva (14,15,16).
Since in the folklore medicine resins of these plants have been used as ocular remedies, in the present study the cytotoxic and antiangiogenesis effects of resins of titled plants (endemic to Iran) were evaluated against cancer and normal cell, using 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and capillary like tube formation assay.
MATERIALS AND METHODS
Plant material and extraction
Oleo gum resins of R. coriaria, P. khinjuk, and P. vera were collected from Markazi province and Bakhtiari Zagross Mountain. Voucher specimens of R. coriaria, P. khinjuk, and P. vera (18004, 14218 and 1130, respectively) are deposited in the department of biology, Isfahan University, Iran.
Frozen oleo resins (500 mg) were ground into powder and then extracted with methanol for 24 h. Extracts were concentrated under reduced pressure to yield dried gummy materials which were stored at 4 °C until the use for their cytotoxic and antiangiogenic effects.
Phytochemical analysis
The essential oils of the oleo gum resins of R. coriaria, P. khinjuk, and P. vera were collected by hydro-distillation method using a clevenger-type apparatus for 3 h according to the method recommended in the British Pharmacopoeia (17). Gas chromatography mass spectrometry (GC/MS) was used for analysis of the essential oils on an Agilent 5975C mass selective detector coupled with an Agilent 7890A GC, equipped with a HP-5 GC capillary column (30 m × 0.25 mm; film thickness 0.25 μm, USA). Oven temperature was programmed from 60 to 280 °C at 4 °C/min. Helium was used as a carrier gas at a flow rate of 2 ml/min. Injector and detector temperatures were set at 280 °C. Components of the oil were identified by comparison of their retention indices (RI) and fragmentation patterns of the mass spectra with those reported in the literature (6) and computer matching with NIST and Wiley275L libraries.
Gas chromatography analysis for standardization of the extracts
α-Pinene (Sigma Aldrich, USA) at concentrations of (5, 50, 100, 250, 500 and 5000 μg/ml) in addition to fenchyl acetate (Sigma Aldrich, USA) at a concentration of 400 μg/ml (used as internal standard) were used to construct the calibration curve. Concentrations of α-pinene in the samples were determined from the regression equations relating measured peak-height ratios of the standards to their concentrations using the minimum square method (R2 value). GC determinations were run on a GC-2550 (Teif Gostar Faraz, Iran) instrument using a TRB1 capillary column (30 m × 0.25 mm; film thickness: 1 μm). The carrier gas was hydrogen with a flow rate of 40 cm3/min. The oven temperature was programmed to 80 °C (hold 2.5 min) to 100 °C at 6 °C/min to 150 °C at10 °C/min to 200 °C at 7 °C/min to 250 °C at 5 °C/min (hold 2.0 min). Injector and detector temperatures were 250 and 280 °C, respectively. The extract (200 mg) was pretreated on Sep-Pak silica 6cc Vac Cartridge (Waters, USA), with hexane:ethyl acetate (90:10) as solvent to absorb the polar constituents. The obtained fraction was concentrated to 1 ml and 2 μl of it was injected to the GC with a spilt less time of 0.6 min.
Cell culture
Human umbilical vein endothelial normal cell (HUVEC) and retinoblastoma cell (Y79) purchased from national cell bank of Pasteur institute (Tehran, Iran) and cultured in roswell park memorial institute (RPMI) 1640 medium (Gibco, USA) with 10% FBS (Gibco) in humidified incubator with 5% CO2.
MTT assay
HUVEC and Y79 cells were seeded at a density of 7 × 105 cell/ml and 6 × 105 cell/ml, respectively and incubated for 24 h. Then 20 μl of various concentrations (from 1 μg/ml to 100 μg/ml) of each gum extracts (Sumac, Vera, Khinjuk) were added and plates were incubated for 48 h. After treatment period, 20 μl of MTT 0.5% (Merck, Germany) was added and further incubated for 3 h. Then supernatant of each well was replaced with 150 μl of DMSO (Merck, Germany) to dissolve the formed formazan crystals.
In the case of Y79 growing in suspension, plate was first centrifuged (Hettich universal, UK) and then the media were replaced with DMSO. The absorbances were determined at 570 nm with an enzyme-linked immunosorbent assay (ELISA) plate reader (Statfax-2100, Awareness USA).
Essential oils were applied in three wells, whilst 6 wells of untreated cells were used as negative control with 100% viability and percent cell survival was calculated as follows (18).
% Cell survival = [(OD of treated group – OD of blank group)/(OD of negative group – OD of blank group)] ×100 (1)
MTT assay was performed in 24 h and 48 h exposure to evaluate if cytotoxic effect was time dependent or not.
Capillary like tube formation assay
Tube formation assay was used to evaluate the angiogenic or antiangiogenic effects of the extracts against the ability of endothelial cells to form tube in matrigel base.
Matrigel (Geltrex®) was added carefully to each well (100 μl) in a 24-well plate (Nunc, Denmark) to form a jelly base with flat surface. Then, the plate was incubated for 30 min in 37 °C allowing gel formation. One hundred μl of HUVEC cell suspension at a density of 105 cells/well were seeded in each well and then treated with different extract so that the final concentrations were 50, 100 and 250 μg/ml and culture medium was added up to 300 μl. Avastin, as an antiangiogenesis standard drug, (25 ng/ml) was used as positive control. Finally plate was incubated at 37 °C for 24 h. After incubation tube formation was observed and photographed with microscope and the images were analyzed with Angioquant V1.33 software (Math Works, Natick, MA) (19).
RESULTS
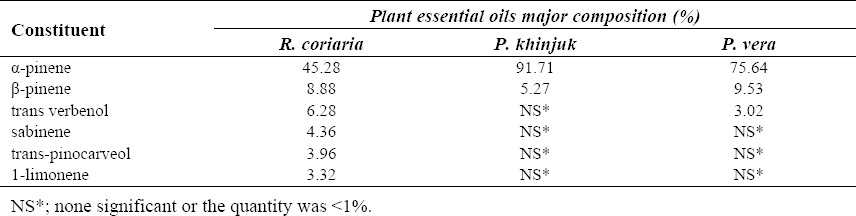
The weight of the gum resins of R. coriaria, P. khinjuk, and P. vera after extraction was 61.7, 80.6, and 76.0% (w/w), respectively. The essential oil of the oleo gum resins of R. coriaria, P. khinjuk, and P. vera were analyzed by GC-MS. Results are shown in Table 1. Using GC, α-pinene as a major component of all oleo gum resins was standardized by internal standard calibration method. Methanolic extract of the oleo gum resins were collected and standardized using α-pinene as the bioactive marker for standardization of the extracts. Alpha-pinene and fenchyl acetate peaks of the extracts appeared at retention times of 7.55 and 13.06 min, respectively. Using calibration curve, the oleo gum resin extracts of R. coriaria, P. khinjuk, and P. vera contained 0.351 ± 0.028, 0.101 ± 0.032 and 1.149 ± 0.074 mg/100 mg of the extracts, respectively (P<0.05). The calibration curve was determined by linear regression in the range of 5-5000 μg/ml. The regression equation was, y=0.014 x-0.825, where x was the concentration of α-pinene in the tested sample (μg/ml) with the correlation co-factor (R2) of 0.999. Three determinations were carried out and the recovery percentage was calculated to be 60.2 ± 3.6%.
Table 1.
Major constituents essential oils of plant resins determined by GC-MS.
Cytotoxicity evaluation
The cytotoxic effects of the studied resin extracts against HUVEC (24 and 48 h exposure) and Y79 (48 h exposure) cells are shown in Figs. 1–3, respectively.
Fig. 1.
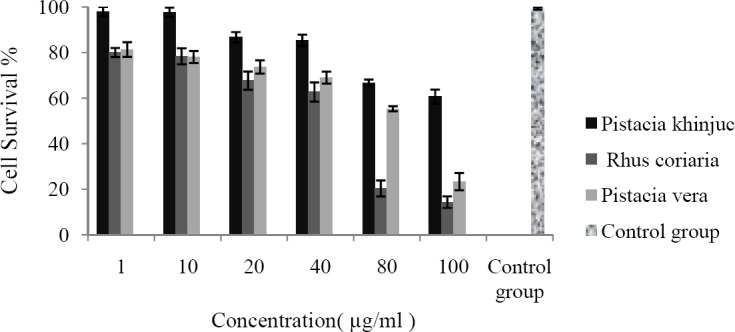
HUVEC cells were seeded in 96-well plates and incubated for 24 h. Cells were then treated with different extracts at various concentrations. Cell survival was measured after 24 h exposure, using MTT cytotoxicity assay. Data are shown as mean ± SD after 3 separate experiments (n = 3).
Fig. 3.
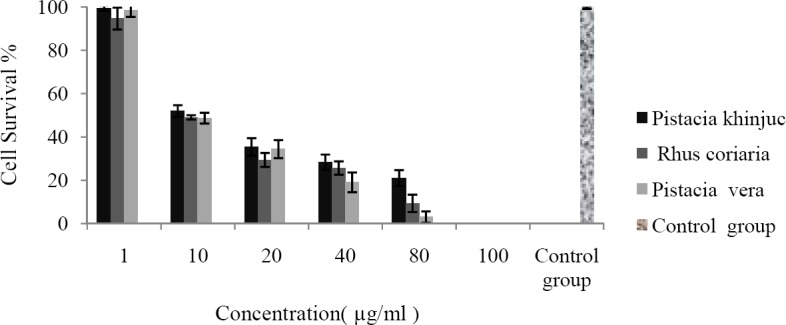
Y79 cells were seeded in 96 well plates and incubated for 24 h. Cells were then treated with different extracts at various concentrations. Cell survival was measured after 48 h exposure, using MTT cytotoxicity assay. Data are shown as mean ± SD after 3 separate experiments (n=3).
Fig. 2.
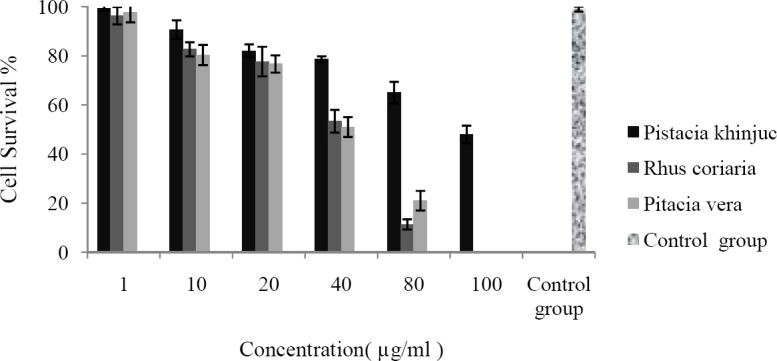
HUVEC cells were seeded in 96-well plates and incubated for 24 h. Cells were then treated with different extracts at various concentrations. Cell survival was measured after 48 h exposure, using MTT cytotoxicity assay. Data are shown as mean ± SD after 3 separate experiments (n=3).
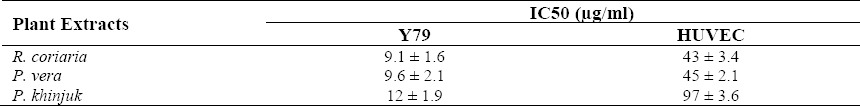
As shown in Table 2 the cytotoxic effects are time and concentration dependent so that IC50 values after 48 h exposure were lower than 24 h exposure or lower concentration (Figs. 1–3). Y79 used as a cancer cell line was more sensitive to the tested extracts than HUVEC which was used as normal cells. Control group which was cell suspension without any treatment assumed 100% survival.
Table 2.
The IC50 value of tested extracts against Y79 and HUVEC cell lines.
Capillary like tube formation assay
The results of tube formation assay based on the ability of endothelial cells to form capillary-like tubular structures in the presence of growth factors, such as VEGF are shown in Fig. 4. VEGF is the key regulator of angiogenesis. Upon quantification of the capillary-tube-like structures using the Angioquant software, R. coriaria, P. vera and P. khinjuk oleo gum resin extracts, inhibited VEGF-induced tube-like structures at concentration of 250 μg/ml to 87.59 ± 1.8, 86.22 ± 1.4, and 54.21 ± 2.8%, respectively. The IC50 values of the antiangiogenic effects of these extracts were 118.86 ± 5.14, 122.72 ± 4.36, and 211.26 ± 15.37 μg/ml, respectively. Avastin (25 ng/ml) showed 57.17 ± 1.76% antiangiogenic effects (Fig. 5).
Fig. 4.
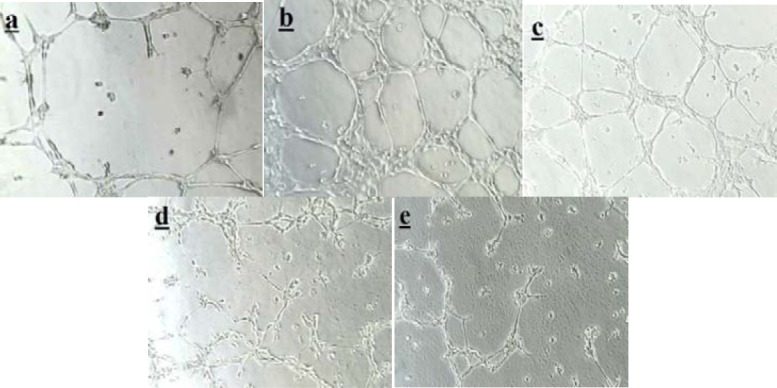
The effect of Rhus coriaria extract on VEGF induced tube formation in HUVEC cells, in comparison with avastin (as an angiogenesis inhibitor). a; VEGF has been used at a concentration of (10 ng/ml) as a positive control, b; Avastin (25 ng/ml) was used as an antiangiogenic compound after tube formation was induced by VEGF. Different concentrations of Rhus coriaria extract were used as a natural antiangiogenic compound after tube formation induced by VEGF, c; 50 μg/ml, d; 100 μg/ml, e; 250 μg/ml.
Fig. 5.
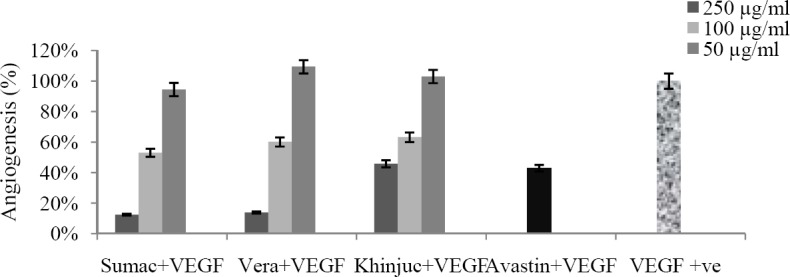
The tube formation effect of different oleo gum resin extracts of tested plants in HUVEC cells. Cells were seeded at a concentration of 1×105 cell/well in a 24 well culture plate pre-coated with matrigel. VEGF used at a concentration of 10 ng/ml (n=3, P<0.05).
Based on the results obtained from these experiments, IC50 of R. coriaria, P. vera, and P. khinjuk was calculated to be 118.86, 122.72 and 211.26 μg/ml, respectively. According to these results, Sumac showed more antiangiogenic effect than the other plants.
DISCUSSION
Biological effects of the Anacardiaceae family plants (endemic to Iran) have not been fully investigated. In an effort to find new natural compounds to cure pathological conditions such as cancer or angiogenesis related disease, 3 plants of this family have been evaluated in the current study. Angiogenesis plays an important role in the survival or metastasis of cancer cells. Increased VEGF-induced angiogenesis have been reported already (20). Antiangiogenic agents derived from plants may have the ability to prevent angiogenesis in cancer diseases and their metastasis.
Unlike previous reports that phellandrene with 52% was the main constituent of essential oils of P. khinjuk and α-pinene with 15% was in the second place (21), in the present study GC/MS measurements of the essential oils showed that α-pinene was the major costituent of resins in all tested plants. Our results were consistent with Haghdosst and coworkers who analyzed P. Atlanta resin oil and found that α-pinene with 46.6% and β-pinene with 9.1% were the major constituents of this resin oil (5).
In this regard, Bhattacharjee and Chatterjee also showed that α-pinene and β-pinene were the principle constituents of cardamom essential oil and exhibited proapoptotic, antiproliferative, anti-invasive, and antiangiogenic effects (22).
R. Coriaria gum extract showed well cytotoxic and antiangiogenic effects. These results were consistent with others, who showed that many plants containing α-pinene and β-pinene in their essential oil had good cytotoxic and antiangigenic effects (11,23,24,25,26).
Similar to our study many investigations reported that extracts and essential oils of gum resins from many medicinal plants exhibited cytotoxic effects (27), antineoplastic activities (28), antimetastastatic effects (29), antiangiogenic effects (30,31) and apoptotic induction (32).Beside α-pinene and β-pinene, compounds like gallic acid from different parts of R. coriaria also showed cytotoxic and antiangiogenic activity (16,33). Loutrari and coworkers (11) showed that mastic oil from P. lentiscus possessed tumor chemopreventive and antiangiogenic properties. They also observed antiproliferative and proapoptotic effects of mastic oil against K562 and inhibition of the release of VEGF from K562 and B16 as mouse melanoma cells with concentration and time dependent manner. We obtained the same results from our gum extracts against Y79 and HUVEC cells (Fig. 5).
CONCLUSION
In conclusion, the essential oils of the tested oleo gum resins contained α-pinene or β-pinene as major constituents. All studied gum resin extracts exhibited more cytotoxic and antiangiogenic effects against cancer cells than normal ones. R. coriaria extract which showed highest cancer cell toxicity, therefore, may have potentials for cancer treatment with minimum normal tissue toxicity.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from the Iranian Ministry of Health and Medical Education. The authors are also grateful to the support of Isfahan Pharmaceutical Sciences Research Center of Isfahan University of Medical Sciences (Isfahan, I.R. Iran). Technical assistance of Miss. Sara Milajerdi is greatly appreciated.
REFERENCES
- 1.Qian X, Zhu L, Hu J, Li M, Xie L, Wang L, et al. Rhizoma paridis ethanol extract selectively inhibits the proliferation of HUVECs comparing to Lovo cells and shows anti-angiogenesis effects in a mouse model. J Ethnopharmacol. 2012;143:256–261. doi: 10.1016/j.jep.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Antiangiogenesis in cancer therapy: Endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13:161–171. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Tayarani-Najaran Z, Emami SA. Cytotoxic plants: potential uses in prevention and treatment of cancer. In: Oner Ozdemir., editor. Current cancer treatment-novel beyond conventional approaches. 1st ed. Istanbul: InTech; 2011. pp. 651–692. [Google Scholar]
- 5.Haghdoost F, Baradaran Mahdavi MM, Zandifar A, Sanei MH, Zolfaghari B, Javanmard SH. Pistacia atlantica resin has a dose-dependent effect on angiogenesis and skin burn wound healing in rat. Evid Based Complement Alternat Med. 2013;2013:ID 893425. doi: 10.1155/2013/893425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alma MH, Nitz S, Kollmannsberger H, Digrak M, Efe FT, Yilmaz N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.) J Agric Food Chem. 2004;52:3911–3914. doi: 10.1021/jf040014e. [DOI] [PubMed] [Google Scholar]
- 7.Taran M, Sharifi M, Azizi E, Khanahmadi M. Antimicrobial activity of the leaves of Pistacia khinjuk. J Med Plants Res. 2010;9:81–85. [Google Scholar]
- 8.Özçelik B, Aslan M, Orhan I, Karaoglu T. Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microbiol Res. 2005;160:159–164. doi: 10.1016/j.micres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Scientific World Journal 2013. 2013 doi: 10.1155/2013/219815. ID 219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistacia vera) hull extracts. Food Chem. 2005;92:521–525. [Google Scholar]
- 11.Loutrari H, Magkouta S, Pyriochou A, Koika V, Kolisis FN, Papapetropoulos A, et al. Mastic oil from Pistacia lentiscus var. chia inhibits growth and survival of human K562 leukemia cells and attenuates angiogenesis. Nutr Cancer. 2006;55:86–93. doi: 10.1207/s15327914nc5501_11. [DOI] [PubMed] [Google Scholar]
- 12.Hong DH, Han SB, Lee CW, Park SH, Jeon YJ, Kim MJ, et al. Cytotoxicity of urushiols isolated from sap of Korean lacquer tree (Rhus vernicifera Stokes) Arch Pharm Res. 1999;22:638–641. doi: 10.1007/BF02975339. [DOI] [PubMed] [Google Scholar]
- 13.Nasar-Abbas SM, Halkman AK. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int J Food Microbiol. 2004;l97:63–69. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Kosar M, Bozan B, Temelli F, Baser KHC. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007;103:952–959. [Google Scholar]
- 15.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem Toxicol. 2010;48:854–858. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Regazzoni L, Arlandini E, Garzon D, Santagati NA, Beretta G, MaffeiFacino R. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J Pharm Biomed Anal. 2013;72:202–207. doi: 10.1016/j.jpba.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Vol. 2. London: HMSO Publication; 1988. British Pharmacopoeia; pp. A137–A138. [Google Scholar]
- 18.Mirian M, Zarghi A, Sadeghi S, Tabaraki P, Tavallaee M, Dadrass O, et al. Synthesis and cytotoxic evaluation of some novel sulfonamide derivatives against a few human cancer cells. Iran J Pharm Res. 2011;10:741–748. [PMC free article] [PubMed] [Google Scholar]
- 19.Dana N, Haghjooy Javanmard SH, Rafiee L. Inhibition of melanoma cell growth and angiogenesis by Punica granatum peel extract. 3rd world congress on cancer sciences and therapy, October 21-23. J Cancer Sci Ther. 2013;5:239. [Google Scholar]
- 20.Ghanadian SM, Ayatollahi AM, Afsharypuor S, Javanmard SH, Dana N. New mirsinane-type diterpenes from Euphorbia microsciadia Boiss. with inhibitory effect on VEGF-induced angiogenesis. J Nat Med. 013;67:327–332. doi: 10.1007/s11418-012-0686-3. [DOI] [PubMed] [Google Scholar]
- 21.Ghasemi-pirbalouti A, Aghaee K. Chemical composition of essential oil of Pistacia khinjuk Stocks grown in Bakhtiari Zagross mountains, Iran. Electr J Biology. 2011;7:67–69. [Google Scholar]
- 22.Bhattacharjee B, Chatterjee J. Identification of proapoptopic, anti-inflammatory, anti-proliferative, anti-invasive and anti-angiogenic targets of essential oils in cardamom by dual reverse virtual screening and binding pose analysis. Asian Pac J Cancer Prev. 2013;14:3735–3742. doi: 10.7314/apjcp.2013.14.6.3735. [DOI] [PubMed] [Google Scholar]
- 23.Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, et al. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritis fibroblast-like synovial cells and in vivo models. Int Immunopharmacol. 2009;9:268–276. doi: 10.1016/j.intimp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Demirci B, Kiyan T, Koparal A, Kaya M, Demirci F, Baser K. The in vivo and in vitro angiogenic evaluation of the essential oil of Echino phora tournefortii. Planta Medica. 2010;76:1346. [Google Scholar]
- 25.Kiyan HT, Demirci B, Baser KHC, Demirci F. The in vivo evaluation of anti-angiogenic effects of Hypericum essential oils using the chorioallantoic membrane assay. Pharm Biol. 2013;52:44–50. doi: 10.3109/13880209.2013.810647. [DOI] [PubMed] [Google Scholar]
- 26.Bostancıoğlu RB, Kürkçüoğlu M, Baþer KH, Koparal AT. Assessment of anti-angiogenic and anti-tumoral potentials of Origanum onites L. essential oil. Food Chem Toxicol. 2012;50:2002–2008. doi: 10.1016/j.fct.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 27.Yazdanpanahia N, Behbahanib M, Yektaeiana A. Effect of Boswellia thurifera gum methanol extract on cytotoxicity and P53 gene expression in human breast cancer cell line. Iran J Pharm Res. 2014;13:719–724. [PMC free article] [PubMed] [Google Scholar]
- 28.Ni X, Suhail MM, Yang Q, Cao A, Fung KM, Postier RG, et al. Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement Altern Med. 2012;13:1–14. doi: 10.1186/1472-6882-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almazari I, Surh YJ. Cancer chemopreventive and therapeutic potential of guggulsterone. Top Curr Chem. 2013;329:35–60. doi: 10.1007/128_2012_344. [DOI] [PubMed] [Google Scholar]
- 30.Lavaud A, Richomme P, Litaudon M, Andriantsitohaina R, Guilet D. Antiangiogenic tocotrienol derivatives from Garcinia amplexicaulis. J Nat Prod. 2013;76:2246–2252. doi: 10.1021/np400598y. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, He Sh, Li Sh, Zhang R, Peng A, Chen L. In vitro and in vivo antiangiogenic activity of caged polyprenylatedxanthones isolated from Garcinia hanburyi Hook. f. Molecules. 2013;11:15305–15313. doi: 10.3390/molecules181215305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Sh, Cha IH, Nam W. Chios mastic gum extracts as a potent antitumor agent that inhibits growth and induces apoptosis of oral cancer cells. Asian Pac J Cancer Prev. 2011;12:1877–1880. [PubMed] [Google Scholar]
- 33.Greenway FL, Liu Zh, Woltering EA. Angiogenic agents from plant extracts, gallic acid, and derivatives. U.S. Patent, 7,709,031, 2010. [Google Scholar]


