Abstract
Objective:
This study aimed to investigate the protective effect of curcumin on chronic ethanol-induced liver injury in mice and to explore its underlying mechanisms.
Materials and Methods:
Ethanol-exposed Balb/c mice were simultaneously treated with curcumin for 6 weeks. Liver injury was evaluated by biochemical and histopathological examination. Lipid peroxidation and anti-oxidant activities were measured by spectrophotometric method. Anti-oxidative genes expression such as NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and superoxide dismutase (SOD) were determined by real-time polymerase chain reaction. The nuclear factor E2-related factor 2 (Nrf2) and the phosphorylation states of specific proteins central to intracellular signaling cascades were measured by western blotting.
Results:
Curcumin treatment protected liver from chronic ethanol-induced injury through reducing serum alanine aminotransferase and aspartate aminotransferase activities, improving liver histological architecture, and reversing lipid disorders indicated by decrease of triglyceride, total cholesterol and low-density lipoprotein-cholesterol levels and increase of High-density lipoprotein-cholesterol levels. Meanwhile, curcumin administration attenuated oxidative stress via up-regulating SOD and glutathione peroxidase activities, leading to a reduction of lipid hydroperoxide production. In addition, curcumin increased Nrf2 activation and anti-oxidative genes expressions such as NQO1, HO-1, and SOD through inducing extracellular signal-regulated kinase (ERK) and p38 phosphorylation.
Conclusion:
Our data suggested that curcumin protected the liver from chronic-ethanol induced injury through attenuating oxidative stress, at least partially, through ERK/p38/Nrf2-mediated anti-oxidant signaling pathways.
Keywords: Curcumin, liver injury, mitogen-activated protein kinase, nuclear factor E2-related factor 2, oxidative stress
INTRODUCTION
Alcoholic liver disease (ALD) is caused by prolonged high alcohol intake and contributes significantly to the prevalence of liver disease worldwide.[1] ALD is primarily driven by alcohol metabolism byproducts that promote steatosis process, which can progress to steatohepatitis, fibrosis, cirrhosis, liver failure, and even hepatocellular carcinoma.[2] Alcohol metabolizes to acetaldehyde and then to acetate by its respective dehydrogenases, leading to nicotinamide adenine dinucleotide production, fatty acid oxidation inhibition, and fat accumulation. Increased NADH production after alcohol metabolism enhances respiratory chain activities, including oxygen consumption and reactive oxygen species (ROS) formation. Alternative metabolism of alcohol by cytochrome P450 enzyme 2E1 leads to excessive ROS production.[3] Therefore, oxidative stress is a feature of alcohol hepatotoxicity and plays an important role in the development of ALD.
Emerging studies demonstrated that anti-oxidants such as Vitamin C, Vitamin E were shown to attenuate alcohol-induced liver injury in both animal models and human studies by enhancing hepatic anti-oxidant capacities.[4] Another promising strategy to combat oxidative stress is by means of induction of endogenous anti-oxidant genes regulated by transcription factor-nuclear factor erythroid 2-related factor 2 (Nrf2), which induces a battery of cytoprotective genes in response to oxidative insults and is considered the master regulator of redox homeostasis.[5] The nuclear accumulation of Nrf2 may also be mediated via Nrf2 phosphorylation by mitogen-activated protein kinase (MAPK) pathways. However, these protein kinase pathways regulate diverse cellular processes, and the precise mechanisms of phosphorylation-dependent Nrf2 activation are not fully understood.[6,7]
Curcumin, a major active component of turmeric, is extracted from powdered dry rhizome of Curcuma longa and it has been used as an anti-oxidant and anti-inflammation in Chinese herbal medicine for hundreds of years.[8,9] Previous studies have confirmed that curcumin and its analogs exhibited powerful hepatoprotective effects against oxidative damage caused by several hepatotoxins including ethanol.[10] However, the underlying mechanisms are poorly understood. Here, we investigated the in vivo hepatoprotective role of curcumin on ethanol-induced liver injury and explored its potential mechanisms.
MATERIALS AND METHODS
Chemicals and reagents
Curcumin (Formula: C21H20O6, molecular weight: 368.4, purity >98.0%) was purchased from National Institutes for Food and Drug Control (Beijing, China). Hong Xing wine was purchased from Beijing Red Star Co., Ltd. (Beijing, China). Detection kits for alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TCH), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were purchased from Jiancheng Institute of Biotechnology (Nanjing, China). Polymerase chain reaction (PCR) primers of heme oxygenase (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), CuZn-SOD, GSH-Px and β-actin were synthesized by Shanghai Shenergy Biocolor BioScience and Technology (Shanghai, China). Trizol, PrimeScript® RT reagent Kit with gDNA Eraser and SYBR® Premix Ex Taq™ were purchased from TaKaRa (Dalian City, Liaoning, China). Anti-bodies for Nrf2, extracellular signal-regulated kinase (ERK), p-ERK, P38, p-P38, JNK, p-JNK, TBP, and β-actin were from Cell Signaling Technology (Beverly, MA, USA). All other chemicals were of analytical grade and were obtained from standard commercial suppliers.
Animals and experimental procedure
Adult Male Balb/C mice (6 weeks old, 25 ± 2 g) were obtained from the Experimental Center of Medical Scientific Academy of Hubei (Hubei, China). Mice were maintained at (22°C ± 2°C), the humidity of 50% ± 5% and a 12-h light-dark cycle with free access to food and water. All procedures were performed according to NIH Guidelines for Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. After 1-week of acclimatization, mice were randomly divided into five groups with ten mice in each group, A: Normal control group (olive oil, 10 mL/kg, orally); B: Curcumin (150 mg/kg, orally) control group; C: Ethanol (56% ethanol, 10 mL/kg, orally) model group; D: Ethanol + curcumin (75 mg/kg, orally) group. E: Ethanol + curcumin (150 mg/kg, orally) group. Mice in C, D, and E groups were given 2.4 g/kg/day ethanol once a day for six consecutive weeks. Homologous curcumin was dissolved in olive oil and given to groups B, D, and E 1 h prior to each ethanol treatment. Twelve hours after the last administration, animals were sacrificed by cervical dislocation under mild anesthesia. Blood samples were collected and centrifuged at 1500 g/min at 4°C for 10 min to obtain serum. Livers were totally excised from the mice. Two pieces of tissues from the same liver lobe in each animal were fixed with formaldehyde for pathological examination, other liver stored at −80°C for the subsequent experiments.
Blood biochemical markers assay
Serum levels of ALT, AST, TG, TCH, LDL-C, and HDL-C were detected colorimetrically using commercially available kits by Olympus AU 400 autoanalyzer according to the manufacturer's instructions.
Histopathological examination
Mice livers from all groups were fixed immediately in 10% neutral buffered formalin, dehydrated in gradual ethanol (50–100%), cleared in xylene and embedded in paraffin. Tissue sections of 5 μm were stained with hematoxylin and eosin and were observed under light microscopy at a magnification of ×200. Pathological assessment involved examining four random fields on each slide according to a method described previously.[11] The number of cells containing fat was estimated as follows: Steatosis <25%, 1; 25–50%, 2; 50–75%, 3; >75%, 4.
Hepatic anti-oxidative enzymes and lipid peroxides assay
superoxide dismutase and GSH-Px activities were measured using commercial assay kits and expressed as U/mg protein. In brief, liver tissues were rinsed with ice-cold saline, homogenized in 100 mM Tris–HCl buffer (pH 7.4) and centrifuged at 3500 ×g at 4°C for 15 min, and then supernatants were used for biochemical assays.[12] SOD activity was obtained following the reduction of nitrite by a xanthine-xanthine oxidase system, a superoxide anion generator. GSH-Px activity was detected by the drop of GSH level, which was determined by a spectrophotometer following the reaction with 5, 5-dithiobes-(2-nitrobenzoic acid). Hepatic lipid peroxidation was evaluated by thiobarbituric acid-reactive substances method, expressed as MDA level and analyzed with a commercial kit according to the manufacturer's instructions. MDA level was calculated using the formula: (Absorbance of measured tube − absorbance of measured blank tube)/(absorbance of standard tube − absorbance of standard blank tube) × standard concentration/sample concentration and expressed as nmol/mg protein.
Western blot analysis
Total proteins and nuclear proteins from liver tissues were extracted using the Total Protein Extraction Kit and Nuclear and Cytosol Fractionation Kit according to the manufacturer's instructions. Total proteins from liver tissues were extracted using the Total Protein Extraction Kit (P1250, Applygen Technologies Inc. Beijing, China). Briefly, liver tissues were homogenized in 100~300 µL of SDS lysis buffer. After incubation on ice for 15 min, then centrifugation at 12,000 g for 15 min, supernatant protein were collected for following analysis. Nuclear proteins were extracted using Nuclear Extraction Kit according to the manufacturer's instructions (P1200, Applygen Technologies Inc. Beijing, China). Briefly, liver tissues were homogenized in 100~300 µL of cell lysis buffer and allowed to swell on ice for 15~20 min with intermittent mixing. Tubes are vortexed to disrupt cell membranes and then centrifuged at 12,000 g at 4°C for 10 min. The pelleted nuclei were washed thrice with the cell lysis buffer and resuspended in the nuclear extraction buffer and incubated on ice for 30 min. Nuclear extract is collected by centrifugation at 12,000 g for 15 min at 4°C. Protein concentrations were analyzed using a BCA protein assay kit. Samples (50 μg protein each) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes by electrophoretic transfer. After being blocked in 5% nonfat milk in TBST for 1 h at room temperature, membranes were incubated overnight at 4°C with the primary anti-bodies directed against Nrf2 (1:500), ERK (1:1000), p-ERK (1:500), P38 (1:1000), p-P38 (1:500), JNK (1:1000), p-JNK (1:500), β-actin (1:1000) and TBP (1:2000), followed by HRP-conjugated anti-rabbit or anti-mouse IgG at room temperature for 1 h. Target protein bands were then observed by the enhanced chemiluminescence and tableted by Kodak film. Relative protein levels were quantified by scanning densitometry and analyzed by Image J (Materialize NV, Leuven, Belgium). Results were normalized to β-actin or TBP expression in each group as a percent of control.
Real-time PCR
Total RNA was extracted from liver tissues by Trizol® reagent according to the manufacturer's protocol and purified by Qiagen RNeasy Mini Kit. Quantity and purity of RNA were assessed by absorbance at 260 nm and 280 nm. cDNA was prepared from the total RNA (1 μg) with a reverse transcriptase (RT) Primer Mix using the PrimeScript RT reagent Kit with gDNA Ersaer according to the instruction. Primer sequences for HO-1, NQO-1, GSH-Px, and CuZn-SOD were given in Table 1. Subsequent PCR amplification was carried out on a Light Cycler system (MJ Research OPTICON 2.0, Biored, America) using 40 cycles of 94°C for 5 s, 58°C~60°C for 30 s and 72°C for 45 s. β-actin was used as an internal control. Relative gene expression was calculated by 2−ΔΔCT method[13] using cycle time (Ct) values and data for normalization.
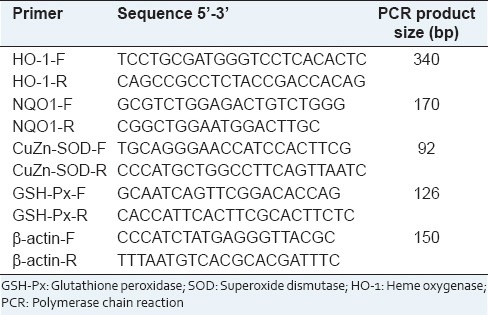
Table 1.
List of primer sequences for real-time PCR
Statistical analyses
All statistical analyses were performed using SPSS 15.0 software (IBM Corporation). Data are expressed as the means ± standard deviation Two-way ANOVA with Bonferroni post-hoc was used for the statistical analysis of our results. Differences with P < 0.05 were taken as statistically significant.
RESULTS
Curcumin decreased serum levels of alanine amino transferase and aspartate aminotransferase in ethanol-treated mice
Serum ALT and AST levels were analyzed to investigate protective effects of curcumin on the chronic alcohol-induced liver injury. As shown in Figure 1, compared to control groups (normal control and curcumin control), serum ALT and AST levels were significantly elevated in ethanol model group (ALT: 58 ± 13 and 65 ± 12 U/L vs. 156 ± 35 U/L, AST: 32 ± 11 and 36 ± 12 U/L vs. 128 ± 31 U/L, P < 0.01). However, serum levels of ALT and AST were significantly decreased after administration of curcumin at the doses of 75 mg/kg and 150 mg/kg (ALT: 125 ± 18 and 103 ± 19 U/L vs. 156 ± 35 U/L, AST: 93 ± 14 and 77 ± 15 U/L vs. 128 ± 31 U/L, P < 0.05 and P < 0.01).
Figure 1.
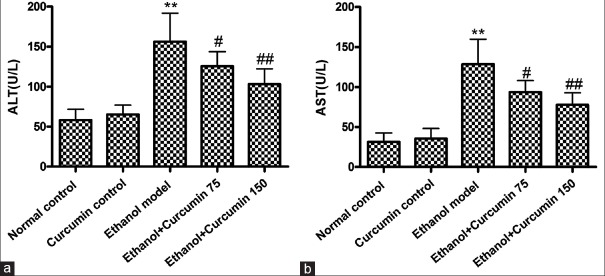
Effects of curcumin on serum alanine amino transferase (a) and aspartate amino transferase (b) activities in mice. **P < 0.01 versus normal control. #P < 0.05 and ##P < 0.01 versus ethanol model. Values are means ± standard deviation, n = 6
Curcumin improved ethanol-induced liver histopathology architecture in mice
As shown in Figure 2, in the normal control group and curcumin control group, liver sections had a normal hepatic cell with preserved cytoplasm, distinct nucleus and central vein [Figure 2a and b]. Photomicrograph of a section of ethanol model mice liver showed loss of cellular boundaries, severe edema and microvesicular steatosis of hepatocytes around the central and interlobular veins [Figure 2c]. In curcumin-treated groups, compared to ethanol model group, a disorder of hepatic cord arrangement has been restored, and edema and microvesicular steatosis of hepatocytes were significantly reduced [Figure 2d–f].
Figure 2.
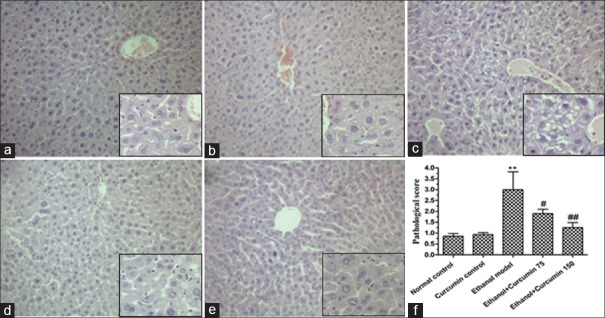
Effects of curcumin on histopathology changes in ethanol-induced liver injury in mice. (a) Normal control group. (b) Curcumin control group. (c) Ethanol model group. (d) Ethanol + 75 mg/kg curcumin. (e) Ethanol + 150 mg/kg curcumin. (f) Pathology was scored as described in “materials and methods”. Arrows indicated appearance of prominent microvesicular steatosis. **P < 0.01 versus normal control. #P < 0.05 and ##P < 0.01 versus ethanol model. Liver sections were stained with H and E, ×200
Curcumin alleviated ethanol-induced lipid disorders in mice
Quantitative assay of lipids was conducted by measuring the concentrations of TG, TCH, HDL-C and LDL-C in serum. As shown in Figure 3, compared to control group, ethanol model group mice showed increased levels of TG, TCH and LDL-C (TG: 0.67 ± 0.10 and 0.66 ± 0.17 mmol/L vs. 1.0 ± 0.16 mmol/L; TCH: 2.44 ± 0.28 and 2.51 ± 0.46 mmol/L vs. 3.85 ± 0.50 mmol/L; LDL-C: 0.48 ± 0.13 and 0.50 ± 0.12 mmol/L vs. 1.37 ± 0.34 mmol/L, P < 0.01) while decreased levels of HDL-C (2.66 ± 0.28 and 2.78 ± 0.26 mmol/L vs. 1.57 ± 0.28 mmol/L, P < 0.01). After administration with curcumin at dosages of 75 mg/kg and 150 mg/kg, TG, TCH and LDL-C levels were significantly decreased (TG: 0.82 ± 0.09 and 0.74 ± 0.09 mmol/L vs. 1.0 ± 0.16 mmol/L; TCH: 3.25 ± 0.30 and 3.0 ± 0.25 mmol/L vs. 3.85 ± 0.50 mmol/L; LDL-C: 0.96 ± 0.14 and 0.79 ± 0.13 mmol/L vs. 1.37 ± 0.34 mmol/L, P < 0.05 or P < 0.01), whereas HDL-C levels were increased when compared with ethanol model group (2.06 ± 0.25 and 2.27 ± 0.29 mmol/L vs. 1.57 ± 0.28 mmol/L, P < 0.05 or P < 0.01).
Figure 3.
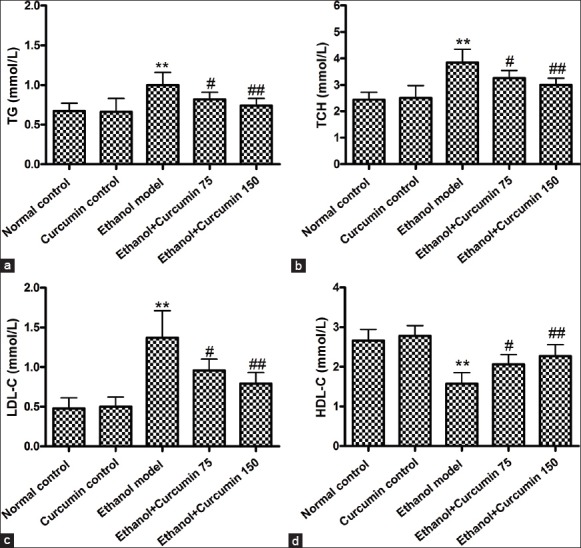
Effects of curcumin on ethanol-induced lipid disorders in mice. (a) Triglyceride contents; (b) total cholesterol levels; (c) low-density lipoprotein-cholesterol levels; (d) high-density lipoprotein-cholesterol levels **P < 0.01 versus normal control. #P < 0.05 and ##P < 0.01 versus ethanol model
Curcumin inhibited ethanol-induced hepatic oxidative stress and lipid peroxidation in mice
Oxidative stress and lipid peroxidation resulting from alcohol metabolism play a critical role in liver injury. In order to evaluate the effects of curcumin on oxidative stress, hepatic SOD and GSH-Px activities as well as MDA contents were determined. As shown in Figure 4, compared to control groups (normal control and curcumin control), SOD and GSH-Px activities in ethanol model group were significantly reduced (SOD: 124.5 ± 8.5 and 132.6 ± 16.3 U/mg vs. 71.1 ± 7.4 U/mg; GSH-Px: 194.8 ± 11.5 and 202.7 ± 15.9 U/mg vs. 119.8 ± 10.4 U/mg; P < 0.01), while MDA contents were significantly increased after ethanol exposure (3.8 ± 0.8 and 4.1 ± 1.2 nmol/mg vs. 13.4 ± 2.7 nmol/mg, P < 0.01). However, curcumin administration at the doses of 75 mg/kg and 150 mg/kg robustly increased SOD and GSH-Px activities (SOD: 90.2 ± 13.3 and 109.3 ± 12.8 U/mg vs. 71.1 ± 7.4 U/mg; GSH-Px: 147.2 ± 8.9 and 169.3 ± 10.8 U/mg vs. 119.8 ± 10.4 U/mg; P < 0.01), and decreased MDA contents (9.2 ± 1.6 and 8.0 ± 1.7 nmol/mg vs. 13.4 ± 2.7 nmol/mg, P < 0.05 or P < 0.01). These data suggested that curcumin might protect the liver against ethanol-induced injury by attenuating oxidative stress.
Figure 4.
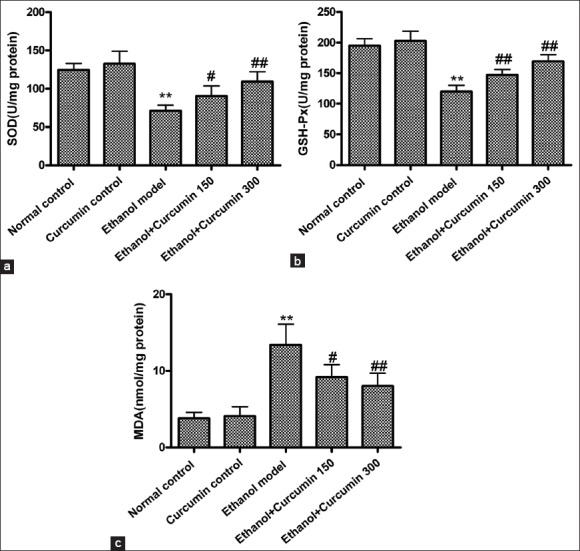
Effects of curcumin on ethanol-induced liver oxidative stress in mice. (a) superoxide dismutase activities; (b) glutathione peroxidase activities; (c) malondialdehyde contents. **P < 0.01 versus normal control. #P < 0.05 and ##P < 0.01 versus ethanol model
Curcumin activated nuclear factor E2-related factor 2-mediated anti-oxidant signaling pathway in ethanol-induced liver injury in mice
Previous studies have confirmed that Nrf2 plays an important role in preventing alcohol-induced oxidative stress and lipid accumulation.[14] Therefore, we investigated whether curcumin administration affected Nrf2-mediated anti-oxidant signaling pathway. As shown in Figure 5, Nrf2 protein levels in nuclei were decreased by 58% after chronic ethanol exposure in comparison with control group, whereas curcumin treatment at the dosages of 75 mg/kg and 150 mg/kg increased Nrf2 nuclear translocation by 58% and 96% respectively compared to ethanol-induced group. Furthermore, mRNA levels of CuZn-SOD, GSH-Px, HO-1 and NQO1, the enzymes downstream of Nrf2 were examined using quantitative real-time PCR. As shown in [Figure 5], mRNA levels of CuZn-SOD, GSH-Px, HO-1, and NQO1 were significantly decreased in ethanol-treated mice compared to normal control mice, whereas curcumin treatment reversed these changes. Our data indicated that curcumin could activate Nrf2-mediated anti-oxidative signaling pathway.
Figure 5.
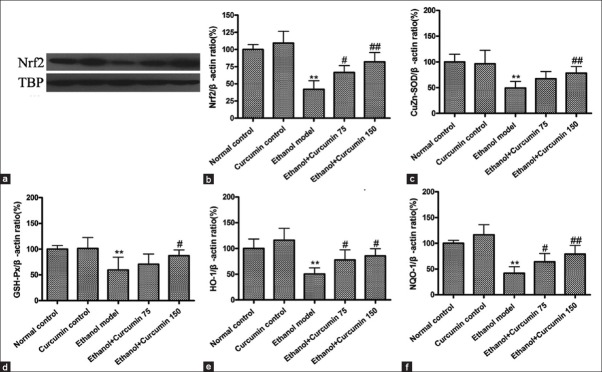
Effects of curcumin on nuclear factor E2-related factor 2 (Nrf2) mediated anti-oxidative signaling pathway in ethanol-induced liver injury in mice. (a) Representative bands of nuclear Nrf2 protein tested by Western blotting; (b) quantification of Nrf2/TBP ratio; TBP was used as internal control for nuclear protein. (c) Measurement of mRNA level of CuZn-superoxide dismutase (CuZn-SOD); (d) Measurement of mRNA level of glutathione peroxidase (GSH-Px); (e) Measurement of mRNA level of heme oxygenase-1 (HO-1); (f) Measurement of mRNA level of NAD(P)H quinone oxidoreductase 1 (NQO1)
Curcumin modulated the phosphorylation of Mitogen-activated protein kinase signaling pathways in ethanol-induced liver injury in mice
Increasing evidence indicates that MAPK signaling pathways regulate Nrf2 activation and facilitate its nuclear accumulation for promoting anti-oxidative gene expression such as HO-1.[15,16] Therefore, phosphorylation of MAPK signaling kinases, indicative of their activation, was detected using western blot analysis. As shown in [Figure 6], compared to control group, chronic ethanol exposure decreased the phosphorylation of ERK, JNK, and p38. However, curcumin treatment at the doses of 75 mg/kg and 150 mg/kg significantly increased the phosphorylation of ERK and p38 but not JNK (P < 0.05 or P < 0.01). These results indicated that curcumin could activate Nrf2-mediated anti-oxidative pathway through the phosphorylation of MAPK signaling pathway.
Figure 6.
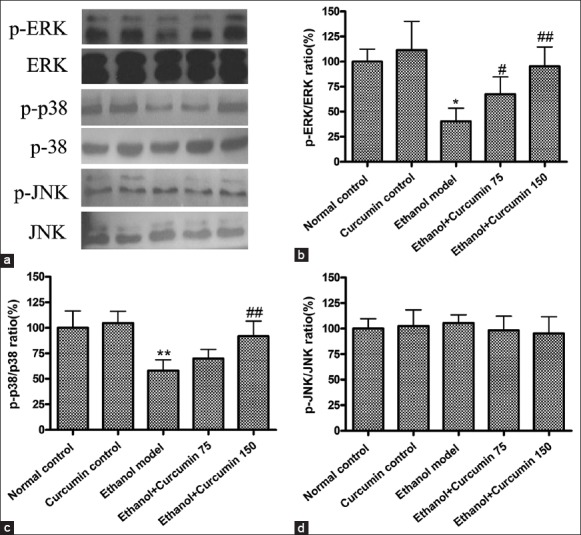
Effects of curcumin on the phosphorylation of mitogen-activated protein kinase (MAPK) signaling pathways in ethanol-induced liver injury. (a) Representative bands of MAPK proteins measured by Western blotting; (b) data quantification of p-ERK; (c) data quantification of p-JNK; (d) data quantification of p-p38. The bands of Western blotting were quantified and expressed as the ratio of p-ERK/ERK, p-JNK/JNK and p-p38/p38 intensity. *P < 0.05 versus normal control group. #P < 0.05 and ##P < 0.01 versus ethanol model group
DISCUSSION
The alcoholic liver disease represents a significant cause for death and disability in most developed countries and is becoming a leading cause of disease in developing countries as well.[2] To the best of our knowledge, ethanol absorbed by gastrointestinal mucosa is almost exclusively metabolized in the liver. The obvious sign of hepatic injury is the leakage of cellular enzymes into plasma. In addition, major parameters of lipid disorders such as elevation of serum TCH, TG and LDL-C levels, and reduction of HDL-C contents were also observed in ethanol-induced liver injury.[17] Many studies have demonstrated that curcumin treatment reverses the elevated serum transaminase (ALT and AST) levels and lipid disorders caused by hepatotoxicity.[17,18] Our present study also found that curcumin supplementation ameliorated ethanol-induced macrovesicular steatosis in liver, reduced serum ALT, AST, TG, TCH, and LDL-C levels, but elevated HDL-C contents, indicating curcumin conferred liver protection from ethanol-induced liver toxicity.
It is now increasingly clear that various mediators have been implicated in ethanol-induced liver injury, but the role of oxidative stress and free radical damage is thought to be of particular importance. Once the imbalance between pro-oxidants and anti-oxidants appears, ROS generated as by-products of oxidative metabolism frequently damage cellular macromolecules such as DNA, lipids, and proteins. A number of studies have been demonstrated that ethanol intake increased the formation of lipid peroxidation product, such as MDA.[19] To counteract this oxidative stress, cells have a variety of anti-oxidant enzymes, including SOD, catalase (CAT), and GSH-Px. Therefore, SOD, CAT, and GPX are part of the key enzymes that defend the cells against oxidative stress. Previous studies have shown that curcumin prevents alcohol-induced liver disease involving decreasing ROS generation and enhancing anti-oxidative capacity.[17,20] Similarly, this study also demonstrated that chronic ethanol exposure resulted in the enhancement of lipid peroxidation, shown as increased MDA contents, decreased GSH-Px and SOD activities in the liver. However, curcumin treatment was found to inhibit significantly the increased MDA content, elevated GSH-Px and SOD activities.
Accumulating evidence has shown that Nrf2, a major regulator of the anti-oxidative response, plays an important role in preventing ethanol-induced oxidative stress and lipid accumulation.[14] Previous studies have shown that livers of Nrf2-null mice are more susceptible to various chemical-and oxidative stress-induced pathologies than wild-type mice[21] Recent studies have suggested that phytochemicals can activate Nrf2 by directly binding to Keap1 through covalent linkages, resulting in the induction of some detoxifying enzymes and related proteins, which play pivotal roles in protecting cells from free radical stress imposed by ROS.[22,23] Nrf2 target key detoxifying enzymes include HO-1, GSH, glutathione-S-transferase, NQO1 and GCLC, enhanced expression of which leads to increased levels of endogenous anti-oxidants such as the major thiol anti-oxidant GSH and reduced quinines.[24,25] A study from Farombi et al. showed that curcumin attenuated dimethylnitrosamine-induced liver injury through Nrf2-mediated induction of HO-1.[26] Similarly, study from Zhao et al. also demonstrated that curcumin led to nuclear accumulation of Nrf2 protein and increased the expression of ARE regulated genes in keratinocytes (HaCaT) treated with sodium arsenite, augmenting cell viability and survival via up-regulating NQO1, HO-1, GCL, and GCLM genes expression.[27] Consistent with previous studies, results from our study showed that curcumin treatment significantly enhanced Nrf2 nuclear expression and increased mRNA expression of subsequent enzymes, such as NQO1, HO-1, and GCLC.
Furthermore, many studies have demonstrated that MAPK cascades have been principally implicated in Nrf2 activation and translocation for highly specialized protein synthesis including HO-1.[28,29] Similarly, increasing natural compounds have been reported to induce Nrf2 activation and detoxifying enzymes expression in different cell types via MAPK pathways. For example, in porcine renal epithelial cells, curcumin induction of HO-1 is mediated by Nrf2 and p38 MAPK.[30] Sulfuretin attenuated tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2/ARE and JNK/ERK MAPK mediated HO-1 expression.[29] We also found that curcumin treatment facilitated the phosphorylation of ERK and p-38 MAPK, but not JNK, indicating curcumin induction of Nrf2-mediated gene expression associated with ERK and p-38 MAPK pathway. These studies suggested that MAPK cascades played crucial roles for Nrf2 activation and induction of ARE-mediated gene expression.
CONCLUSIONS
This study showed that curcumin induced Nrf2 activation and up-regulated detoxifying genes expression such as NQO1, HO-1, and GCLC through ERK/p38-MAPK pathways, leading to inhibition of chronic ethanol-induced liver oxidative stress and lipid peroxidation. Our study indicated that curcumin might be a potential bioactive agent for the prevention of oxidative injuries.
ACKNOWLEDGMENTS
This study was supported by the natural science foundation of Hubei Province, China (No. 2013CFB388).
Footnotes
Source of Support: This study was supported by the natural science foundation of Hubei Province (No. 2013CFB388)
Conflict of Interest: None declared.
REFERENCES
- 1.Lieber CS. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: Classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089–105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Yanardag R, Ozsoy-Sacan O, Ozdil S, Bolkent S. Combined effects of vitamin C, vitamin E, and sodium selenate supplementation on absolute ethanol-induced injury in various organs of rats. Int J Toxicol. 2007;26:513–23. doi: 10.1080/10915810701707296. [DOI] [PubMed] [Google Scholar]
- 5.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–46. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–39. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MA, Ahamed M, Ahmad J, Majeed Khan MA, Musarrat J, Al-Khedhairy AA, et al. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem Toxicol. 2012;50:641–7. doi: 10.1016/j.fct.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Patwardhan RS, Checker R, Sharma D, Kohli V, Priyadarsini KI, Sandur SK. Dimethoxycurcumin, a metabolically stable analogue of curcumin, exhibits anti-inflammatory activities in murine and human lymphocytes. Biochem Pharmacol. 2011;82:642–57. doi: 10.1016/j.bcp.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari H, Rao MV. Curcumin supplementation protects from genotoxic effects of arsenic and fluoride. Food Chem Toxicol. 2010;48:1234–8. doi: 10.1016/j.fct.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989;13:15–9. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang MJ, Xiao JH, Wang Y, Yan YL, Yang J, Wang JL. 2, 3,5,4’- Tetrahydroxystilbene-2-O-beta-D-glucosideimproves gastrointestinal motility disorders in STZ-induced diabetic mice. PLoS One. 2012;7:e50291. doi: 10.1371/journal.pone.0050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabatabaeian H, Hojati Z. Assessment of HER-2 gene overexpression in Isfahan province breast cancer patients using Real Time RT-PCR and immunohistochemistry. Gene. 2013;531:39–43. doi: 10.1016/j.gene.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262:321–9. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Tobón-Velasco JC, Vázquez-Victorio G, Macías-Silva M, Cuevas E, Ali SF, Maldonado PD, et al. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: Involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radic Biol Med. 2012;53:1024–40. doi: 10.1016/j.freeradbiomed.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Park EJ, Kim YM, Park SW, Kim HJ, Lee JH, Lee DU, et al. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264-7 cells and CLP-induced septic mice. Food Chem Toxicol. 2013;55:386–95. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Rong S, Zhao Y, Bao W, Xiao X, Wang D, Nussler AK, et al. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine. 2012;19:545–50. doi: 10.1016/j.phymed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Naik SR, Thakare VN, Patil SR. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Exp Toxicol Pathol. 2011;63:419–31. doi: 10.1016/j.etp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, et al. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–23. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 20.Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol 2009. 2009:981963. doi: 10.1155/2009/981963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Ichihara S, Valentine WM, Itoh K, Yamamoto M, Sheik Mohideen S, et al. Increased susceptibility of Nrf2-null mice to 1-bromopropane-induced hepatotoxicity. Toxicol Sci. 2010;115:596–606. doi: 10.1093/toxsci/kfq075. [DOI] [PubMed] [Google Scholar]
- 22.Hwang YP, Choi JH, Choi JM, Chung YC, Jeong HG. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem Toxicol. 2011;49:2081–9. doi: 10.1016/j.fct.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Jeong GS, Lee DS, Li B, Byun E, Kwon DY, Park H, et al. Protective effect of sauchinone by upregulating heme oxygenase-1 via the P38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med. 2010;76:41–7. doi: 10.1055/s-0029-1185906. [DOI] [PubMed] [Google Scholar]
- 24.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 25.Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279–87. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 27.Zhao R, Yang B, Wang L, Xue P, Deng B, Zhang G, et al. Curcumin protects human keratinocytes against inorganic arsenite-induced acute cytotoxicity through an NRF2-dependent mechanism. Oxid Med Cell Longev 2013. 2013:412576. doi: 10.1155/2013/412576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q. Gastrodin protects against MPP(+)-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int. 2014;75:79–88. doi: 10.1016/j.neuint.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee DS, Kim KS, Ko W, Li B, Jeong GS, Jang JH, et al. The cytoprotective effect of sulfuretin against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2/ARE and JNK/ERK MAPK-mediated heme oxygenase-1 expression. Int J Mol Sci. 2014;15:8863–77. doi: 10.3390/ijms15058863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]


