Abstract
Background:
Diabetic nephropathy (DN) is one of the most important complications of diabetes mellitus. Now-a-days, cystatin C (CysC) is introduced as a new marker for diagnosis of renal damages; however, use of this marker in clinical laboratories is still controversial. The present study was aimed to evaluate the diagnostic value of serum CysC for early detection or monitoring treatment of kidney damages in the Kurdish people with type 2 diabetes mellitus.
Materials and Methods:
Glomerular filtration rate (GFR) was estimated by Modification of Diet in Renal Disease formula. Serum CysC and urine microalbumin were also measured in 126 diabetic and healthy subjects. Blood glycated hemoglobin (Hb) also measured in all healthy and diabetic patients. Two independent samples t-test, Mann-Whitney U-test, one-way ANOVA, and Kruskal-Wallis test, as well as Pearson/Spearman correlation coefficient statistical tests were used as appropriate.
Results:
Serum CysC was higher (1312.41 ng/ml) in diabetic patients with GFR <60 ml/min than other subjects (993.25 ng/ml) (patients with normal kidney function and healthy subjects). A borderline significant correlation between CysC and estimating GFR (rs = −0.16, P = 0.05) but highly significant with microalbumin (rs = 0.22, P = 0.014) was observed. Serum CysC sensitivity, negative and positive predictive values were 100 and 4%.
Conclusion:
CysC cover variation of GFR and urine microalbumin, but it cannot be used as a surrogating marker of glycated Hb. According to our results, it seems that serum CysC is a useful marker for screening of DN; but it cannot be used for monitoring of treatment in diabetic patients.
Keywords: Cystatin C, diabetic nephropathy, diagnostic value, estimating glomerular filtration rate, hemoglobin A1c, microalbumin
INTRODUCTION
The incidence of diabetes mellitus is increasing worldwide. Diabetic nephropathy (DN) is one of the most severe complications of type 2 diabetes mellitus (T2DM). DN usually accompanied by proteinuria and decline in renal function. One of the most important leading causes of the end stage renal failure in developed countries is DN and it increases cardiovascular disease risk by 20-40-fold. In the past two decades, the prevalence of the end stage renal failure has increased dramatically.[1] So, the reliable diagnostic tools and treatment strategies are needed.
Many tests have been used to detect early renal impairment in diabetic patients; however, accurate markers to detect early changes in kidney function and kidney injury are controversial. Measurement of glomerular filtration rate (GFR) is the most common test for determining the kidney function and serum creatinine is widely used for the rapid estimation of GFR. According to the National Kidney Foundation for the diagnosis and stratification of chronic kidney diseases guidelines,[2] renal function is moderately decreased if GFR is 60 ml/min and severely decreased if GFR is 30 ml/min. In addition, proteinuria is present in patients with advanced stage of DN. The urine albumin excretion rate is a useful standard tool for detecting early stage of DN and monitoring its progression and it has been widely accepted as one of the earliest and most sensitive markers detecting renal damage and predicting further cardiovascular morbidity.[3,4,5] But urinary albumin excretion does not necessarily reveal patients with kidney failure.[6]
Human cystatin C (CysC) is a nonglycosylated, low molecular weight, a basic protein that belongs to the superfamily of cysteine proteinase inhibitors. It is steadily expressed in most tissues and is present in relatively high concentrations in body fluids. In the kidney, it is removed from the bloodstream by filtration and is completely reabsorbed and catabolized in proximal tubules.[7] The uniqueness of CysC has made it an endogenous indicator for GFR evaluation. CysC production is not affected by age, sex and muscle mass.[7,8,9] This marker is powerfully associated with GFR measures by intravenous infusion of iothalamate.[10,11]
Early detection of renal damages can remove the consequences of DN. Therefore, the early diagnosis and treatment of DN are critical.[12,13] As a result, powerful markers for the staging and monitoring of DN are very necessary. CysC is one of the most important new markers for diagnosis of kidney disease; but, use of this marker as a routine diagnostic tool in clinical laboratories is still controversial. In addition, few data exist on CysC levels in different ethnic groups, and there are no other studies that investigate the role of this marker in the Kurdish population. The increase of urinary albumin excretion more than 25 μg/ml and decrease of GFR <60 ml/min are considered as markers of renal impairment. Moreover, hemoglobin A1c (HbA1c) is currently the most commonly used marker for the determination of the glycemic status in people with diabetes and it is frequently used to guide therapy and especially medical treatment of people with diabetes. For this reason, we measured urinary albumin, GFR, HbA1c, and CysC to evaluate the possible correlation between these markers. Finally, for the first time we evaluated the diagnostic value of CysC for detection of the early onset of DN in patients with T2DM in Kurdish population.
MATERIALS AND METHODS
Study population
This cross-sectional study was conducted on diabetic patients who admitted to the diabetes center of Sanandaj Tohid Hospital (Kurdistan, Iran). The study group consisted of 126 patients (28 male and 98 female) aged 51.27 ± 1.32 (range, 18-78), who had been diagnosed with T2DM according to the World Health Organization Diagnostic Criteria for Diabetes Mellitus.[14,15] For evaluating the correlation between Cys C and kidney function, we estimated GFR in both groups according to Modification of Diet in Renal Disease (MDRD) formula,[16] and then we divided all patients into two groups based on estimating GFR (eGFR). Patients with renal insufficiency had GFR lower than or equal to 60 ml/min and patients with the normal renal function had GFR higher than 60 ml/min. Fifty aged matched healthy subjects (25 male and 25 female) without a known history of DM, aged 47.2 ± 9.7 (range, 30-67) voluntarily participated in the study as controls. Control subjects had normal eGFR and they were statistically similar in terms of age with patients (P > 0.05). Furthermore, for assessment of Cys C diagnostic efficacy in the diagnosis of early renal damages, we measured urine microalbumin. According to corresponding manufacturers’ recommendations, patients with two early morning urine microalbuminuria levels >25 μg/ml, were considered to have DN. Subjects with simultaneous cardiovascular, hepatic, or other medical conditions were excluded from the study. Written informed consent for participation was obtained, and the project was approved by the Research Ethics Committee of Kurdistan University of Medical Sciences (Iran) and conformed to the Declaration of Helsinki. All patients and control individuals were from Kurdistan, a province in western Iran with a population that is Kurds.
Urinary and blood sample collection and analysis: Fasting blood samples were collected; serum were separated and stored at −70°C pending simultaneous analysis. Whole blood was employed for measuring of HbA1c and serum samples were used for determination of creatinine and CysC using commercial kits, according to the manufacturer's instructions.
The early-morning urine specimen was used for urinary albumin assay. The urine samples were collected in sterile bottles without any preservatives and the physical, chemical, and microscopic properties of the urine were recorded by general urine analysis then stored at −80°C pending analysis.
Serum CysC (BioVendor, Czechia) and urinary albumin (Orgentec Diagnostika, Mainz, Germany) were determined based on standard sandwich enzyme-linked immunosorbent assay technology. The concentration of serum CysC and urine albumin were expressed as ng/ml and μg/ml, respectively.
The serum creatinine was measured on same day by an autoanalyzer (RA 1000, Bayer, Ireland) using the Jaffe’ method according to the manufacturer's specifications (Pars Azmoon, Tehran, Iran) and then GFR estimated by MDRD equation from serum creatinine level.[17,18,19,20]
Glycated Hb was measured colorimetrically using Pishtaz Teb Commercial Kit (Pishtaz Teb Diagnostics, Tehran, Iran).
Statistical analysis
Results of serum CysC and urine microalbumin were compared with eGFR levels. Statistical analyzes were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Since holding the normality assumption is crucial when testing hypothesis, the nonparametric one-sample Kolmogorov-Smirnov test was applied to determine this assumption. Results were presented as mean ± standard deviation (SD) if normality assumption met; otherwise median ± intermediate quartile range (IQR) was used. Wherever applicable, the independent samples t-test or Mann-Whitney statistical tests were used to compare mean/median differences between two experimental groups. One-way ANOVA followed by post-hoc, Tukey's, and Dunnett's tests were used to analyze mean differences between more than two groups. If normality assumption was violated, Kruskal-Wallis test was used instead. The Pearson/Spearman correlation coefficient was determined to show the association between two variables. In all performed hypothesis tests, a P < 0.05 was considered as statistically significant.
To establish a sensitivity specificity relationship, receiver operating characteristic (ROC) curves were constructed. Cut-off values that provided the best combination of sensitivity and specificity indices were determined by ROC curve analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio were also calculated.[21,22]
RESULTS
GFR levels were determined for all subjects in the study. With reference to MDRD equation, 26 patients were identified with eGFR above 60 ml/min and 100 subjects below 60 ml/min. The average levels of CysC (both mean and median) were significantly higher in patients with eGFR <60 ml/min compare to those with eGFR >60 ml/min or healthy subjects (Wilcoxon-Mann-Whitney test, P = 0.007). Twenty to 80 years old subjects were classified into six groups in term of age (each 10 years were considered as a separate group). We found CysC levels were independent of sex and age. Furthermore, no statistically significant result was observed between urine microalbumin and eGFR levels (Wilcoxon-Mann-Whitney test, P = 0.196), sex and patients’ age. The full details of statistical analyzes are given in Table 1. In this table, figures in brackets represent either SD for the mean or IQR if the median is reported as the central tendency index of data.
Table 1.
Serum CysC and urine microalbumin concentrations based on eGFR
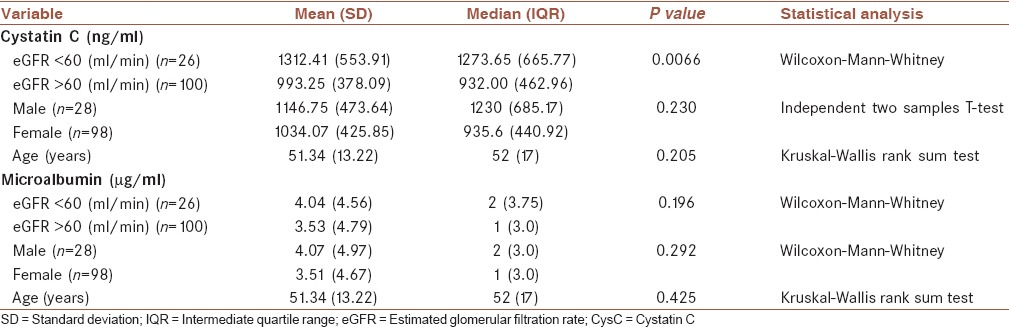
Table 2 shows the correlation between CysC and urine microalbumin, HbA1c and eGFR. There was an inverse correlation between serum CysC and eGFR (rs = −0.16, P = 0.05). Besides, the association between CysC and urine microalbumin with HbA1C levels was investigated using Spearman correlation coefficient, rs The results showed a decrease in the urine microalbumin as HbA1c decreased, rs = 0.17. However, this wasn’t recognized as statistically significant (P = 0.06). Similar conclusion could be made about the association between CysC and HbA1C (rs = 0.12, P = 0.18). It should be noted that Spearman correlation showed a significant association between CysC and urine microalbumin levels (rs = 0.22, P = 0.014).
Table 2.
Correlation between CysC with urine and serum markers in diabetic patients

For determination of diagnostic efficacy of CysC, we used microalbumin = 25 μg/ml for the early stage of DN. Table 3 shows the diagnostic value of this marker. Using a cut-off level of 886 ng/ml for serum CysC, sensitivity and specificity and their confidence intervals (CIs) were respectively 100% (CI: 0.4-1.0) and 38% (CI: 0.30-0.46). Areas under the curve for serum CysC was 0.61 (CI: 0.35-0.87) [Figure 1].
Table 3.
The diagnostic value for serum CysC

Figure 1.
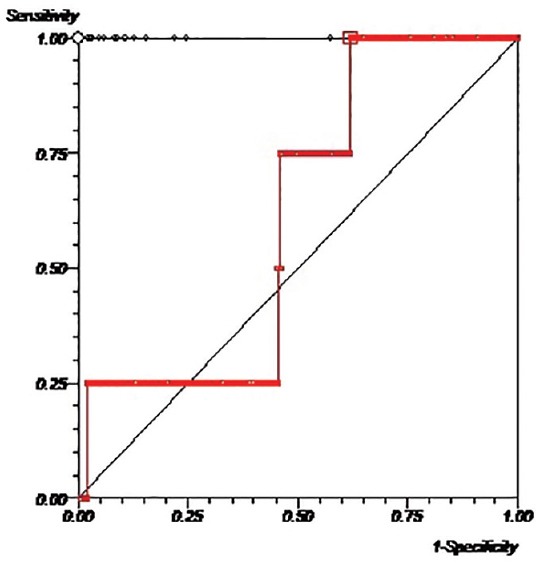
ROC curves for serum cystatin C. ROC = receiver operating characteristic
The point estimates of PPV and NPV for serum CysC is given in Table 2.
DISCUSSION
In this study, we assessed the diagnostic value of serum CysC for diagnosis of early renal damages in a Kurdish population with T2DM.
Our results show that there is no correlation between CysC and HbA1c, so CysC cannot be used for monitoring of diabetic patients. One of the most important forms of Hb that is measured primarily to recognize the average plasma glucose concentration over prolonged periods of time is glycated HbA1c. In diabetes mellitus, higher amounts of glycated Hb, which indicates poorer control of blood glucose levels, have been associated with cardiovascular disease, nephropathy, and retinopathy.[23] The major damages of the glomerular membrane such as the charge selectivity and barrier integrity mainly determine by urinary microalbumin and this marker is considered as the standard test for detection of glomerular damage.[24,25]
The increase of urinary albumin excretion and decrease of eGFR are two important markers of early stage renal damages. We showed that CysC has a high correlation with these markers for diagnosis of renal damage. In healthy people, urinary concentration of albumin is very low. Increase in urinary excretion of albumin occurs in diabetes mellitus following of glomerular damage.[26] Chronic hyperglycemia and poor blood glucose control lead to the basement membrane thickening, followed by increase albumin outflow to the urine and this stage is called microalbuminuria.[27] It is believed that microalbumin excretion may be affected by position, exercise, urinary tract infection, stress response, and protein intake. Moreover, albumin excretion rate has a day-to-day deviation of up to 40%.[28] Studies from various laboratories demonstrated that patients with diabetic mellitus have a higher concentration of urinary albumin that is directly correlated with worsening prognosis.[27,29,30,31] Likewise, the results of the present study showed a significant increase in urine albumin concentration in DM patients in comparison to the controls which confirms the contribution of microalbuminuria in the clinical complication of DM. Moreover, a clear increasing trend in microalbumin level was observed from DM patients with eGFR >60 ml/min to DM patients with eGFR <60 ml/min. This observation is in line with the results of other studies indicating that level of microalbumin as a marker of glomerular damage is correlated with the GFR as a marker of glomerular function.
CysC, also called microprotein, is a homocysteine protease inhibitor synthesized by various nucleated cells. Our results showed that the great majority of diabetic patients had a significant increase in serum CysC. The serum levels of CysC significantly increased in DM patients with eGFR <60 ml/min as compared to DM patients with eGFR >60 ml/min and healthy subjects and showed a positive correlation with microalbuminuria. In addition, our results showed a significant negative correlation between CysC and eGFR. Similar to other studies,[31,32,33] our results suggests that CysC may be involved in the development of DN in diabetic patients.
While the gold standard for calculating GFR is measured by intravenous infusion of exogenous markers; one of the limitations of our study is the calculation of GFR based on endogenous markers. However, GFR estimated by MDRD formula has a great sensitivity and specificity and almost can reflect the actual value of GFR.[16]
In line with previous studies,[34] our results showed that CysC is a better marker for evaluation of kidney damages in DM patients and has a high diagnostic value for screening of DN. Although, it seems to be a better interpretation of CysC in the diagnosis of early renal damages, further studies in other population with larger sample size are needed. Furthermore, sensitivity is reported for CysC in our study is more than other studies.[35,36,37] In other words, among the other studies, we showed the highest sensitivity for this marker in kidney damages. A highly sensitive test means that there are few false negative results, and thus fewer cases of the disease are missed.
CONCLUSION
Serum CysC levels increased significantly in association with decreasing GFR in diabetic patients as compared to control subjects and have a direct correlation with microalbuminuria. Serum CysC concentration was higher in DM patients with eGFR <60 ml/min as compared to DM patients with eGFR >60 ml/min and healthy subjects and has an inverse correlation with this marker. However, there was not any correlation between CysC and HbA1c. In summary, our results suggested that measurement of serum CysC concentrations is a useful marker for screening of DN in diabetic patients; but, it cannot be used for monitoring of these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
MJ contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. NAA contributed in the conception of the work, analysis and interpretation of data, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. SA contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MA contributed in the conception and design of the work, conducting the study, analysis and interpretation of data, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This article is a part of M.Sc thesis and was approved by Sanandaj Branch of Islamic Azad University (Code No. 61540231922007). The authors wish to thank all patients and health stuffs who participated in this study.
REFERENCES
- 1.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 4.Ladeia AM, Ladeia-Frota C, Pinho L, Stefanelli E, Adan L. Endothelial dysfunction is correlated with microalbuminuria in children with short-duration type 1 diabetes. Diabetes Care. 2005;28:2048–50. doi: 10.2337/diacare.28.8.2048. [DOI] [PubMed] [Google Scholar]
- 5.Ritz E, Schmieder RE, Pollock CA. Renal protection in diabetes: Lessons from ONTARGET. Cardiovasc Diabetol. 2010;9:60. doi: 10.1186/1475-2840-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: The Japan Diabetes Clinical Data Management study (JDDM15) Nephrol Dial Transplant. 2009;24:1212–9. doi: 10.1093/ndt/gfn603. [DOI] [PubMed] [Google Scholar]
- 7.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function — A review. Clin Chem Lab Med. 1999;37:389–95. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 8.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C — A new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–81. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 9.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59:587–92. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 10.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–12. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 12.Sämann A, Pofahl S, Lehmann T, Voigt B, Victor S, Möller F, et al. Diabetic nephropathy but not HbA1c is predictive for frequent complications of Charcot feet — Long-term follow-up of 164 consecutive patients with 195 acute Charcot feet. Exp Clin Endocrinol Diabetes. 2012;120:335–9. doi: 10.1055/s-0031-1299705. [DOI] [PubMed] [Google Scholar]
- 13.Sämann A, Wolf G. Diabetic nephropathy. Internist (Berl) 2012;53:1195–206. doi: 10.1007/s00108-012-3129-z. [DOI] [PubMed] [Google Scholar]
- 14.Davies M. New diagnostic criteria for diabetes mellitus. Prevalence is reduced using these criteria rather than 1895 WHO criteria. BMJ. 1999;318:532. [PubMed] [Google Scholar]
- 15.Deckers JG, Schellevis FG, Fleming DM. WHO diagnostic criteria as a validation tool for the diagnosis of diabetes mellitus: A study in five European countries. Eur J Gen Pract. 2006;12:108–13. doi: 10.1080/13814780600881268. [DOI] [PubMed] [Google Scholar]
- 16.Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Liu C, et al. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of Diet in Renal Disease study equation? Diabetes Care. 2005;28:838–43. doi: 10.2337/diacare.28.4.838. [DOI] [PubMed] [Google Scholar]
- 17.Nyman U, Grubb A, Sterner G, Björk J. The CKD-EPI and MDRD equations to estimate GFR. Validation in the Swedish Lund-Malmö Study cohort. Scand J Clin Lab Invest. 2011;71:129–38. doi: 10.3109/00365513.2010.543143. [DOI] [PubMed] [Google Scholar]
- 18.Vervoort G, Klein Gunnewiek JM, Willems HL, Wetzels JF. Effect of creatinine assay standardization on the performance of Cockcroft-Gault and MDRD formula in predicting GFR. Nephrol Dial Transplant. 2006;21:2998–9. doi: 10.1093/ndt/gfl276. [DOI] [PubMed] [Google Scholar]
- 19.Herget-Rosenthal S, Bökenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007;40:153–61. doi: 10.1016/j.clinbiochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Abdi M, Ahmadi A, Roshany D, Khodadadi I, Javid S, Shahmohammad-Nezhad S, et al. Diagnostic value of serum adenosine deaminase activity in HIV infected patients of Kurdish population. Clin Lab. 2013;59:757–62. doi: 10.7754/clin.lab.2012.120631. [DOI] [PubMed] [Google Scholar]
- 22.Sigari N, Mohsenpour B, Nikkhoo B, Ghaderi B, Afkhamzadeh A, Azadi NA, et al. Determination of the best prognostic value of serum tumor markers in patients with suspected lung cancer in an Iranian population. Clin Lab. 2014;60:23–7. doi: 10.7754/clin.lab.2013.121003. [DOI] [PubMed] [Google Scholar]
- 23.Larsen ML, Hørder M, Mogensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1021–5. doi: 10.1056/NEJM199010113231503. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 25.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 26.Amin AP, Whaley-Connell AT, Li S, Chen SC, McCullough PA, Kosiborod MN, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: Results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013;61(4 Suppl 2):S12–23. doi: 10.1053/j.ajkd.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waanders F, Visser FW, Gans RO. Current concepts in the management of diabetic nephropathy. Neth J Med. 2013;71:448–58. [PubMed] [Google Scholar]
- 28.Mathiesen ER, Oxenbøll B, Johansen K, Svendsen PA, Deckert T. Incipient nephropathy in type 1 (insulin-dependent) diabetes. Diabetologia. 1984;26:406–10. doi: 10.1007/BF00262210. [DOI] [PubMed] [Google Scholar]
- 29.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 30.Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post-hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18:1540–6. doi: 10.1681/ASN.2006050445. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Wang Q, Wang Z, Xiao Z, Liu L. Diagnostic value of the combined measurement of serum hcy, serum cys C, and urinary microalbumin in type 2 diabetes mellitus with early complicating diabetic nephropathy. ISRN Endocrinol 2013. 2013:407452. doi: 10.1155/2013/407452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López Gómez JM, Sacristán Enciso B, Micó M, Arias Meneses F, de Sande Medel F, Alejo S. Serum cystatin C and microalbuminuria in the detection of vascular and renal damage in early stages. Nefrologia. 2011;31:560–6. doi: 10.3265/Nefrologia.pre2011.Jul.10834. [DOI] [PubMed] [Google Scholar]
- 33.Xia LH, Bing XG, An XT. Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal. 2004;18:31–5. doi: 10.1002/jcla.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlemoine C, Beauvieux MC, Rigalleau V, Baillet L, Barthes N, Derache P, et al. Interest of cystatin C in screening diabetic patients for early impairment of renal function. Metabolism. 2003;52:1258–64. doi: 10.1016/s0026-0495(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 35.Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl. 2014;25:582–8. doi: 10.4103/1319-2442.132194. [DOI] [PubMed] [Google Scholar]
- 36.Pirgakis KM, Makris K, Dalainas I, Lazaris AM, Maltezos CK, Liapis CD. Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28:1649–58. doi: 10.1016/j.avsg.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Yim HE, Yim H, Bae ES, Woo SU, Yoo KH. Predictive value of urinary and serum biomarkers in young children with febrile urinary tract infections. Pediatr Nephrol. 2014;29:2181–9. doi: 10.1007/s00467-014-2845-0. [DOI] [PubMed] [Google Scholar]


