Abstract
Background:
In developing countries it is important to the exploration of available and safe regimens for medical abortion. The present study was designed to assess the effect of letrozole compared to placebo pretreatment followed by sublingual misoprostol for therapeutic abortion in eligible women with gestational age less than 17 weeks.
Materials and Methods:
In this randomized control trail, 130 women eligible for legal abortions were randomly divided into two groups of case and controls. Cases received daily oral dose of 10 mg letrozole 10 mg letrozole for three days followed by sublingual misoprostol. Controls received daily oral dose of placebo followed by sublingual misoprostol. The dose of misoprostol was administrated according to ACOG guidelines based on patients’ gestational age. The rate of complete abortion, induction-of-abortion time, and side-effects were assessed as main outcomes.
Results:
Complete abortion was observed in 46 (76.7%) letrozole group and 26 (42.6%) controls (P < 0.0001). Also, in 14 subjects of letrozole group and 35 subjects in placebo group, the placenta was not delivered during follow-up and curettage was performed. The mean interval induction-to-abortion was 5.1 h in letrozole group and 8.9 h in control (P < 0.0001). The cumulative rates of the induction-of-abortion time were a significant difference between the two groups (P < 0.0001). The incidence and severity of side-effects was comparable for the two groups (P = 0.9).
Conclusion:
Letrozole could be a quite beneficial adjuvant to misoprostol for induction of complete abortion in those who are candidates for legal medical abortion.
Keywords: Letrozole, medical abortion, misoprostol
INTRODUCTION
Abortion, spontaneous or induced, is a common complication of pregnancy. The World Health Organization reported that annually about 79 million unintended pregnancies excluding miscarriages are occur worldwide.[1] And annually, about 46 million induced abortions occur in the world.[2,3] The estimation of the total number of abortions is quite difficult, especially in developing countries, and it is usually underreported due to the legal restrictions a huge number of the inducted abortions are performed in nonhygienic situations. The abortion might load an undesirable cost to the families and health care system. Serious complications such as maternal death, uterine rupture, and sepsis may occur after abortions that are not taken place under medical observation.[4] The safety of the procedure has a dramatic importance in order to achieving a nonlife threatening result.
Induced abortion can be performed by medical and surgical methods. Medical abortion is the induction of early abortion by consumption of special of medications and is named successful if it is completed without the need of any surgical intervention. Also, the medical approach is a safe and effective alternative to surgical methods with a high level of patient satisfaction.[8,9]
Prostaglandins and their analogs are widely used for medical induced abortion. Misoprostol, a prostaglandin E1 analog, is used widely for early abortion and has been shown to be a better alternative to other prostaglandin substances due to feasibility, simple and easy administration, low price, stability at room temperature, and fewer systemic side-effects.[10,11] Sublingual and vaginal are two common routes of misoprostol administration with different pharmacokinetics and effectiveness. Sublingual misoprostol reaches its peak concentration in a short time and vaginal route has less adverse effects after administration.[12] The range of reported successes rate of abortion induction with misoprostol is quite different in several studies (between 37% and 86%) depending on the regimen, route of administration, and dosage used. However, in combination with other drugs was more effective.[13,14,15] Letrozole, aromatase inhibitors, in combined with vaginal misoprostol shown that was more effective than misoprostol alone with lower, but not significant induction-of-abortion time in termination of pregnancy.[16] Letrozole is a third generation aromatase inhibitor and its action is suppressing estrogen production.[17,18]
Mifepristone in combination with misoprostol is the most common medical abortion regimen with the rate of complete abortion up to 95%.[8,19,20,21] The widespread use of mifepristone is limited by the fact that mifepristone is expensive and is not available in many countries; so, a cheaper and easily available alternative need to be find. Also, the exploration of new regimens to achieve a safe abortion, especially in developing countries is important. The aim of this study is to assess the effect of pretreatment with letrozole followed by sublingual misoprostol in medical abortion.
MATERIALS AND METHODS
This randomized, placebo-controlled clinical trial was conducted between June and December, 2014, on 130 women who were candidate of medical abortion and eligible for legal abortion with gestational age <17 weeks, attending Al-Zahra and Beheshti Hospitals in Isfahan, Iran. The Ethics Committee of Isfahan University of Medical Sciences investigates and approves this study, research project number was 393899. Written informed consent was obtained from all women. Eligibility was defined as age more than 18 years old, gestational age <17 weeks (confirmed by ultrasound scanning on day-1 of the study), hemoglobin levels >10 g/l, diastolic pressure lower 95 mm Hg; no history or evidence of adrenal pathology, steroid-dependent cancer, porphyria, thromboembolism, severe or recurrent liver disease or pruritus of pregnancy.[16] Also, subjects were excluded from recruitment if they had history or evidence of bronchial asthma, arterial hypertension, regular use of prescription drugs before admission to the study, presence of an intrauterine device, breastfeeding, and any abnormal values in pretreatment blood or liver tests.[16] During the treatment period women with the presence of an acute illness of any nature; the use of drugs other than those prescribed by the investigator for the treatment of possible therapy-related side-effects were excluded from the final analysis.
After admission into the study, eligible women were randomly divided into two groups, each group 65 subjects, using Random-Maker Software “Random Allocation (Saghaei, 2004).” Case group; include women who received pretreatment daily oral dose of 10 mg letrozole (Iran Hormone Company, Iran) for three days. Control group; include women who received daily oral placebo for three days.[16]
Placebo tablets were of the same appearance as letrozole tablets which manufactured in Isfahan Faculty of Pharmacy. In both groups, the first dose of letrozole or placebo tablets were taken on day-1 under the supervision of the project nurse, and then they took the second dose herself on day-2.[16] The third dose of letrozole or placebo was given on admission to our hospital on day-3 followed by sublingual misoprostol (Pfizer Company, USA) for cases and controls, according to ACOG guidelines based on patients gestational age.[8] They stayed in the hospital for 4 h and then discharged if vaginal bleeding was not heavy and abdominal pain was not severe.
Collected data included age, complete abortion, the induction-to-abortion interval, missed abortion; vaginal bleeding duration, and severity of side-effects. The complete abortion and induction-to-abortion interval were the primary outcomes. Complete abortion was defined if no emergency or elective curettage was necessary until next menstruation. Other remaining cases were classified as incomplete abortion, and curettage was done in these women. Induction-to-abortion interval was defined as the interval between administration of misoprostol and abortion. To assess vaginal bleeding duration, subjects were reminded to keep a record of duration and amount of vaginal bleeding. Side-effects in our study were nausea, vomiting, diarrhea, fatigue, dizziness, headache, lower abdominal pain, fever, rash, and chills or shivering.[9] To assess the severity of side-effects, patients were asked to report mild, moderate or severity of occurred side-effects based on physician opinion.
The sample size was calculated as 130 subjects using the comparison of proportion formula with two-sided log-rank test, α = 0.05, and 84% of power. All statistical analyses were done using SPSS version 20 (SPSS Inc, Chicago, IL, USA). Descriptive data are reported as mean ± standard deviation, or number (percent) as appropriate. Independent sample t-test, Chi-square test, and Kaplan-Meier were used for data analysis. The level of significance is considered to be <0.05.
RESULTS
One hundred and thirty women were eligible and randomly assigned into two intervention groups. During following period, five letrozole group and four controls did not return for the follow-up visit and were excluded. Finally, 60 women in letrozole Group 1 (misoprostol + letrozole) and 61 women in control Group 2 (misoprostol + placebo) completed the study and analyzed [Figure 1]. Baseline characteristics between studied groups are shown in Table 1, which were not significantly different between groups.
Figure 1.
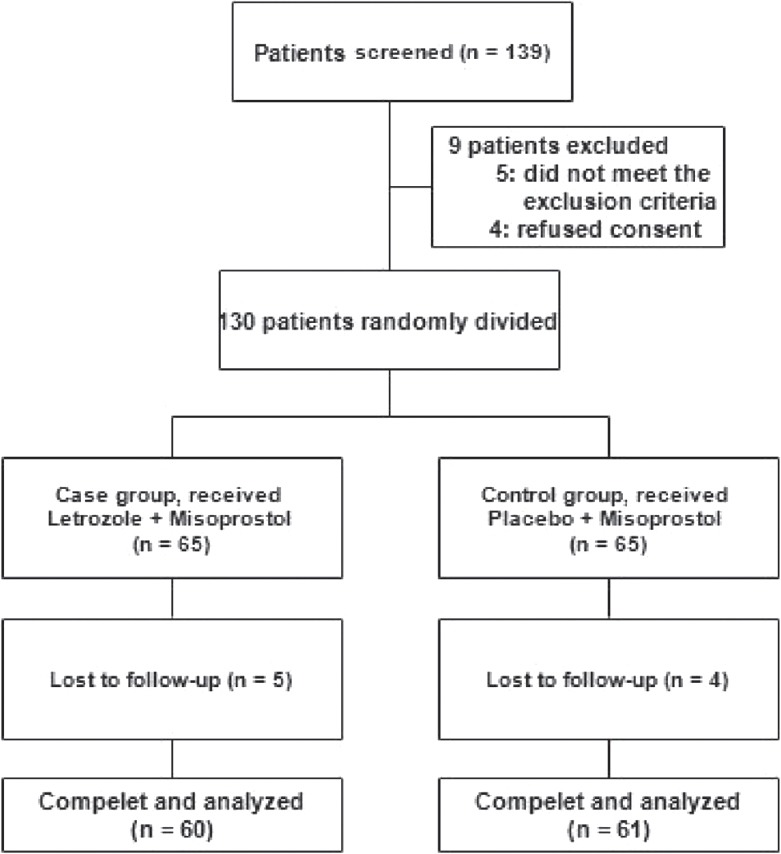
Patients study flow diagram
Table 1.
Baseline characteristics in studied groups

The complete abortion was seen in 59% of all studied women. Treatment outcomes in both groups are shown in Table 1. Complete abortion was observed in 46 subjects in letrozole group which was significantly more than 26 subjects in controls (76.7% and 42.6%, respectively, P < 0.001). The mean dosage of misoprostol in controls was higher than letrozole group [Table 2], but was not statistically significant (922.6 ± 235.9 mcg and 853.3 ± 270.2 mcg respectively, P = 0.13). The mean interval for induction-to-abortion duration in letrozole group was significantly lower than in control group (5.1 ± 1.7 h and 8.9 ± 2 h respectively, P < 0.001). The induction-to-abortion time in letrozole group and controls are presented in Figure 2. As shown in the figure, there was a significant difference between the two regimens in terms of the induction-of-abortion duration. The estimate median (95% CI) in letrozole group was 5 h (4.06-5.93) and in controls was 11 h (9.65-12.35), (P < 0.001). The most common side-effects in both groups were gastrointestinal disturbances and abdominal pain. The incidence of side-effects was comparable for the two groups (P = 0.86), also the severity of side-effects was not significantly different between groups (P = 0.9).
Table 2.
Treatment outcomes and complications in studied groups
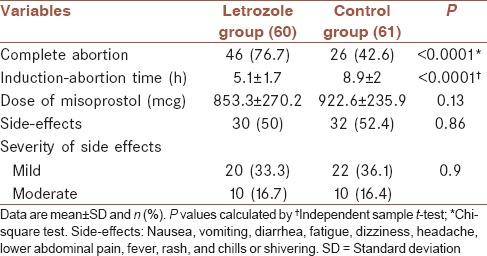
Figure 2.
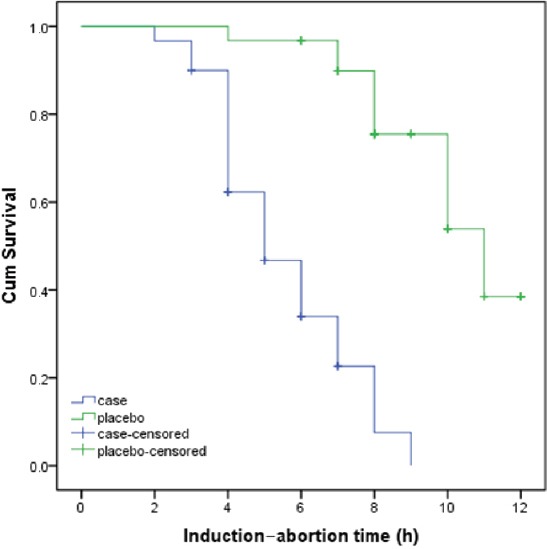
Kaplan-Meier estimates of the abortion in both letrozole and control groups
DISCUSSION
The present study is aimed to assess letrozole as a new synergistic agent in combination with sublingual misoprostol compared with misoprostol alone for medical abortion. Our results demonstrated a complete abortion rate of 76.7% in women requesting medical abortion with the regimen of letrozole and sublingual misoprostol with lower induction-to-abortion time compared to 42.6% in women with the regimen of placebo and sublingual misoprostol.
Previous studies reported different results, after vaginal administration of misoprostol alone or in combination with other drugs. One study reported 56.3% of complete abortion after first administration of 800 mcg of misoprostol vaginally, and 92.4% after administration of the second dose (1600 mcg).[21] Other study reported about 82.9% of complete abortion within 24 h after vaginal administration of misoprostol (800 mcg).[22] Also, the rate of complete abortion between 68% and 81% was reported in previous studies using misoprostol alone.[23,24,25,26] The rate complete abortion in the present study was 42.6% in women who received sublingual misoprostol alone, which was lower than previous reports. The variations between findings may explain by different doses and gestational age in studied women.
The use of letrozole combined with vaginal or sublingual misoprostol is assessed in some studies and different results are reported. In Lee et al. study[16] after use of letrozole (10 mg for 3 days) combined with a single dose of vaginal misoprostol (800 mcg), complete abortion rate was 86.9% in pregnancies up to 49 days gestation and 93.3% up to 63 days gestation which was higher than in vaginal misoprostol with placebo. In a pilot study by Yeung et al, 7-day course of letrozole followed by vaginal misoprostol shows a very high complete abortion rate about 95%.[27] Another pilot study explored the regimen of vaginal misoprostol (800 mcg) following pretreatment with mifepristone (200 mg) and 3 days of letrozole (10 mg/daily) as a new regimen with complete abortion rate of 98%.[28]
The results of the present study demonstrated that complete abortion rate was 76.7% with sublingual misoprostol after pretreatment with 3 days of letrozole (10 mg) which was lower than previous studies,[16,27,28] however, this was significantly more than complete abortion rate in women who received placebo and sublingual misoprostol.
These variations may explain by differences in gestational age in studied women, duration of letrozole, and different doses. However like previous studies letrozole in combination with misoprostol is more effective than misoprostol alone or with placebo.
The regimen used in the present study was well tolerated with no major adverse events or specific side-effects and the comparable to those in the placebo group. Our study was similar to some previous studies that show similar side-effects between letrozole and placebo group or vaginal and sublingual routes of misoprostol.[13,15,29,30] The sublingual route appears to be more convenient and less uncomfortable and higher percentage of women preferred it.[31,32] So, the differences between findings can be a reason to perform more randomized trial for use of the regimen with letrozole and misoprostol.
In our study, the mean of induction-to-abortion time in letrozole group (5.1 h) appear to be significantly shorter than the control group (8.9 h). Lee et al. reported the median of 9.7 h in letrozole group compared to 10.9 in the control group.[15] Other study reported a lower median of induction-to-abortion in letrozole group compared to control group 9.1 h versus 90 h, respectively.[33] Some previous reports show the median of 5.1 h, 7.5 h, 8.2 h, and 8.7 h, after the addition of letrozole pretreatment of misoprostol.[16,27,28,33]
CONCLUSION
Our results showed that a 3-day course of letrozole (10 mg/daily) followed by sublingual misoprostol was associated with a higher complete abortion rate in women with gestational age <17 weeks compared to placebo followed by misoprostol. However, further studies using different doses and longer duration may be warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors contributed equally in this work, carried out the design and coordinated the study, participated in most of the monitored data collection and data collection, drafted and revised the paper. Also, authors read and approved the final manuscript for publication.
Acknowledgments
Financial support was provided by the Isfahan University of Medical Sciences, Isfahan, Iran, (no, 393899).
REFERENCES
- 1.Sedgh G, Bankole A, Oye-Adeniran B, Adewole IF, Singh S, Hussain R. Unwanted pregnancy and associated factors among Nigerian women. Int Fam Plan Perspect. 2006;32:175–84. doi: 10.1363/3217506. [DOI] [PubMed] [Google Scholar]
- 2.Sedgh G, Henshaw S, Singh S, Ahman E, Shah IH. Induced abortion: Estimated rates and trends worldwide. Lancet. 2007;370:1338–45. doi: 10.1016/S0140-6736(07)61575-X. [DOI] [PubMed] [Google Scholar]
- 3.Shah I, Ahman E. Unsafe abortion in 2008: Global and regional levels and trends. Reprod Health Matters. 2010;18:90–101. doi: 10.1016/S0968-8080(10)36537-2. [DOI] [PubMed] [Google Scholar]
- 4.Shaikh Z, Abbassi RM, Rizwan N, Abbasi S. Morbidity and mortality due to unsafe abortion in Pakistan. Int J Gynaecol Obstet. 2010;110:47–9. doi: 10.1016/j.ijgo.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Hosseini SH. The development of Australian abortion laws in a worldwide trend, free and legal practices of abortion and a glance at abortion laws in Iran. J Reprod Infertil. 2007;4:398–409. [Google Scholar]
- 6.Behjati A, Akhoundi MM, Sadeghi MR, Sadriardekani H. The necessity of a comprehensive study on abortion in Iran. J Reprod Infertil. 2005;4:299–320. [Google Scholar]
- 7.Rustamnezhad M, Asadzadeh F, Mustafazadeh F, Karami R, Kazemzadeh R. Study on abortion cases referred to the Forensic Medicine Center of Ardebil city. Health Care J. 2011;11:38–42. [Google Scholar]
- 8.ACOG. ACOG practice bulletin. Clinical management guidelines of obstetrician-gynecologists. Number 67, October 2005. Medical management of abortion. Obstet Gynecol. 2005;106:871–82. doi: 10.1097/00006250-200510000-00051. [DOI] [PubMed] [Google Scholar]
- 9.Winikoff B, Sivin I, Coyaji KJ, Cabezas E, Xiao B, Gu S, et al. Safety, efficacy, and acceptability of medical abortion in China, Cuba, and India: A comparative trial of mifepristone-misoprostol versus surgical abortion. Am J Obstet Gynecol. 1997;176:431–7. doi: 10.1016/s0002-9378(97)70511-8. [DOI] [PubMed] [Google Scholar]
- 10.Schaub B, Fuhrer P, Sainte-Rose D. Randomized study of sulprostone versus misoprostol in the cervical preparation before elective abortion in nulliparous women. J Gynecol Obstet Biol Reprod (Paris) 1995;24:505–10. [PubMed] [Google Scholar]
- 11.Ho PC, Blumenthal PD, Gemzell-Danielsson K, Gómez Ponce de León R, Mittal S, Tang OS. Misoprostol for the termination of pregnancy with a live fetus at 13 to 26 weeks. Int J Gynaecol Obstet. 2007;99(Suppl 2):S178–81. doi: 10.1016/j.ijgo.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Tanha FD, Golgachi T, Niroomand N, Ghajarzadeh M, Nasr R. Sublingual versus vaginal misoprostol for second trimester termination: A randomized clinical trial. Arch Gynecol Obstet. 2013;287:65–9. doi: 10.1007/s00404-012-2508-y. [DOI] [PubMed] [Google Scholar]
- 13.von Hertzen H, Piaggio G, Wojdyla D, Nguyen TM, Marions L, Okoev G, et al. Comparison of vaginal and sublingual misoprostol for second trimester abortion: Randomized controlled equivalence trial. Hum Reprod. 2009;24:106–12. doi: 10.1093/humrep/den328. [DOI] [PubMed] [Google Scholar]
- 14.Kapp N, Borgatta L, Stubblefield P, Vragovic O, Moreno N. Mifepristone in second-trimester medical abortion: A randomized controlled trial. Obstet Gynecol. 2007;110:1304–10. doi: 10.1097/01.AOG.0000289577.32274.a5. [DOI] [PubMed] [Google Scholar]
- 15.Lee VC, Tang OS, Ng EH, Yeung WS, Ho PC. A prospective double-blinded, randomized, placebo-controlled trial on the use of letrozole pretreatment with misoprostol for second-trimester medical abortion. Contraception. 2011;84:628–33. doi: 10.1016/j.contraception.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Lee VC, Ng EH, Yeung WS, Ho PC. Misoprostol with or without letrozole pretreatment for termination of pregnancy: A randomized controlled trial. Obstet Gynecol. 2011;117(2 Pt 1):317–23. doi: 10.1097/AOG.0b013e3182073fbf. [DOI] [PubMed] [Google Scholar]
- 17.Mitwally MF, Casper RF. Single-dose administration of an aromatase inhibitor for ovarian stimulation. Fertil Steril. 2005;83:229–31. doi: 10.1016/j.fertnstert.2004.07.952. [DOI] [PubMed] [Google Scholar]
- 18.Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85:277–84. doi: 10.1016/j.fertnstert.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 19.Ashok PW, Penney GC, Flett GM, Templeton A. An effective regimen for early medical abortion: A report of 2000 consecutive cases. Hum Reprod. 1998;13:2962–5. doi: 10.1093/humrep/13.10.2962. [DOI] [PubMed] [Google Scholar]
- 20.Schaff EA, Eisinger SH, Stadalius LS, Franks P, Gore BZ, Poppema S. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1–6. doi: 10.1016/s0010-7824(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 21.Behnamfar F, Mahdian M, Rahimi F, Samimi M. Misoprostol abortion: Ultrasonography versus Beta-hCG testing for verification of effectiveness. Pak J Med Sci. 2013;29:1367–70. doi: 10.12669/pjms.296.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yilmaz B, Kelekci S, Ertas IE, Ozel M, Sut N, Mollamahmutoglu L, et al. Randomized comparison of second trimester pregnancy termination utilizing saline moistened or dry misoprostol. Arch Gynecol Obstet. 2007;276:511–6. doi: 10.1007/s00404-007-0374-9. [DOI] [PubMed] [Google Scholar]
- 23.Wong KS, Ngai CS, Yeo EL, Tang LC, Ho PC. A comparison of two regimens of intravaginal misoprostol for termination of second trimester pregnancy: A randomized comparative trial. Hum Reprod. 2000;15:709–12. doi: 10.1093/humrep/15.3.709. [DOI] [PubMed] [Google Scholar]
- 24.Wong KS, Ngai CS, Wong AY, Tang LC, Ho PC. Vaginal misoprostol compared with vaginal gemeprost in termination of second trimester pregnancy. A randomized trial. Contraception. 1998;58:207–10. doi: 10.1016/s0010-7824(98)00099-7. [DOI] [PubMed] [Google Scholar]
- 25.Tang OS, Chan CC, Kan AS, Ho PC. A prospective randomized comparison of sublingual and oral misoprostol when combined with mifepristone for medical abortion at 12-20 weeks gestation. Hum Reprod. 2005;20:3062–6. doi: 10.1093/humrep/dei196. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharjee N, Saha SP, Ganguly RP, Patra KK, Jha T, Barui G, et al. A randomized comparative study on vaginal administration of acetic acid-moistened versus dry misoprostol for mid-trimester pregnancy termination. Arch Gynecol Obstet. 2012;285:311–6. doi: 10.1007/s00404-011-1949-z. [DOI] [PubMed] [Google Scholar]
- 27.Yeung TW, Lee VC, Ng EH, Ho PC. A pilot study on the use of a 7-day course of letrozole followed by misoprostol for the termination of early pregnancy up to 63 days. Contraception. 2012;86:763–9. doi: 10.1016/j.contraception.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Chai J, Ho PC. A pilot study on the combined use of letrozole, mifepristone and misoprostol in termination of first trimester pregnancy up to 9 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2013;171:291–4. doi: 10.1016/j.ejogrb.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Milani F, Sharami SH, Arjmandi S. Comparison of sublingual and vaginal misoprostol for second-trimester pregnancy terminations. J Family Reprod Health. 2014;8:41–4. [PMC free article] [PubMed] [Google Scholar]
- 30.von Hertzen H, Huong NT, Piaggio G, Bayalag M, Cabezas E, Fang AH, et al. Misoprostol dose and route after mifepristone for early medical abortion: A randomised controlled noninferiority trial. BJOG. 2010;117:1186–96. doi: 10.1111/j.1471-0528.2010.02636.x. [DOI] [PubMed] [Google Scholar]
- 31.Saxena P, Salhan S, Sarda N. Role of sublingual misoprostol for cervical ripening prior to vacuum aspiration in first trimester interruption of pregnancy. Contraception. 2003;67:213–7. doi: 10.1016/s0010-7824(02)00517-6. [DOI] [PubMed] [Google Scholar]
- 32.Tang OS, Lau WN, Chan CC, Ho PC. A prospective randomised comparison of sublingual and vaginal misoprostol in second trimester termination of pregnancy. BJOG. 2004;111:1001–5. doi: 10.1111/j.1471-0528.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee VC, Tang OS, Ng EH, Yeung WS, Ho PC. A pilot study on the use of letrozole with either misoprostol or mifepristone for termination of pregnancy up to 63 days. Contraception. 2011;83:62–7. doi: 10.1016/j.contraception.2010.05.014. [DOI] [PubMed] [Google Scholar]


