Abstract
Background:
These findings from several observational studies, investigated the association between red meat consumption and gliomas, were inconsistent. We conducted a systematic review and meta-analysis of observational studies to summarize available date on the relation between meat intake and risk of glioma.
Materials and Methods:
A systematic literature search of relevant reports published until May 2014 of the PubMed/Medline, ISI Web of Knowledge, Excerpta Medica database, Ovid database, Google Scholar, and Scopus databases was conducted. From 723 articles yielded in the preliminary literature search, data from eighteen publications (14 case-control, three cohort, and one nested case-control study) on unprocessed red meat, processed meat, and/or total red meat consumption in relation to glioma in adults were included in the analysis. Quality assessment of studies was performed. Random effects model was used to conduct the meta-analysis.
Results:
We found a positive significant association between unprocessed red meat intake and risk of glioma (relative risk [RR] = 1.30; 95% confidence interval [CI]: 1.08-1.58) after excluding three studies with uncertain type of brain cancer. This analysis included only one cohort study which revealed no relation between unprocessed red meat intake and glioma (RR = 1.75; 95% CI: 0.35-8.77). Consumption of processed meats was not related to increased risk of glioma in population-based case-control studies (RR = 1.26; 95% CI: 1.05-1.51) and reduced risk in hospital-based case-controls (RR = 0.79; 95% CI: 0.65-0.97). No significant association was seen between processed red meat intake and risk of glioma in cohort studies (RR: 1.08; 95% CI: 0.84-1.37). Total red meat consumption was not associated with risk of adult glioma in case-control or cohort studies.
Conclusion:
In this meta-analysis of 18 observational studies, we found a modest positive association between unprocessed red meat intake and risk of gliomas based almost entirely on case-control studies. Processed red meat was overall not associated with risk of gliomas in case-control or cohort studies.
Keywords: Glioma, meta-analysis, processed meat, red meat, risk factor
INTRODUCTION
Although brain tumors are uncommon, they are associated with significant mortality and morbidity; the estimated 5-year survival rate of these cancers in adults is only 34%.[1,2] About 81% of malignant brain tumors are gliomas.[2] Astrocytoma, ependymoma, and oligodendroglioma are the three main types of gliomas.[2] The cause of glioma remains largely unknown; established risk factors include increasing age, male sex, white race, and inherited syndromes.[3] Several occupations, environmental carcinogens, and dietary intakes have also been reported to be associated with an elevated glioma risk.[4,5]
A variety of dietary factors including Vitamin C, fruits, vegetables, carotenoids, and alcohol consumption, to glioma have been investigated in epidemiological studies; but the evidence is inconsistent.[6,7,8,9] Meat consumption has been given particular attention in relation to glioma. However, most studies of the association between meat consumption and gliomas used case-control study designs, and findings from observational cohort studies are scarce. Furthermore, findings on meat glioma associations have been inconsistent. Several case-control studies found no linkage between intakes of total red meats,[8] cured meats,[8,9,10] individual red meat items,[4,9] and risk of glioma. However, in an international case-control study that pooled data from eight study centers in six countries,[10] marginally significant positive associations were found between intake of red meat and glioma. A meta-analysis of six observational studies in 2003 suggested a 48% increased risk of glioma in adults with high intakes of cured meat.[11] However, that study was limited[11] in the inclusion of all available documents, including a cohort study[12] and two case-control studies.[8,13] In addition, the authors of the meta-analysis considered data on total N-nitroso compounds (NOC) along with data on cured meat intake in relation to gliomas. Moreover, findings from two large prospective cohort studies,[14,15] published after 2003, revealed no significant association between intake of red or processed meats and risk of glioma. Due to the rarity of this disease, most studies addressing the possible contribution of meat consumption to glioma included relatively few cases which limit the statistical power of any individual study to detect associations. Given the limitations of the existing meta-analysis[11] and publication of several other studies,[8,10,13,14,15,16,17,18] we conducted a systematic review and meta-analysis of observational studies to assess the relationship between red and processed meat intake and risk of glioma.
MATERIALS AND METHODS
Search strategy
We performed a systematic review and meta-analysis of observational studies assessing the relation between meat intake and risk of glioma. This review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis guideline.[19] Two authors (PS and AE) independently conducted a systematic literature search of the PubMed/Medline, ISI Web of Knowledge, Excerpta Medica database, Ovid database, Google Scholar, and Scopus databases for relevant reports published from database inception through May 2014. Our search strategy was based on the following MeSH terms: (“Glioma” or “glioblastoma” or “oligodendroglioma” or “oligoastrocytoma” or “ependymoma” or “glial cell tumor” “astrocytoma” or “brain cancer” or “brain tumor” or “brain neoplasm” or “intracranial tumor”) and (“meat” or “diet” or “risk factor” or “n-nitroso” or “nitroso compounds” or “nitrates” or “nitrosamines”). No language limitation was imposed. In additional, a manual search of relevant studies’ references was performed to find additional reports.
Inclusion criteria
We adopted the following inclusion criteria:
All studies considered the red and processed meat intake as exposure and glioma or total brain tumors in adults as an outcome;
Studies reported that the estimates of relative risk (RR), odds ratio (OR) or hazard ratio (HR), with corresponding 95% confidence interval (CIs).
“Unprocessed red meat” was defined as unprocessed beef, pork, lamb, or game, with or without fish and poultry. Studies considered as poultry and fish intake in relation to glioma were not included in this analysis. “Processed meat” was defined as types of meat that processed by smoking, salting, curing. Bacon, sausages, hot dogs, salami, and ham were included in the category of “processed meat.” “Total red meat” was defined as unprocessed red meat and processed meat combined. We did not exclude studies that reported the ORs or RRs for total meat (red and white meat combined) consumption only; however, the analysis was done with and without these studies.
Excluded studies
When more than one report based on the same study population[20,21] was published, only the most comprehensive publication[20] was included in this meta-analysis. We also excluded papers that examined specifically childhood gliomas,[22,23,24,25,26,27,28,29,30,31] because a previously published meta-analysis has examined almost all these papers.[32] Some studies did not report ORs or RRs for meat intake were not included in this meta-analysis.[33,34] These data could not be obtained even through a request from the authors. The report by Schwartzbaum et al.[35] was removed due to reporting ORs for nitrite intake rather than meat intake.
Data extraction
The data extraction and evaluation of study quality were conducted independently by two reviewers (PS and AE). The following data were extracted:First author's last name, year of publication, country, study design, gender, follow-up years, and person-year for prospective studies, number of subjects (cases, controls or cohort size), criteria for diagnosis of glioma, exposure variables (red meat, processed meat, total meat or other meat items), method of exposure assessment (food frequency (FFQ) questionnaire, other tools), factors used for matching cases and controls, covariates adjusted for, and the risk estimates with corresponding 95% CIs. Some studies[4,17,36,37] had reported risk estimates for separately processed meats items. To include in the meta-analysis, we combined the risk estimates of these items in each study and the combined effect size was used as the ORs for processed meat intake. A cohort study[14] included in the present study had provided RRs for total meat with and without white meat; in this case, we used only RRs for total meat without white meat in our analysis. For the dose-response meta-analysis, we tried to gather this information from each study: The number of cases and the total number of participants (or person-time) for each category, the RRs with CIs for at least two exposure categories; and the mean or median meat consumption for each category.
Assessment of methodological quality
The quality of included studies was examined by using the Newcastle-Ottawa Scale (NOS).[38] For cohort and case-control studies included in the analysis, we used their own specific methods. The NOS assigns a maximum of nine points to each study: Four for selection, two for comparability, and three for assessment of outcomes (for cohort study) or exposures (for case-control study). In the current analysis, when a study got more than median stars, it was considered as relatively high quality; otherwise, it was deemed to have low quality.[38] Any discrepancies were resolved by discussion.
Statistical analysis
Main outcome variables were measures of RRs and ORs for the association between unprocessed, processed, total red meat intake, and glioma. Reported RRs and ORs (and their 95% CIs) were used to calculate log RR and its standard error. Since the prevalence of glioma was relatively low, ORs and HRs were directly considered as RRs. Using a random effects model takes between study variation into account, the overall effect size was calculated. The sources of between study heterogeneity were evaluated by Cochran's Q and I square (I2) tests. I2 values range between 0% and 100%, and I2 values of 25, 50, and 75% are referred to as low, moderate, and high estimates, respectively. These sources of heterogeneity were detected by subgroup analysis. Between subgroup heterogeneity was examined through fixed effects model. Sensitivity analysis was done to examine the extent to which inferences might depend on a particular study. The publication bias was assessed by visual inspection of Begg's funnel plots. Formal statistical assessment of funnel plot asymmetry was done by Egger's regression asymmetry test. Statistical analysis was conducted using STATA version 11.2 (STATA Corp., College Station, Texas, USA). P < 0.05 was considered as statistically significant.
RESULTS
Study characteristics
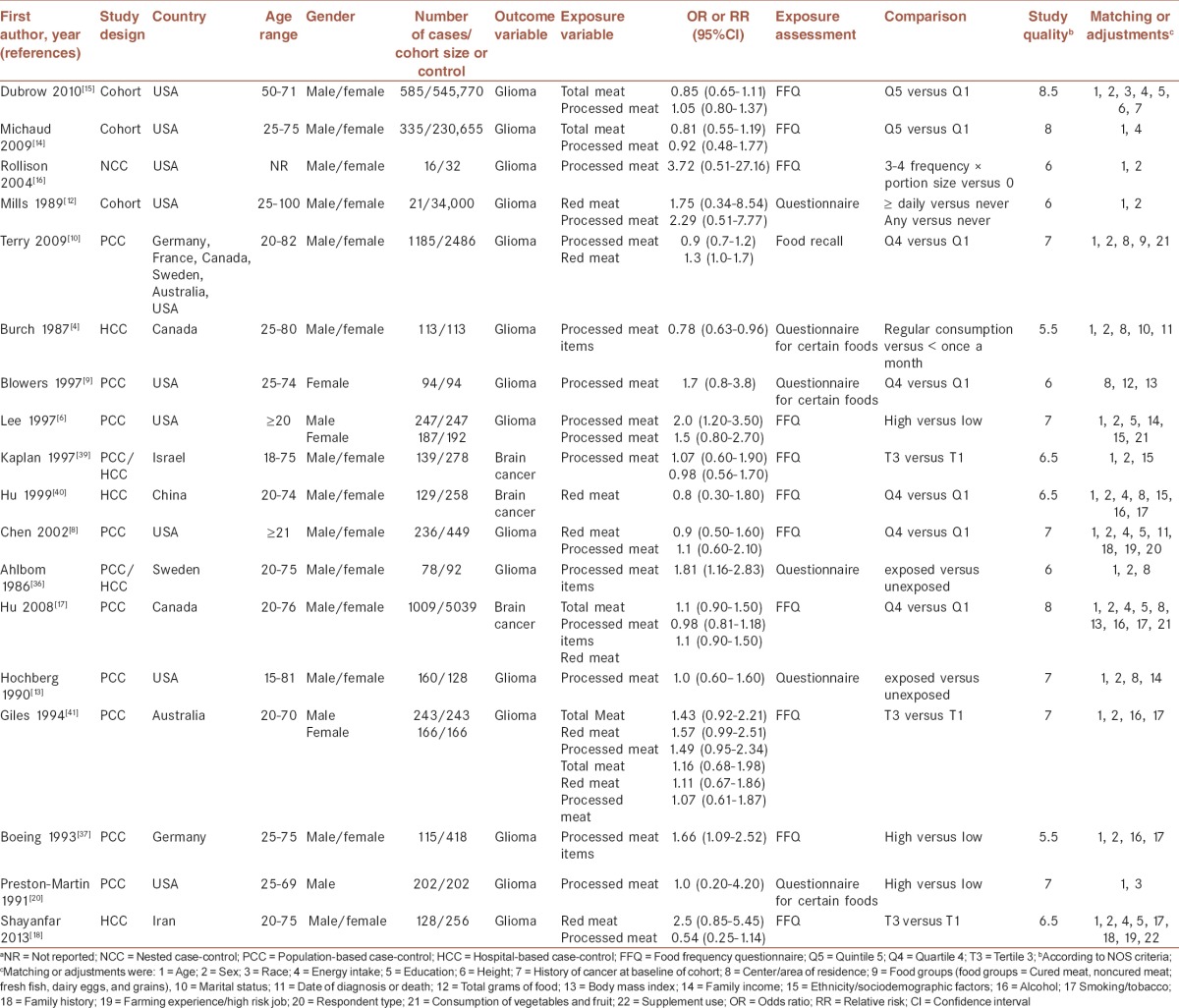
The preliminary literature search yielded 723 unique articles. Of these, 29 articles and three additional reports, identified from the reference lists, were considered for further review [Figure 1]. After the full-text review, 18 articles (including 14 case-control studies,[4,6,8,9,10,13,17,18,20,36,37,39,40,41] three cohort studies,[12,14,15] and 1 nested case-control study[16] had presented data on unprocessed red meat (n = 8 studies), processed meat (n = 17 studies), and/or total red meat (n = 4 studies) consumption in relation to glioma fulfilled our inclusion criteria [Figure 1]. The characteristics of these studies are presented in Table 1.
Figure 1.
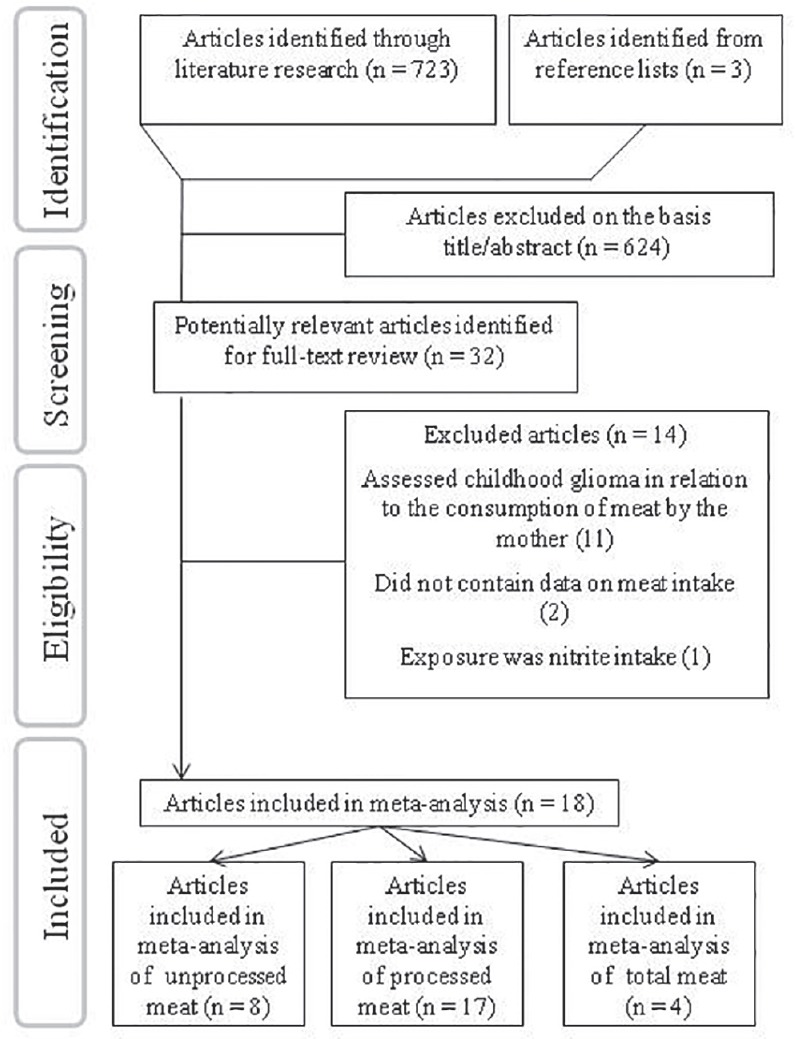
The flow diagram of study selection
Table 1.
Characteristics of studies included in the meta-analysisa
Findings from systematic review
Fifteen studies provided RRs or ORs for gliomas, and three had considered brain cancer among adults in general. Jointly these studies had included 8,25,731 individuals aged 15-100 years (4,441 glioma cases); one report did not specify the number of glioma cases separately from other brain cancer subtypes.[17] These studies were published between 1986 and 2013. Of 18 studies, nine originated from the USA,[6,8,9,12,13,14,15,16,20] two from Canada,[4,17] two from Europe,[36,37] one from Australia,[41] one from Israel,[39] one from Iran,[18] one from China,[40] and one from the USA, Europe and Australia.[10] Of these articles, one study was done on men,[20] one on women,[9] and others had included either gender,[4,6,8,10,12,13,14,15,16,17,18,36,37,39,40,41] but only two studies had reported their findings separately for men and women.[6,41] Most studies had verified cases through histological examination; however, some studies had used cancer registries. Eight studies had provided data on unprocessed red meat intake;[8,10,12,18,33,39,40,41] 14 on processed red meat intake;[6,8,9,10,11,12,13,14,15,16,18,20,39,41] four on only processed red meat items[4,17,36,37] and four on total meat intake.[14,15,17,41] Reported RRs for unprocessed red meat intake were in the range of 0.80-2.50, for processed red meat intake ranged from 0.54 to 3.72, and for total red meat intake was between 0.85 and 1.43. Among 14 case-control studies included in this review, three studies had recruited controls from hospitals (hospital-based),[4,18,40] and nine were population-based studies had recruited controls from the general population.[6,8,9,10,13,17,20,37,41] The remaining two case-control studies had enrolled controls from the general population and also from the hospitals.[36,39] Mean follow-up duration for cohort and nested case-control studies ranged from 6 to 24 years.[12,14,15] Almost all studies were matched for age and sex (if applicable) or had adjusted for their effects, except two studies[9,14] that have considered only one of these confounders. Six studies had controlled for total energy intake,[8,14,15,17,18,40] others did not. The area of residence (n = 6 studies), education (n = 5 studies), and smoking/tobacco (n = 4 studies) were also controlled for in some studies. Meat consumption was assessed by either FFQ or structured questionnaires. In terms of study quality, nine studies were awarded seven or more stars,[6,8,10,13,14,15,17,20,41] indicating that the overall quality of the studies was relatively high. The remaining nine studies were considered to have low quality according to NOS criteria.
Unprocessed red meat intake and risk of glioma
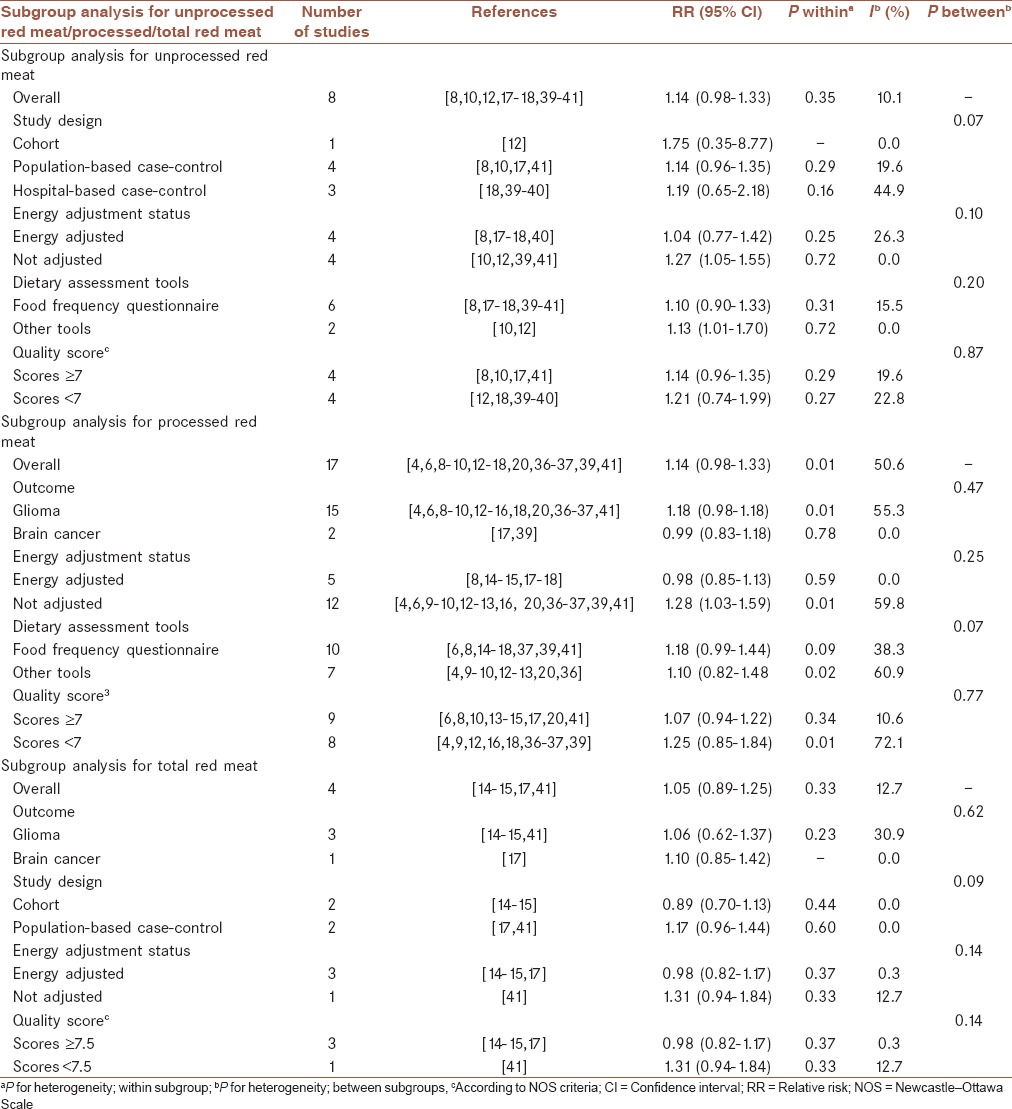
Eight studies,[8,10,12,18,33,39,40,41] which provided nine estimates, analyzing unprocessed red meat consumption were included in this meta-analysis. The summary RR for these nine effect sizes was 1.14 (95% CI: 0.98-1.33) for the highest versus the lowest category of unprocessed red meat intake [Figure 2], with little evidence of between study heterogeneity (P for heterogeneity = 0.35, I2 = 10.1%), indicating a trend toward positive association between unprocessed red meat intake and risk of brain cancers. Subgroup analysis based on the main outcome revealed a significant association for glioma (summary RR: 1.30; 95% CI: 1.08-1.58, P for heterogeneity = 0.47, I2 = 0.0%), and no association for total brain cancer (RR = 0.99, 95% CI: 0.82-1.19, P for heterogeneity = 0.89, I2 = 0.0%) [Figure 2]. The results from further analysis according to study design, adjustment (or nonadjustment) for energy intake, dietary assessment tools and study quality are presented in Table 2. When we confined the analysis to the studies that had controlled for energy intake, no significant link was found between unprocessed red meat consumption and brain cancers (1.04; 0.77-1.42), whereas a significant positive association was seen in papers that had not adjusted for energy intake (1.27; 1.05-1.55). When we examined whether type of dietary assessment tools used in different studies can explain the source of between study variability, we found that the pooled RRs were 1.10 (95% CI: 0.90-1.33, I2 = 15.5%, P for heterogeneity = 0.31) and 1.13 (95% CI: 1.01-1.70, I2 = 0.0%, P for heterogeneity = 0.72), for studies that had used FFQ questionnaire and other dietary assessment tools, respectively. Subgroup analysis based on study design and study quality revealed that these factors could not explain between study heterogeneity. When we excluded the two studies[12,18] had included fish and poultry intake in the category of unprocessed red meat, the findings did not change significantly (summary RR for glioma: 1.26; 95% CI: 1.03-1.53, and for brain cancers: 0.99; 0.82-1.19). Sensitivity analysis indicated that no single study influenced the findings. In addition, no evidence of publication bias was found (P = 0.68, Begg's test).
Figure 2.
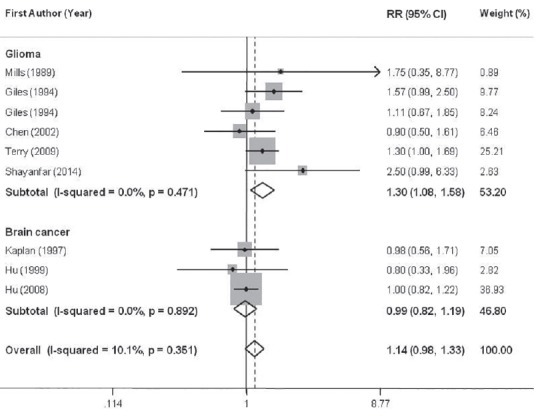
Forest plots of the association between intake of unprocessed red meat and glioma risk, stratified by study outcome. Relative risk stands for relative risk
Table 2.
Results of subgroup-analysis for unprocessed red meat, processed meat, and total meat consumption and risk of glioma
Consumption of processed meats and risk of glioma
In a meta-analysis of the 17 included studies,[6,8,9,10,11,12,13,14,15,16,18,20,39,41] provided 19 effect sizes, the overall combined RR for the highest versus the lowest category of processed meat intake and risk of glioma were 1.14 (95%CI: 0.98-1.33) indicating a trend toward a positive association between processed meat consumption of risk of brain cancers, with moderate evidence of between study heterogeneity (P for heterogeneity = 0.01, I2 = 50.6%) [Figure 3]. Subgroup analysis based on study design was done to examine this as a source of heterogeneity; study design could completely explain between study heterogeneity; high intake of processed meat intake was associated with a 26% greater risk of glioma in population-based (95%CI: 1.05-1.51) and a 21% lower risk in hospital-based case-control studies (95%CI: 0.65-0.97). No significant association was seen in prospective studies (RR: 1.08; 95%CI: 0.84-1.37). There was no statistically significant heterogeneity between cohort (P for heterogeneity = 0.40, I2 = 0.0%), population-based case-control (P for heterogeneity = 0.06, I2 = 46.0%), and hospital-based case-control (P for heterogeneity = 0.36, I2 = 2.2%) subgroups. The findings of subgroup analysis based on energy adjustment status, main outcome, dietary assessment tools, and study quality are provided in Table 2. In further analysis according to adjustment for energy, no significant association was observed for energy adjusted RRs (RR = 0.98, 95% CI: 0.85-1.13, I2 = 0.0%, P for heterogeneity = 0.59), while a significant association was detected for unadjusted RRs (RR = 1.28, 95% CI: 1.03-1.59, I2 = 59.8%, P for heterogeneity = 0.01). The associations did not vary significantly by study outcome, dietary assessment tools, and study quality. Sensitivity analysis revealed that no single study substantially influenced the findings. No asymmetry was seen in Begg's funnel plot (P = 0.51), and no evidence of significant publication bias was found using Egger's test (P = 0.07).
Figure 3.
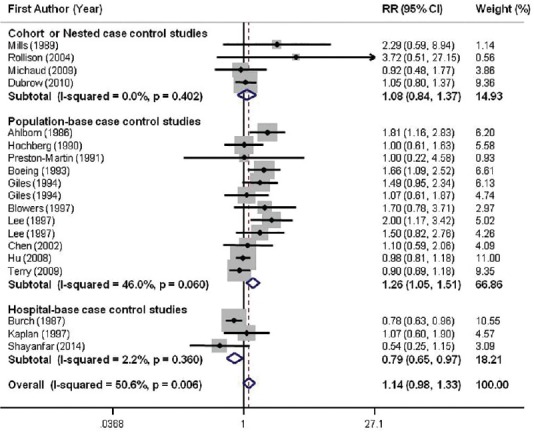
Forest plots of the association between intake of processed meats and glioma risk, stratified by study design. Relative risk stands for relative risk
Total meat intake and risk of glioma
Combining five effect sizes provided from four studies[14,15,17,41] for total meat intake, we found an overall summary RR of 1.05 (95%CI: 0.89-1.25) for glioma; with little evidence of between study heterogeneity (P for heterogeneity = 0.33, I2 = 12.7%) [Figure 4]. The results of the further analysis are shown in Table 2. Excluding the study[17] reported total processed and unprocessed red meat in combination with white meat intake did not significantly change the summary RR for glioma (RR = 1.06, 95% CI: 0.82-1.37). No asymmetry was seen in Begg's funnel plot, indicating no evidence of publication bias (P = 0.63, Begg's test).
Figure 4.
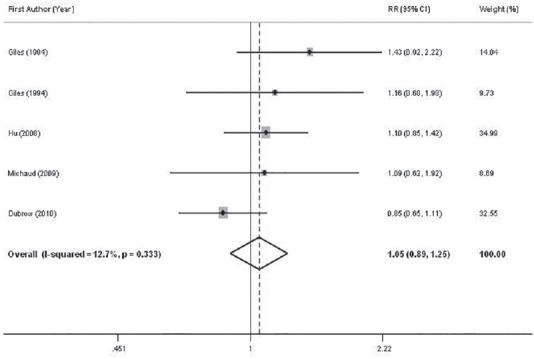
Forest plots of the association between intake of total red meat and glioma risk. Relative risk stands for relative risk
DISCUSSION
Findings from this meta-analysis indicated a positive significant association between red meat intake and risk of gliomas (after excluding studies with unspecified brain cancers). In addition, we found that processed red meat intake was associated with increased risk of gliomas in population-based case-control studies, but with reduced odds in hospital-based case-control studies. The risk of gliomas was not related to unprocessed, processed, or total red meat consumption in cohort studies.
Although gliomas are uncommon, they are associated with significant morbidity and mortality in adult populations. As diet is a potentially modifiable behavior, studying the association between processed and unprocessed red meat intake and gliomas might result in an important disease prevention strategy. Previous meta-analysis of observational studies has shown that processed, unprocessed or total red meat intake was associated with an increased risk of esophageal[42] and lung cancer,[43] colorectal adenomas,[44] bladder,[45] and renal cancer.[46] The findings of the present meta-analysis also revealed a positive significant association between unprocessed red meat consumption and increased risk of glioma. In line with our study, the above mentioned meta-analysis has also included several case-control reports and a few number of cohort studies. In general, positive findings on diet-cancer relations obtained from case-control studies have often not been confirmed in prospective cohort studies, for example the relationships between fruits and vegetables, or dietary fat and risk of specific cancers[47,48] which were observed in case-control studies, were not confirmed in prospective cohort studies. Findings from case-control studies might be misleading due to several methodological limitations that have clearly been stated in other publications.[47,48,49,50] The null overall finding on the association between consumption of processed and total red meat and risk of glioma in our meta-analysis were in agreement with the results of cohort studies.[12,14,15,16] Meta-analysis of prospective studies on other types of cancers has also failed to find a significant association between red and processed meat intake and risk of ovarian[51] and prostate cancer.[52] In line with our findings, some other meta-analysis of other cancers have also documented no significant association in prospective studies, despite a positive relation between processed meat intake and risk of esophageal and gastric cancer in case-control investigations.[49,50]
In the present meta-analysis, we found a positive association between unprocessed red meat intake and risk of gliomas based almost entirely on case-control studies; but not in cohort studies. Lack of finding a significant association between red meat intake and risk of cancer in prospective studies might be explained by the controlling for total energy intake in these studies. On the other hand, most case-control studies had reached a significant association did not consider total energy intake in their analysis. When we did the analysis separately for studies had controlled for energy intake and those that had not adjusted for, we found a positive association between unprocessed and processed meat intake and risk of adult glioma for publications that had not taken total energy intake into account. This association was not observed for studies that had controlled for energy intake. Therefore, it seems that the association between red meat intake and risk of glioma (or even other cancers) in the case-control studies is mainly due to energy intake rather than specifically red meat intake. Another hypothesis is the macronutrient composition of red meat might be involved in the pathophysiology of cancers; however, the key role of NOC cannot be excluded. Whether the association between unprocessed or processed red meat consumption and glioma can be mediated through total energy intake warrants further investigations, although this may simply be an artifact in case-control studies.
When investigating red meat-cancer relations, dietary intakes of antioxidants should be considered as potential confounding factors because they have been suggested to protect against gliomas. Any association between high red meat intake and risk of glioma might be in part attributed to low intake of Vitamins E and C that have been shown to protect cells against oxidative damage and are hypothesized to reduce the risk of cancer.[53] It is worth noting that only 4 (out of 18) studies[6,10,17,18] included in this meta-analysis, had controlled for intake of antioxidants.
Available studies on the red meat and gliomas associations have not provided any information about dose-response relations. Demonstration and characterization of dose-response relations may suggest a firmer causal association. Eight studies (out of 18) included in the present meta-analysis had quantified meat intake as a dichotomous variable of “high” versus “low” intake or as “any” versus “never” consumption. Others had categorized participants according to tertiles or quartiles of red meat intake. As most studies did not provide any information on the mean or median of red meat intake in each category, it was impossible for us to do a dose-response analysis. Future investigations should take this important point into consideration.
As random effects models give larger weights to small extreme studies, some investigators believe that fixed effects model, which assumes a single common (or “fixed”) effect underlies every study, should be used to summarize associations across studies in the meta-analysis.[54] In the present study, we used random effects models to calculate the overall effect size. We also repeated the analysis by using the fixed effects model; although the CIs were relatively narrow, the results did not change significantly.
Several potential mechanisms for the association between red and processed meat intake and risk of glioma have been suggested. Preservation, cooking, and/or processing methods might result in the high levels of mutagens and carcinogens including NOC, heterocyclic amines, and polycyclic aromatic hydrocarbons; many of them have been shown to induce tumors at several sites.[55] In experimental studies, intravenous administration of NOCs such as N-methyl-N-nitroso urea and N-ethyl-N-nitroso urea, could induce glioma.[56] Heme iron, which is abundant in red meat, has also been shown to stimulate endogenous NOC production.[57] In addition, high levels of saturated fat present in red meat may influence the penetration of blood-brain barrier and increase membrane permeability to carcinogens.[58]
The strength of the present meta-analysis lies in a large sample size (8,25,731 subjects and 4441 glioma cases) and no significant evidence of publication bias. We used a prospectively defined protocol, explicit study inclusion criteria and comprehensive literature search. Furthermore, our findings were stable and robust in sensitivity analysis. However, several limitations should be noted. Although epidemiological data have shown the greater incidence of glioma in men than in women,[59] a gender-stratified separate was not possible in this meta-analysis since only two studies had provided results separately for men and women. In addition, total energy intake had not been considered as a confounding factor in several included studies. Residual confounding by other inadequately measured covariates could also be of concern. Another limitation is that red meat consumption was self-reported through questionnaires, which might inevitably lead to some misclassification of exposure. Moreover, most publications were of case-control design. Thus, the possible recall and selection bias may confound the relationship. On the other hand, we were unable to analyze data by various types of glioma (e.g. glioblastoma, astrocytoma, etc.) because data were scant. Brain tumors are characterized by a striking variation in biology and natural history. The observed differences in their biological behavior imply the existence of differences at the genetic and cellular level, with possible distinct etiologies based on the tissue of origin. Failure to stratify on histology, even among the gliomas, may mask or attenuate a causal association when one in fact exists.
In this meta-analysis of observational studies, we found a positive association between unprocessed red meat intake and risk of gliomas based almost entirely on case-control studies. Processed meats and total red meat intake were not related to the risk of gliomas in case-control or cohort studies. Further studies, particularly well-designed prospective studies, are needed to evaluate these relationships.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
PS contributed to conception, design, search, statistical analysis, data interpretation, manuscript drafting, approval of the final version of the manuscript, and agreed for all aspects of the work. WW contributed to conception, design, data interpretation, manuscript editing, approval of the final version of the manuscript, and agreed for all aspects of the work. AE contributed to conception, design, search, statistical analysis, data interpretation, manuscript drafting, supervision the study approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This study was financially supported by Research Council of the Food Security Research Center (No. 294075), Isfahan University of Medical Sciences, Isfahan, Iran. The authors would like to appreciate the generous cooperation of Prof. Jinfu Hu for providing extra information we required from her studies.
REFERENCES
- 1.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(7 Suppl):1953–68. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng JH, Merchant E, Tseng MY. Effects of socioeconomic and geographic variations on survival for adult glioma in England and Wales. Surg Neurol. 2006;66:258–63. doi: 10.1016/j.surneu.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Burch JD, Craib KJ, Choi BC, Miller AB, Risch HA, Howe GR. An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst. 1987;78:601–9. [PubMed] [Google Scholar]
- 5.Beall C, Delzell E, Rodu B, Sathiakumar N, Lees PS, Breysse PN, et al. Case-control study of intracranial tumors among employees at a petrochemical research facility. J Occup Environ Med. 2001;43:1103–13. doi: 10.1097/00043764-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA) Cancer Causes Control. 1997;8:13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 7.Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr. 2007;85:877–86. doi: 10.1093/ajcn/85.3.877. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Ward MH, Tucker KL, Graubard BI, McComb RD, Potischman NA, et al. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control. 2002;13:647–55. doi: 10.1023/a:1019527225197. [DOI] [PubMed] [Google Scholar]
- 9.Blowers L, Preston-Martin S, Mack WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA) Cancer Causes Control. 1997;8:5–12. doi: 10.1023/a:1018437031987. [DOI] [PubMed] [Google Scholar]
- 10.Terry MB, Howe G, Pogoda JM, Zhang FF, Ahlbom A, Choi W, et al. An international case-control study of adult diet and brain tumor risk: A histology-specific analysis by food group. Ann Epidemiol. 2009;19:161–71. doi: 10.1016/j.annepidem.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huncharek M, Kupelnick B, Wheeler L. Dietary cured meat and the risk of adult glioma: A meta-analysis of nine observational studies. J Environ Pathol Toxicol Oncol. 2003;22:129–37. doi: 10.1615/jenvpathtoxoncol.v22.i2.60. [DOI] [PubMed] [Google Scholar]
- 12.Mills PK, Preston-Martin S, Annegers JF, Beeson WL, Phillips RL, Fraser GE. Risk factors for tumors of the brain and cranial meninges in Seventh-Day Adventists. Neuroepidemiology. 1989;8:266–75. doi: 10.1159/000110193. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8:55–60. doi: 10.1007/BF00182087. [DOI] [PubMed] [Google Scholar]
- 14.Michaud DS, Holick CN, Batchelor TT, Giovannucci E, Hunter DJ. Prospective study of meat intake and dietary nitrates, nitrites, and nitrosamines and risk of adult glioma. Am J Clin Nutr. 2009;90:570–7. doi: 10.3945/ajcn.2008.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubrow R, Darefsky AS, Park Y, Mayne ST, Moore SC, Kilfoy B, et al. Dietary components related to N-nitroso compound formation: A prospective study of adult glioma. Cancer Epidemiol Biomarkers Prev. 2010;19:1709–22. doi: 10.1158/1055-9965.EPI-10-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollison DE, Helzlsouer KJ. Processed meat consumption and adult gliomas in a Maryland cohort. Cancer Causes Control. 2004;15:99–100. doi: 10.1023/b:caco.0000016675.85386.54. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, La Vecchia C, DesMeules M, Negri E, Mery L. Cancer Registries Epidemiology Research Group. Meat and fish consumption and cancer in Canada. Nutr Cancer. 2008;60:313–24. doi: 10.1080/01635580701759724. [DOI] [PubMed] [Google Scholar]
- 18.Shayanfar M, M-Shirazi M, Rashidkhani B, Esmaillzadeh A, Houshiar-Rad A, Doaei S, et al. The association between food groups and adult gliomas: A casecontrol study in adult with gliomas. J Health Syst Res. 2013 Nutrition supplement: 1491-502 [in Persian] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preston-Martin S, Mack W. Gliomas and meningiomas in men in Los Angeles County: Investigation of exposures to N-nitroso compounds. IARC Sci Publ. 1991;107:197–203. [PubMed] [Google Scholar]
- 21.Preston-Martin S, Henderson BE. N-nitroso compounds and human intracranial tumours. IARC Sci Publ. 1984;57:887–94. [PubMed] [Google Scholar]
- 22.Sarasua S, Savitz DA. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer Causes Control. 1994;5:141–8. doi: 10.1007/BF01830260. [DOI] [PubMed] [Google Scholar]
- 23.Searles Nielsen S, Mueller BA, Preston-Martin S, Farin FM, Holly EA, McKean-Cowdin R. Childhood brain tumors and maternal cured meat consumption in pregnancy: Differential effect by glutathione S-transferases. Cancer Epidemiol Biomarkers Prev. 2011;20:2413–9. doi: 10.1158/1055-9965.EPI-11-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston-Martin S, Yu MC, Benton B, Henderson BE. N-Nitroso compounds and childhood brain tumors: A case-control study. Cancer Res. 1982;42:5240–5. [PubMed] [Google Scholar]
- 25.Preston-Martin S, Pogoda JM, Mueller BA, Holly EA, Lijinsky W, Davis RL. Maternal consumption of cured meats and vitamins in relation to pediatric brain tumors. Cancer Epidemiol Biomarkers Prev. 1996;5:599–605. [PubMed] [Google Scholar]
- 26.Pogoda JM, Preston-Martin S, Howe G, Lubin F, Mueller BA, Holly EA, et al. An international case-control study of maternal diet during pregnancy and childhood brain tumor risk: A histology-specific analysis by food group. Ann Epidemiol. 2009;19:148–60. doi: 10.1016/j.annepidem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubin F, Farbstein H, Chetrit A, Farbstein M, Freedman L, Alfandary E, et al. The role of nutritional habits during gestation and child life in pediatric brain tumor etiology. Int J Cancer. 2000;86:139–43. doi: 10.1002/(sici)1097-0215(20000401)86:1<139::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Kuijten RR, Bunin GR, Nass CC, Meadows AT. Gestational and familial risk factors for childhood astrocytoma: Results of a case-control study. Cancer Res. 1990;50:2608–12. [PubMed] [Google Scholar]
- 29.Giordana MT. Epidemiology and pathology of brain tumors in childhood. Minerva Med. 1984;75:1401–6. [PubMed] [Google Scholar]
- 30.Bunin GR, Kushi LH, Gallagher PR, Rorke-Adams LB, McBride ML, Cnaan A. Maternal diet during pregnancy and its association with medulloblastoma in children: A children's oncology group study (United States) Cancer Causes Control. 2005;16:877–91. doi: 10.1007/s10552-005-3144-7. [DOI] [PubMed] [Google Scholar]
- 31.Bunin GR, Kuijten RR, Boesel CP, Buckley JD, Meadows AT. Maternal diet and risk of astrocytic glioma in children: A report from the Childrens Cancer Group (United States and Canada) Cancer Causes Control. 1994;5:177–87. doi: 10.1007/BF01830264. [DOI] [PubMed] [Google Scholar]
- 32.Huncharek M, Kupelnick B. A meta-analysis of maternal cured meat consumption during pregnancy and the risk of childhood brain tumors. Neuroepidemiology. 2004;23:78–84. doi: 10.1159/000073979. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Johnson KC, Mao Y, Guo L, Zhao X, Jia X, et al. Risk factors for glioma in adults: A case-control study in northeast China. Cancer Detect Prev. 1998;22:100–8. doi: 10.1046/j.1525-1500.1998.cdoa22.x. [DOI] [PubMed] [Google Scholar]
- 34.Ryan P, Lee MW, North B, McMichael AJ. Risk factors for tumors of the brain and meninges: Results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51:20–7. doi: 10.1002/ijc.2910510105. [DOI] [PubMed] [Google Scholar]
- 35.Schwartzbaum JA, Fisher JL, Goodman J, Octaviano D, Cornwell DG. Hypotheses concerning roles of dietary energy, cured meat, and serum tocopherols in adult glioma development. Neuroepidemiology. 1999;18:156–66. doi: 10.1159/000026207. [DOI] [PubMed] [Google Scholar]
- 36.Ahlbom A, Navier IL, Norell S, Olin R, Spännare B. Nonoccupational risk indicators for astrocytomas in adults. Am J Epidemiol. 1986;124:334–7. doi: 10.1093/oxfordjournals.aje.a114393. [DOI] [PubMed] [Google Scholar]
- 37.Boeing H, Schlehofer B, Blettner M, Wahrendorf J. Dietary carcinogens and the risk for glioma and meningioma in Germany. Int J Cancer. 1993;53:561–5. doi: 10.1002/ijc.2910530406. [DOI] [PubMed] [Google Scholar]
- 38.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 39.Kaplan S, Novikov I, Modan B. Nutritional factors in the etiology of brain tumors: Potential role of nitrosamines, fat, and cholesterol. Am J Epidemiol. 1997;146:832–41. doi: 10.1093/oxfordjournals.aje.a009201. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, La Vecchia C, Negri E, Chatenoud L, Bosetti C, Jia X, et al. Diet and brain cancer in adults: A case-control study in northeast China. Int J Cancer. 1999;81:20–3. doi: 10.1002/(sici)1097-0215(19990331)81:1<20::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Giles GG, McNeil JJ, Donnan G, Webley C, Staples MP, Ireland PD, et al. Dietary factors and the risk of glioma in adults: Results of a case-control study in Melbourne, Australia. Int J Cancer. 1994;59:357–62. doi: 10.1002/ijc.2910590311. [DOI] [PubMed] [Google Scholar]
- 42.Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: A systematic review and dose-response meta-analysis. Nutr Rev. 2013;71:257–67. doi: 10.1111/nure.12028. [DOI] [PubMed] [Google Scholar]
- 43.Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, Yang YS, et al. Meat consumption and risk of lung cancer: Evidence from observational studies. Ann Oncol. 2012;23:3163–70. doi: 10.1093/annonc/mds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Yu E, Gao X, Song N, Liu L, Wei X, et al. Red and processed meat intake and risk of colorectal adenomas: A meta-analysis of observational studies. Int J Cancer. 2013;132:437–48. doi: 10.1002/ijc.27625. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Jiang H. Meat intake and risk of bladder cancer: A meta-analysis. Med Oncol. 2012;29:848–55. doi: 10.1007/s12032-011-9985-x. [DOI] [PubMed] [Google Scholar]
- 46.Faramawi MF, Johnson E, Fry MW, Sall M, Zhou Y. Consumption of different types of meat and the risk of renal cancer: Meta-analysis of case-control studies. Cancer Causes Control. 2007;18:125–33. doi: 10.1007/s10552-006-0104-9. [DOI] [PubMed] [Google Scholar]
- 47.Washington, DC: AICR; 2007. World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. [Google Scholar]
- 48.Willett WC. 3th ed. New York: Oxford University Press; 2012. Nutritional Epidemiology. [Google Scholar]
- 49.Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: Meta-analysis. World J Gastroenterol. 2013;19:1020–9. doi: 10.3748/wjg.v19.i7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, et al. Red and processed meat intake is associated with higher gastric cancer risk: A meta-analysis of epidemiological observational studies. PLoS One. 2013;8:e70955. doi: 10.1371/journal.pone.0070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallin A, Orsini N, Wolk A. Red and processed meat consumption and risk of ovarian cancer: A dose-response meta-analysis of prospective studies. Br J Cancer. 2011;104:1196–201. doi: 10.1038/bjc.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J. 2010;9:50. doi: 10.1186/1475-2891-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur J Nutr. 2008;47(Suppl 2):51–9. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- 54.Maslova E, Bhattacharya S, Lin SW, Michels KB. Caffeine consumption during pregnancy and risk of preterm birth: A meta-analysis. Am J Clin Nutr. 2010;92:1120–32. doi: 10.3945/ajcn.2010.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 56.Maekawa A, Mitsumori K. Spontaneous occurrence and chemical induction of neurogenic tumors in rats — Influence of host factors and specificity of chemical structure. Crit Rev Toxicol. 1990;20:287–310. doi: 10.3109/10408449009089866. [DOI] [PubMed] [Google Scholar]
- 57.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 58.Hattori T, Andoh T, Sakai N, Yamada H, Kameyama Y, Ohki K, et al. Membrane phospholipid composition and membrane fluidity of human brain tumour: A spin label study. Neurol Res. 1987;9:38–43. doi: 10.1080/01616412.1987.11739769. [DOI] [PubMed] [Google Scholar]
- 59.Qi ZY, Shao C, Zhang X, Hui GZ, Wang Z. Exogenous and endogenous hormones in relation to glioma in women: A meta-analysis of 11 case-control studies. PLoS One. 2013;8:e68695. doi: 10.1371/journal.pone.0068695. [DOI] [PMC free article] [PubMed] [Google Scholar]


