Abstract
Pain is an unpleasant experience and effects daily routine negatively. Although there are various drugs, many of them are not entirely successful in relieving pain, since pain modulation is a complex process involving numerous mediators and receptors. Therefore, it is a rational approach to identify the factors involved in the complex process and develop new agents that act on these pain producing mechanisms. In this respect, the involvement of the imidazoline receptors in pain modulation has drawn attention in recent years. In this review, it is aimed to focus on the imidazoline receptors and their ligands which contribute to the pain modulation. It is demonstrated that imidazoline-2 (I2) receptors are steady new drug targets for analgesics. Even if the mechanism of I2 receptor is not well known in the modulation of pain, it is known that it plays a role in tonic and chronic pain but not in acute phasic pain. Moreover, the I2 receptor ligands increase the analgesic effects of opioids in both acute and chronic pain and prevent the development of opioid tolerance. So, they are valuable for the chronic pain treatment and also therapeutic coadjuvants in the management of chronic pain with opiate drugs due to the attenuation of opioid tolerance and addiction. Thus, the use of the ligands which bind to the imidazoline receptors is an effective strategy for relieving pain. This educational forum exhibits the role of imidazoline receptors and ligands in pain process by utilizing experimental studies.
KEY WORDS: Acute pain, chronic pain, imidazoline receptors, imidazoline receptor ligands
Introduction
Pain is defined as an unpleasant sensory and emotional experience arising from any part of the body. It is associated with actual or potential tissue damage or described in terms of such damage by International Association for the Study of Pain. It is an “experience” and in this respect, it differs from “nociception.” Nociception is called a neural process that provides transduction and transmission of a noxious stimulus to the brain via pain pathways. The pain arises from a complicated interaction between signaling systems, modulation of higher centers, and individual perception.[1,2]
The whole human population experiences pain in varying degrees and daily routine is affected negatively. Pain may be occurred acutely or chronically related with various disturbances such as lesions, traumatic injury, tumors, inflammatory diseases, Parkinson's disease, and diabetes.[3,4] Since different mechanisms involve in the pathophysiology of acute and chronic pain and even nociceptive and neuropathic pain, the management strategies and current drug classes also vary. Although there are too many analgesic agents, there are certain problems such as tolerability, tolerance, abstinence syndrome, insufficiency, possible drug interactions, and side effects. Thereby, the development of new analgesic compounds is still going on. In this respect, the development and the use of imidazoline receptor ligands have gradually drawn attention since the role of imidazoline receptors in pain modulation was identified. For instance, various ligands which bind to imidazoline-2 (I2) receptors, the imidazoline receptor subtype which is predominantly involved in pain modulation, have been synthesized 2-(4,5-dihydroimidazole-2-il) quinoline hydrochloride (BU224), 2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI), 4-chloro-2-(imidazoline-2-yl) isoindoline (RS-45041), etc., in last decades and all of them have been reported to exhibit antinociceptive properties as discussed in this review.[5,6,7] Whereas the single use of imidazoline receptor ligands is effective in tonic and chronic pain, combined usage of other analgesic drugs such as morphine and clonidine is also effective in the potentiation of both acute and chronic pain conditions such as neuropathic pain. In fact, it is known that the I2 receptor agonism is one the mechanisms of neuropathic pain control, and the ligands that use this mechanism are in the phase 2 and phase 3 studies.[8,9,10] In this review, we document the role of I2 receptors and ligands in antinociception and the relevant experimental studies performed by various researchers.
Imidazoline Receptors and Their Biologic Roles
Although the term imidazoline receptor has not yet been adopted by major professional societies including “International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification” since these receptors have not yet been cloned and the signaling pathway not characterized, this term is widely used in the literature.[11] Therefore, it is also frequently called as imidazoline binding sites; however, the term imidazoline receptor is employed in this review.
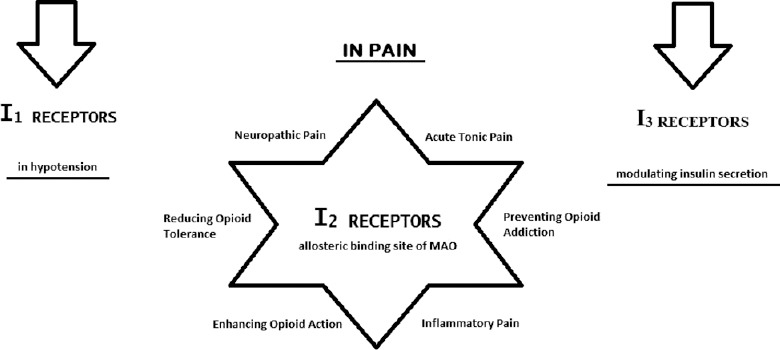
The presence of imidazoline receptors, with high affinity for imidazoline subdivision containing compounds, first became apparent in the mid-1970. The hypotensive effect induced by clonidine, α2-adrenoceptor agonist, and microinjection into the rat brainstem was not mimicked by norepinephrine.[12] The imidazoline receptors are broadly located in the mammalian cells of the central nervous system (CNS) and peripheral nervous system[13] and contribute to cardiovascular system activity, gastric acid secretion, insulin release, antinociception, Alzheimer's, and Parkinson's disorders.[14] According to a general opinion, there are three main classes of heterogeneous imidazoline receptors, as seen in Figure 1. Imidazoline-1 (I1) receptors constitute a family of nonadrenergic high-affinity binding sites for some ligands such as clonidine and idazoxan. These receptors handle the centrally mediated hypotension occurred with clonidine-like drugs.[15] They are located in the plasma membranes in the brain, heart, kidney, liver, and pancreas. The I2 receptors bind imidazolines and guanidines and have a lower affinity for 2-aminoimidazolines such as clonidine.[16] The I2 receptors are mitochondrial, not G-protein coupled, and allosteric binding sites, possibly with a modulatory function, on monoamine oxidase-A and -B (MAO-A and -B). These enzymes are found in the neurons and astroglia cells and have a critical role in the inactivation of neurotransmitters. Thereby, the I2 receptors may be useful as a therapeutic agent in various neurological diseases in which neurodegeneration is observed.[14,15] Besides, it is known that these subtypes contribute to reduce body temperature, exert control over central noradrenergic and hypothalamic-pituitary-adrenal axis activity, and regulate the small intestinal motility.[17,18] More remarkably, it has been shown that the I2 receptors take part in the antinociception in several acute and chronic pain models and the ligands acting on the I2 receptors may be assessed as novel analgesics.[19] The imidazoline-3 (I3) receptors whose biological importance investigated further, are present in the pancreatic beta islet cells and modulate insulin secretion.[16] We focus on the I2 receptor subtypes in this educational forum because involvement of this subtype in antinociception is well determined.
Figure 1.
The imidazoline receptors in pain
Imidazoline-2 Receptors in Pain Modulation
Pain modulation is a highly complex process, including numerous interacting peripheral and central mechanisms. The activation of the peripheral nociceptors or mediators that are released by the damaged tissue is required for the perception of pain. The activation-triggered signal is conveyed with the afferent transmission to the spinal cord with Aδ and C nerve fibers and transmission parts through the dorsal horn (DH) to the higher centers via parallel ascending pain pathways.[20] The impulses originated from the brain stem nuclei, descend to the spinal level and affect the transmission of pain signals at the DH.[9,21] The relative balance between descending inhibition and facilitation can be changed by the type and intensity of the stimulus and also by the time following an injury.[8] This complex process is regulated by the interaction of various chemicals and receptors over an extensive network from the periphery to the CNS. The rate of participation of the chemicals and receptor types in the modulation depends on the types of pain and noxious stimulus.
The imidazoline receptors that are taken part in this extensive receptor networks have gradually gained important in the recent years. When the roles of imidazoline receptors are evaluated in terms of pain, I2 subtypes attract the attention.[22] the I2 receptors are distributed ubiquitously throughout the brain regions such as the arcuate nucleus, caudate nucleus, putamen, globus pallidus, substantia nigra, interpeduncular nucleus, area postrema, mammillary peduncle, ependyma, lateral mammillary nucleus, and the pineal gland. Low to moderate densities are found in the cerebral cortex, thalamus, hippocampus, amygdala, inferior olivary nucleus, ependymal, and various periphery regions.[16] The I2 receptors are characterized by their high affinity for imidazolines and guanidines and medium affinity for imidazolidines. Also, they have two subtypes as I2A and I2B, which differ in terms of their sensitivity to amiloride.[23] The I2 receptor is first located on the outer membranes of mitochondria and as allosteric sites on enzyme MAO-A and MAO-B.[24] However, the imidazoline binding site on MAO is separate from the active domain of the enzyme that recognizes the mechanism-based inhibitors, and it is not equally available in all the tissues.[25] The I2 receptors have the same molecular weight as MAO, and the amino acid sequencing of purified I2 receptors is similar to MAO.[26]
Two MAO isoforms play a fundamental role in the metabolism of monoamine neurotransmitters such as serotonin, noradrenaline, and dopamine.[27] As the I2 receptor is thought to be a modulatory site on the MAO protein, the activity of I2 receptors may involve in several neurological disorders.[24,27] Another fascinating development with I2 receptors is that its effect on nociception since these monoamines appear to play a significant role in specific CNS structures implicated in pain modulation spinal cord, cerebral cortex, etc., and are involved in the antinociception of several drugs such as antidepressants which are commonly used for the management of pain.[27] The ligands binding to the I2 receptors can be considered as inhibitors of MAO-A and MAO-B since they inhibited monoamine oxidation,[28] and this inhibition could explain some biological effects of imidazoline receptor ligands such as pain modulation.[29] In fact, it is well identified that increased levels of monoamines such as serotonin and noradrenaline contributes to pain control, especially in the CNS regions related to pain. The drugs such as MAO inhibitors, serotonin noradrenaline reuptake inhibitors, and selective serotonin reuptake inhibitors that provide the enhancement of levels of these neurotransmitters are successfully being used to relieve various pain conditions.[30,31,32]
Some evidence that shows the I2 receptors contribute to the modulation of pain [Figure 1] reports that the ligands that affect I2 receptors are effective for tonic pain, neuropathic pain, but little effective for acute pain.[11] Moreover, the activation of I2 receptors has been suggested to be a way of enhancing opioid analgesic actions.[10] When they are combined with opioids, the I2 receptor ligands increase the analgesic effects of opioids in both acute and chronic pain. In chronic use, tolerance and addiction to the opioids can develop. However, the I2 receptor ligands can reduce the development of this opioid tolerance or prevent from deprivation syndrome that is triggered by the antagonists in animals.[11] When all of these findings are considered together, ligands that affect the I2 receptors can be beneficial in monotherapy or combination therapy with opioids to overcome pain. Therefore, various new selective ligands are being developed for this receptor types.
Imidazoline-2 Receptor Ligands in Pain
Although the I2 receptors and their signaling pathways have not yet been characterized, the majority of the I2 receptors are widely accepted as being allosteric sites on MAO. Therefore, the I2 receptor ligands are described as allosteric modulators. Allosteric modulation is the modulation of a protein via binding a ligand to the domain that is distinct from the active site of the protein. Conformational change occurs by this binding process. Ligands that improves the activity of the protein are referred as allosteric activators or agonists, whereas those that decrease the activity of the protein are referred as allosteric inhibitors or antagonists.[33] In principle, it is not precisely known whether a ligand is an I2 receptor agonist, an antagonist, or an inverse agonist in a constitutively active system. Each and every ligand has two properties (i.e., affinity and efficacy) that govern its effects related to certain receptors in a bioassay.[34] It is possible to have a knowledge of the relative efficacy of these ligands by systematic comparison. Ligand efficacy values may vary depending upon assays, however, the rank order of their efficacy remains unchanged with few exceptions (e.g., functional selectivity).[11]
Idazoxan, agmatine, and clonidine are ligands that bind to the I2 receptors as well as α2 and/or I1 receptors with varying affinities. Due to the proven effect of the I2 receptors in pain, further studies are required to discover selective analgesic I2 receptor ligands and to elucidate their physiological functions. Over the last few decades, investigators have attempted to synthesize and recognize selective ligands for the I2 binding sites. For instance, the I2 receptor ligand 2-BFI (pKi = 8.47 for I2 in rabbit kidney membrane with [3 H] 2-BFI) which has high affinity for both I2A and I2B sites has been used to characterize these sites in several species, including humans.[26] It is also extremely useful for binding and autoradiographic studies.[35] BU series have also been investigated in the previous years. It has been proved that quinolone compound BU224 has high affinity (pKi = 8.43 for I2 in rabbit kidney membrane with [3 H] 2-BFI)[26] for the I2 receptors over I1 receptors and α2-adrenoceptors, among all the compounds of BU series.[26,35] RS-45041-190 is also a selective ligand with a high affinity (pKi = 9.37 for I2 in rabbit kidney membrane with [3 H] idazoxan)[36] for the I2 site which exhibits comparable data to that observed using 2-BFI in membrane-binding and autoradiographic studies.[26]
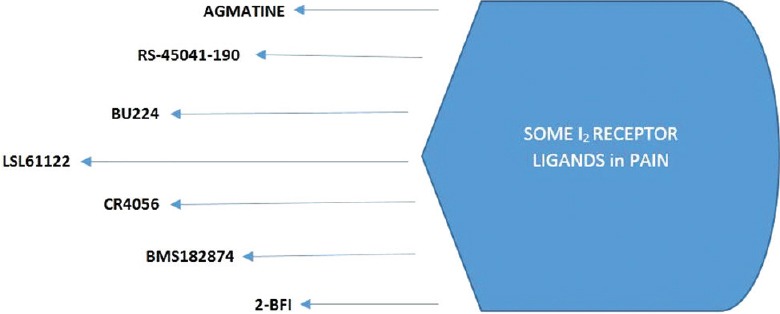
As mentioned before, the imidazoline receptor ligands are declared to modulate certain processes which involve MAO activities in CNS.[29] The studies reveal that among all of the imidazoline receptor ligands those mentioned in this review; 2-BFI, BU224, 2-phenyl-6-(1H-imidazol-1il) quinazoline (CR4056), idazoxan, and RS-45041 more selectively inhibit MAO-A while LSL60101 and LSL61122 inhibit MAO-B.[13,36,37,38] After this part of the review, several studies will be conducted with the mentioned ligands [Figure 2] that have a higher affinity for the I2 receptors and the provided results will be included.
Figure 2.
The imidazoline imidazoline-2 receptor ligands in pain
It should first be noted that clonidine that mediated the discovery of the presence of imidazoline receptors, is a α2-adrenoceptor agonist that binds not only to α2 adrenoceptors but also to imidazoline receptors in particular to I1 receptors compared to I2 receptors; however, its antinociceptive activity occurs via α2-adrenoceptors rather than imidazoline receptors.[39] Agmatine is a nonselective and most extensively studied endogenous imidazoline receptor agonist, and has a moderate affinity to α2-adrenoceptors as well as all subtypes of imidazoline receptors (pKi < 5 for I2 in rabbit kidney membrane with [3 H] 2-BFI).[26,40] Its effect on spinal nociception is known. It is indicated that this endogenous substance prevents reflex respond to the noxious stimulus through nonadrenergic receptors in mice.[29] It does not show a significant effect on acute phasic pain while it is vice versa in the acute tonic (differs from acute phasic pain in terms of using chemicals to induce noxious stimuli), inflammatory, and neuropathic pain. The effectiveness of agmatine also depends on the administration route in acute pain. Although agmatine showed weak effectiveness in a few studies,[41] in many others, it was not efficient when administered systemically in acute phasic pain.[42] Spinal and supraspinal agmatine administration also does not reduce the thermal threshold, similar to systemic agmatine treatment. For example, in a study, i.t. (spinal) and also i.c.v. (supraspinal) agmatine administration did not produce analgesic effects in the tail-flick test in mice.[43] Conversely, agmatine is effective in inflammatory pain. The acute systemic agmatine treatment markedly decreased mechanical allodynia induced by complete Freund's adjuvant (CFA), chronic pain model, in mice.[44] Agmatine injections by systemic and supraspinal route have also been demonstrated to be effective in relieving hyperalgesia and/or allodynia in several neuropathic pain models.[22]
Some other similar substances have also showed the same results. Systemic administration of the I2 receptor ligands as phenyzoline, 2-BFI, 2-(2-benzofuranyl) imidazole hydrochloride (LSL 60101); the analog of 2-BFI, 2-styryl-2-imidazoline; valldemossine or tracizoline (LSL 61122) also did not produce antinociception in the acute pain model.[29,45] In addition, researches showed that the I2 receptor ligands such as RS-45041-190, CR4056, 2-BFI, BU224 were effective in inflammatory and neuropathic pain.[6,7,46]
RS-45041-190, the first selective and high-affinity ligand, showed significant results on the carrageenan-induced thermal and mechanic hyperalgesia tests when administered systemically (i.p.), not spinally (i.t.) in rats.[7] CR4056 (moderate affinity I2 receptor ligand (IC50=596 nM, in rat whole brain membrane with [3 H] 2-BFI),[13] is a new, highly selective I2 receptor ligand which inhibits MAO-A activity more selectively than MAO-B. In a study, the effectiveness of CR4056 was evaluated in various models of pain. CR4056 was found active in CFA-induced model of inflammation. In acute capsaicin-evoked pain model, CR4056 blocked the paw-induced mechanic hyperalgesia.[13] This effect was antagonized by idazoxan (pKi = 7.22 for I2 in rabbit kidney membrane with [3 H] 2-BFI),[26] which is both a α2-adrenoceptor and I2 receptor antagonist. CR4056 also significantly reduced the mechanic hyperalgesia and allodynia in neuropathic pain models.[13] Similarly, in another study, CR4056 oral administration dose-dependently reversed the allodynia in bortezomib-induced peripheral neuropathy model that is painful.[46] The postoperative pain is apparently distinct from pure inflammatory or neuropathic pain. More recently, Lanza et al.,[47] have investigated the antinociception induced by CR4056 in a rat model of postoperative pain and Caselli et al.,[48] in a rat model of joint pain. Acute administration of CR4056 was found effective in relieving postoperative pain and joint pain as well as inflammatory and neuropathic pain. Even naproxen showed low and nonremarkable antinociception compared to antinociception induced by CR4056 in these pain models. In addition, in joint pain model driven by both nociceptive and neuropathic mechanisms, the rats treated for 7 days (from days 14 to 21) after the induction of cartilage degeneration with CR4056, showed a significant reduction of both basal pain behaviors (allodynia and hyperalgesia). This result demonstrates either a long lasting effect or even an actual symptom modifying effect. The joint pain model is related to osteoarthritis that is driven by both nociceptive and neuropathic mechanisms.[48] Shortly, these studies present a new opportunity for the management of inflammatory, neuropathic, postoperative, and joint pain based on the selective interactions with the central I2 receptors. The phase II trials on neuropathic pain for CR4056 are available and still going on. The effect of BU224, a high affinity selective I2 receptor ligand, has been assessed electrophysiologically on the nociceptive neurons’ responses in the spinal DH. Spinal injection of BU-224 by i.t. route attenuated the nociceptive responses of DH neurons, creating a dose-dependent inhibition of C-fiber-induced responses, Aδ-fibre-evoked responses, post discharge, and winding-up of the cells. Idazoxan (i.t.) completely and significantly antagonized these effects while the nonselective α2-adrenoceptor antagonist yohimbine and the highly selective α2-adrenoceptor antagonist atipamezole only partially antagonized.[5] When we consider the results, it is possible to say that BU224 has a high affinity for the spinal I2 receptors, as well as it has an insignificant action at the spinal α2-adrenoceptor receptors. It is obvious that the I2 receptor ligands are more effective in chronic pain models than acute phasic pain models as mentioned with agmatine. Chronic pain usually means a persistent pain lasting 3 months or more, and pharmacotherapy of chronic pain always comprises repeated dose. A possible result of repeated doses of analgesics is a gradual decrease in analgesic effect, in other words, development of analgesic tolerance. From this point of view, in a research performed by Li et al.,[6] antihyperalgesic effects of 2-BFI and CR4056 have also been tested in repeating treatments, in addition to single doses in chronic constriction injury (CCI)-induced neuropathic pain and CFA-induced inflammatory pain models. The antinociceptive tolerance did not develop against repeated administration (daily for 7–9 days) of 2-BFI or CR4056 in CFA-treated or CCI rats. It is possible to say that the repeated dose regimen for the I2 receptor ligands in chronic pain treatment may be useful as monotherapy or adjunctive therapy without tolerance and addiction. It has been also shown that three high selective I2 receptor ligands; 2-BFI, BU224, and LSL 61122 possess antihyperalgesic effects in the rat models of CFA evoked inflammatory pain in this study. These drugs reduced mechanical and thermal hyperalgesia in a dose-dependent manner. Also, the I2 receptor antagonist idazoxan antagonized the antihyperalgesic effects of 2-BFI in CFA-treated and CCI rats.[6] These data also support the beneficial roles of the I2 receptor ligands in pain control. Pain models contain lots of irrelevant and interacting compounds. Although most parts of preclinical pain studies focus on sensorial relieving pain, the sensorial and emotional compounds should be studied together. For instance, the effects of the I2 receptor ligands such as 2-BFI, BU224, and CR4056 on escape/avoidance behaviors on CFA injected rats were studied connectedly to the affective pain in the same study. This method is suitable to measure the dissociable components of effective pain which is different from the sensory pain. It was observed that 2-BFI, BU224, and CR4056 increase the escape/avoidance behavior in the hyperalgesia increasing doses.[6] These results exhibit that the I2 receptor ligands may be effective against the affective components of pain. More recently, Thorn et al.[49] have studied the antinociception induced by 2-BFI and phenyzoline, high affinity ligand for I2 receptors (pKi = 8.60 in rabbit kidney membrane with [3 H]-idazoxan),[45] using the von Frey filament test in rats with CFA-induced inflammatory pain. Providing antinociception by 2-BFI was not surprising, however, phenyzoline also produces antinociception in this chronic model as in acute phasic pain under weak noxious stimulus, as reported previously in a study by Sampson et al.[50]
The role of imidazoline receptors for enhancing morphine antinociception is also known. Even some I2 receptor ligands as agmatine are not effective alone in acute phasic pain, they potentiate morphine antinociception.[42] In a study, performed in the warm water, tail withdrawal procedure in rats by using selective I2 receptor ligand 2-BFI along with agmatine, it was observed that these two ligands increase the antinociceptive effects of morphine and tramadol. In contrast, another selective I2 receptor ligand BU224 failed to increase the antinociceptive efficacy but prevented agmatine and 2-BFI ligands to increase the morphine and tramadol-induced antinociception. The reason that BU224 acts differently from other imidazoline receptor ligands is its lower efficacy in spite of its high affinity.[51] This contradictory situation may be confusing, but it should be noted that affinity and efficacy are distinct terms from each other. A ligand that shows low efficacy may bind its binding site with high affinity.[52] In yet another study, the combination of 2-BFI and morphine produced additive effects on mechanical hyperalgesia in CFA-treated rats.[6] These results suggest that the combination of the I2 receptor ligands and opioids may be effective in chronic pain treatment. However, the previous studies indicated the I2 receptors to contribute potentiation mechanism, and I1 and I2 receptors’ contribution of potentiation mechanism was not clearly understood. More recently, a study which has been done to understand which subtype contributes to potentiation mechanism showed that a significant oxycodone-induced antinociceptive respond could not be reversed by efaroxan (I1 receptor antagonist) but could be reversed by BU224 (I2 receptor antagonist). As a similar way, endothelin ETA receptor antagonist 5-(Dimethylamino) -N-(3,4-dimethyl-5- isoxazolyl)-1-naphthalenesulfonamide-induced potentiation of oxycodone antinociception was reversed by BU224 but not efaroxan.[53] So, it is thought that the I2 receptors participate in the potentiation, the I1 receptors do not. In yet another study performed for understanding the importance and mechanisms of potentiation, agmatine, high selective and more powerful substances such as 2-BFI, LSL 60101 (pKi = 6.45 for I2 in rat cerebral cortex with [3 H] idazoxan),[54] LSL 61122 (pKi = 8.74 for I2 in rabbit kidney with [3 H] idazoxan),[55] and aganodine have been tested. It was observed that central (i.c.v.) or peripheral (s.c.) administration of the I2 receptor ligands (but not I1 or α2 adrenoreceptor) potentiate the morphine-evoked supraspinal antinociception in mice. The enhanced morphine antinociception via the I2 receptor ligands was reversed by idazoxan, BU224, and isothiocyanatobenzylimidazoline, an irreversible I2 antagonist. In the same study, it has also been shown how the augmentation of morphine antinociception by the I2 agonists’ changes in mice with pertussis toxin impaired guanosine triphosphate-binding Gi-Go proteins. The potentiation ability effect was blocked. Therefore, the contribution of Gi-Go transducer proteins in the modulation of morphine antinociception induced by the I2 receptors cannot be disrespected.[29] Most recently, Thorn et al.[49] studied 2-BFI and phenyzoline with oxycodone as combinations, separately. 2-BFI and oxycodone produced additive interactions while phenyzoline and oxycodone produced synergistic interactions for their effects on mechanical hyperalgesia in CFA-treated rats.
The imidazoline receptors are also important for the tolerance developed with opioids as well as improvement of opioid analgesia as mentioned before. For instance, agmatine prevents or decreases the tolerance development against morphine or other opioids.[56] Additionally, α-difluoromethylornithine and aminoguanidine, which may affect the metabolism of endogenous agmatine, were found effective in the inhibition of acute morphine tolerance in tail-flick test.[57] Similarly, Boronat et al.,[58] have assessed the role of imidazoline receptors in opioid (morphine and pentazocine) tolerance in rats by the administration of idazoxan. The tail-flick test was used for evaluating the antinociception. Idazoxan completely prevented the morphine tolerance, but 2-methoxy-idazoxane and RS-15385-197, selective α2-adrenoceptor antagonists, did not and it remarkably reduced tolerance to pentazocine.[58] In contrary, Su et al.,[59] showed that idazoxan promoted the development of tolerance to morphine and induced the abstinence syndrome in morphine-dependent mice and rats similar to naloxone. The chronic concurrent administration of 2-BFI, LSL 60101, and LSL 61122, selective and potent I2 receptor ligands, and morphine, also prevented or attenuated morphine tolerance.[58] In the light of the positive outcomes, it is supported that the I2 receptor ligands as promising therapeutic coadjuvants in the management of chronic pain with opiate drugs since these agents prevent tolerance development and enhance opioid analgesia.
Conclusion
All the studies show us that the I2 receptors are also steady, new drug targets for analgesics. Even if the mechanism of the I2 receptor is not well known in the modulation of pain, it is known that it plays a role in tonic and chronic pain but not in the acute phasic pain. Additionally, when they are combined with opioids in both acute and chronic pain, the I2 receptor ligands increase the antinociceptive actions of opioids. The development of tolerance and addiction induced by chronic administration of opioids is one of the major problems in the clinic. However, the I2 receptor ligands can reduce the opioid tolerance development or prevent from deprivation syndrome in the combination therapy. They are valuable for the chronic pain treatment and also valuable as therapeutic coadjuvants of opiates, because of the attenuation of opioid tolerance and addiction.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Gamze Ulugbay for contributing to prepare the paper. No organizations funded this article. All diagrams in this paper are original and designed by the authors of this paper.
References
- 1.Merskey H, Bogduk N. Part III: Pain terms, A current list with definitions and notes on usage. In: Merskey H, Bogduk N, editors. Classification of Chronic Pain, IASP Task Force on Taxonomy. 2nd ed. Seattle, WA: IASP Press; 1994. pp. 209–14. [Google Scholar]
- 2.Steeds CE. The anatomy and physiology of pain. Surgery. 2009;27:507–11. [Google Scholar]
- 3.Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999;40:111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010:07–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Diaz A, Mayet S, Dickenson AH. BU-224 produces spinal antinociception as an agonist at imidazoline I2 receptors. Eur J Pharmacol. 1997;333:9–15. doi: 10.1016/s0014-2999(97)01118-7. [DOI] [PubMed] [Google Scholar]
- 6.Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–90. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jett MF, Hedley LR, Dillon MP, Eglen RM, Hunter JC. Behavioral effects of RS-45041-190, a selective I2 imidazoline ligand, in rats. Ann N Y Acad Sci. 1999;881:369–71. doi: 10.1111/j.1749-6632.1999.tb09383.x. [DOI] [PubMed] [Google Scholar]
- 8.Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM. 3rd ed. Melbourne: ANZCA and FPM; 2010. Acute Pain Management: Scientific Evidence; pp. 1–6. [Google Scholar]
- 9.Schaible HG. Peripheral and central mechanisms of pain generation. Handb Exp Pharmacol. 2007;177:3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 10.Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs. 2014;19:329–41. doi: 10.1517/14728214.2014.915025. [DOI] [PubMed] [Google Scholar]
- 11.Li JX, Zhang Y. Imidazoline I2 receptors: Target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet P, Feldman J, Velly J, Bloch R. Role of the ventral surface of the brain stem in the hypotensive action of clonidine. Eur J Pharmacol. 1975;34:151–6. doi: 10.1016/0014-2999(75)90235-6. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, et al. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–25. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dardonville C, Rozas I, Callado LF, Meana JJ. I(2)-imidazoline binding site affinity of a structurally different type of ligands. Bioorg Med Chem. 2002;10:1525–33. doi: 10.1016/s0968-0896(01)00420-5. [DOI] [PubMed] [Google Scholar]
- 15.Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Annu Rev Pharmacol Toxicol. 1996;36:511–44. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- 16.Smith KL, Jessop DS, Finn DP. Modulation of stress by imidazoline binding sites: Implications for psychiatric disorders. Stress. 2009;12:97–114. doi: 10.1080/10253890802302908. [DOI] [PubMed] [Google Scholar]
- 17.Pypendop BH. Small Animal Critical Care Medicine. 2015:866–71. [Google Scholar]
- 18.Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I2 receptor agonists in rats. Br J Pharmacol. 2012;166:1936–45. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Y, Zhang Y, Li JX. Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol. 2015;749:133–41. doi: 10.1016/j.ejphar.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aydin ON, Unal F. Functional endoscopic sinus surgery under sphenopalatine block or general anaesthesia: Effects on postoperative pain. Ağrı. 2002;14:55–9. [Google Scholar]
- 21.Hudspith MJ, Siddall PJ, Munglani R. 2nd ed. St Louis: Mosby; 2006. Physiology of pain. Foundations of Anesthesia: Basic Sciences for Clinical Practice; pp. 267–85. [Google Scholar]
- 22.Aricioglu F, Korcegez E, Bozkurt A, Ozyalcin S. Effect of agmatine on acute and mononeuropathic pain. Ann N Y Acad Sci. 2003;1009:106–15. doi: 10.1196/annals.1304.010. [DOI] [PubMed] [Google Scholar]
- 23.Olmos G, Alemany R, Boronat MA, Garcia-Sevilla JA. Pharmacologic and molecular discrimation of I2-imidazoline receptor subytpes. Imidazoline Receptors and their Endogenous Ligands Current Concepts and Therapeutic Potentiation. In: Göthert M, Molderings GJ, Reis DJ, editors. Vol. 881. New York: The New York Academy of Science; 1999. pp. 144–60. [DOI] [PubMed] [Google Scholar]
- 24.Lowry JA, Brown JT. Significance of the imidazoline receptors in toxicology. Clin Toxicol (Phila) 2014;52:454–69. doi: 10.3109/15563650.2014.898770. [DOI] [PubMed] [Google Scholar]
- 25.Raddatz R, Savic SL, Lanier SM. Imidazoline binding domains on MAO-B. Localization and accessibility. Ann N Y Acad Sci. 1999;881:26–31. doi: 10.1111/j.1749-6632.1999.tb09337.x. [DOI] [PubMed] [Google Scholar]
- 26.Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, et al. ‘Seeing through a glass darkly’: Casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci. 1998;19:381–90. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- 27.Villarinho JG, Fachinetto R, de Vargas Pinheiro F, da Silva Sant’Anna G, Machado P, Dombrowski PA, et al. Antidepressant-like effect of the novel MAO inhibitor 2-(3,4-dimethoxy-phenyl)-4,5-dihydro-1H-imidazole (2-DMPI) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:31–9. doi: 10.1016/j.pnpbp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Bour S, Iglesias-Osma MC, Marti L, Duro P, Garcia-Barrado MJ, Pastor MF, et al. The imidazoline I2-site ligands BU 224 and 2-BFI inhibit MAO-A and MAO-B activities, hydrogen peroxide production, and lipolysis in rodent and human adipocytes. Eur J Pharmacol. 2006;552:20–30. doi: 10.1016/j.ejphar.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Blázquez P, Boronat MA, Olmos G, García-Sevilla JA, Garzón J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: A model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130:146–52. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micó JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–54. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Marks DM, Shah MJ, Patkar AA, Masand PS, Park GY, Pae CU. Serotonin-norepinephrine reuptake inhibitors for pain control: Premise and promise. Curr Neuropharmacol. 2009;7:331–6. doi: 10.2174/157015909790031201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villarinho JG, Pinheiro Kde V, Pinheiro Fde V, Oliveira SM, Machado P, Martins MA, et al. The antinociceptive effect of reversible monoamine oxidase-A inhibitors in a mouse neuropathic pain model. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:136–42. doi: 10.1016/j.pnpbp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 34.Kenakin T, Onaran O. The ligand paradox between affinity and efficacy: Can you be there and not make a difference? Trends Pharmacol Sci. 2002;23:275–80. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- 35.Hudson AL, Gough R, Tyacke R, Lione L, Lalies M, Lewis J, et al. Novel selective compounds for the investigation of imidazoline receptors. In: Géthert M, Molderings GJ, Reis DJ, editors. Imidazoline Receptors and their Endogenous Ligands Current Concepts and Therapeutic Potentiation. Vol. 881. New York: The New York Academy of Science; 1999. pp. 81–91. [DOI] [PubMed] [Google Scholar]
- 36.Brown CM, MacKinnon AC, Redfern WS, Williams A, Linton C, Stewart M, et al. RS-45041-190: A selective, high-affinity ligand for I2 imidazoline receptors. Br J Pharmacol. 1995;116:1737–44. doi: 10.1111/j.1476-5381.1995.tb16656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozaita A, Olmos G, Boronat MA, Lizcano JM, Unzeta M, García-Sevilla JA. Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver. Br J Pharmacol. 1997;121:901–12. doi: 10.1038/sj.bjp.0701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macinnes N, Duty S. Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway. Br J Pharmacol. 2004;143:952–9. doi: 10.1038/sj.bjp.0706019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabetkasaie M, Vala S, Khansefid N, Hosseini AR, Sadat Ladgevardi MA. Clonidine and guanfacine-induced antinociception in visceral pain: Possible role of α2/I2 binding sites. Eur J Pharmacol. 2004;501:95–101. doi: 10.1016/j.ejphar.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Raasch W, Schäfer U, Chun J, Dominiak P. Biological significance of agmatine, an endogenous ligand at imidazoline binding sites. Br J Pharmacol. 2001;133:755–80. doi: 10.1038/sj.bjp.0704153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhalla S, Rapolaviciute V, Gulati A. Determination of a(2)-adrenoceptor and imidazoline receptor involvement in augmentation of morphine and oxycodone analgesia by agmatine and BMS182874. Eur J Pharmacol. 2011;651:109–21. doi: 10.1016/j.ejphar.2010.10.090. [DOI] [PubMed] [Google Scholar]
- 42.Yesilyurt O, Uzbay IT. Agmatine potentiates the analgesic effect of morphine by an α2-adrenoceptor-mediated mechanism in mice. Neuropsychopharmacology. 2001;25:98–103. doi: 10.1016/S0893-133X(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 43.Roerig SC. Spinal and supraspinal agmatine activate different receptors to enhance spinal morphine antinociception. Ann N Y Acad Sci. 2003;1009:116–26. doi: 10.1196/annals.1304.011. [DOI] [PubMed] [Google Scholar]
- 44.Paszcuk AF, Gadotti VM, Tibola D, Quintão NL, Rodrigues AL, Calixto JB, et al. Anti-hypernociceptive properties of agmatine in persistent inflammatory and neuropathic models of pain in mice. Brain Res. 2007;1159:124–33. doi: 10.1016/j.brainres.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 45.Gentili F, Cardinaletti C, Carrieri A, Ghelfi F, Mattioli L, Perfumi M, et al. Involvement of I2-imidazoline binding sites in positive and negative morphine analgesia modulatory effects. Eur J Pharmacol. 2006;553:73–81. doi: 10.1016/j.ejphar.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 46.Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, et al. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–67. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171:3693–701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caselli G, Comi E, Ferrari F, Mauri V, Catapano L, Lanza M, Lanza M, Rovati LC. Analgesic efficacy of CR4056, a novel I2-imidazoline receptor ligand, in the rat monosodium iodoacetate model of osteoarthritic pain. Osteoarthritis Cartilage. 2015;23:A358. [Google Scholar]
- 49.Thorn DA, Siemian JN, Zhang Y, Li JX. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in a rat model of inflammatory pain: Interactions with oxycodone. Psychopharmacology. 2015;232:3309–18. doi: 10.1007/s00213-015-3983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampson C, Zhang Y, Del Bello F, Li JX. Effects of imidazoline I2 receptor ligands on acute nociception in rats. Neuroreport. 2012;23:73–7. doi: 10.1097/WNR.0b013e32834e7db3. [DOI] [PubMed] [Google Scholar]
- 51.Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX. Effects of imidazoline I2 receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol. 2011;670:435–40. doi: 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- 52.Kenakin TP. The relative contribution of affinity and efficacy to agonist activity: Organ selectivity of noradrenaline and oxymetazoline with reference to the classification of drug receptors. Br J Pharmacol. 1984;81:131–41. doi: 10.1111/j.1476-5381.1984.tb10753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhalla S, Ali I, Lee H, Andurkar SV, Gulati A. Potentiation of oxycodone antinociception in mice by agmatine and BMS182874 via an imidazoline I2 receptor-mediated mechanism. Pharmacol Biochem Behav. 2013;103:550–60. doi: 10.1016/j.pbb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Alemany R, Olmos G, Escribá PV, Menargues A, Obach R, García-Sevilla JA. LSL 60101, a selective ligand for imidazoline I2 receptors, on glial fibrillary acidic protein concentration. Eur J Pharmacol. 1995;280:205–10. doi: 10.1016/0014-2999(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 55.Pigini M, Bousquet P, Brasili L, Carrieri A, Dontenwill M, Gentili F, et al. Binding of tracizolines to the imidazoline receptor. Role of lipophilicity in quantitative structure-activity relationship models. Ann N Y Acad Sci. 1999;881:118–22. doi: 10.1111/j.1749-6632.1999.tb09351.x. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Li X, Pei G, Qin BY. Effects of agmatine on tolerance to and substance dependence on morphine in mice. Zhongguo Yao Li Xue Bao. 1999;20:232–8. [PubMed] [Google Scholar]
- 57.Lu G, Su RB, Li J, Qin BY. Modulation by a-difluoromethyl-ornithine and aminoguanidine of pain threshold, morphine analgesia and tolerance. Eur J Pharmacol. 2003;8(478):139–44. doi: 10.1016/j.ejphar.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 58.Boronat MA, Olmos G, García-Sevilla JA. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br J Pharmacol. 1998;125:175–85. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su RB, Li J, Gao K, Pei G, Qin BY. Influence of idazoxan on analgesia, tolerance, and physical dependence of morphine in mice and rats in vivo. Acta Pharmacol Sin. 2000;21:1011–5. [PubMed] [Google Scholar]


