Abstract
Objectives:
Magnesium historically has been used for treatment and/or prevention of eclampsia. Considering the low body mass index of Indian women, a low-dose magnesium sulfate regime has been introduced by some authors. Increased blood levels of magnesium in neonates is associated with increased still birth, early neonatal death, birth asphyxia, bradycardia, hypotonia, gastrointestinal hypomotility. The objective of this study was to assess safety of low-dose magnesium sulfate regimen in neonates of eclamptic mothers treated with this regimen.
Materials and Methods:
This was a cross-sectional observational study of 100 eclampsia patients and their neonates. Loading dose and maintenance doses of magnesium sulfate were administered to patients by combination of intravenous and intramuscular routes. Maternal serum and cord blood magnesium levels were estimated. Neonatal outcome was assessed.
Results:
Bradycardia was observed in 18 (19.15%) of the neonates, 16 (17.02%) of the neonates were diagnosed with hypotonia. Pearson Correlation Coefficient showed Apgar scores decreased with increase in cord blood magnesium levels. Unpaired t-test showed lower Apgar scores with increasing dose of magnesium sulfate. The Chi-square/Fisher's exact test showed significant increase in hypotonia, birth asphyxia, intubation in delivery room, Neonatal Intensive Care Unit (NICU) care requirement, with increasing dose of magnesium sulfate. (P ≤ 0.05).
Conclusion:
Several neonatal complications are significantly related to increasing serum magnesium levels. Overall, the low-dose magnesium sulfate regimen was safe in the management of eclamptic mothers, without toxicity to their neonates.
KEY WORDS: Eclampsia, low-dose magnesium sulfate, serum magnesium level, neonatal outcome
Introduction
Eclampsia remains an important cause of maternal and perinatal morbidity and mortality worldwide. Incidence of eclampsia is one in 2000 deliveries in developed countries and one in 100-1700 deliveries in developing countries.[1,2] In India, the incidence of eclampsia ranges from 1 in 500 to 1 in 30.[3] Perinatal mortality rate in neonates of eclamptic mothers is 59 in 1000 in developed countries.[4] In India, the perinatal mortality of neonates of eclamptic mothers, is very high to the extent of about 30–50%.[5]
Magnesium sulfate is the anticonvulsant drug of choice for both prevention and treatment of eclampsia. Exactly how magnesium sulfate might control eclamptic convulsions is unclear. Magnesium may cause cerebral vasodilatation with subsequent reduction of cerebral ischemia or block some of the neuronal damage associated with ischemia, or both. A possible mechanism for vasodilatation is calcium antagonism and relaxation of vascular smooth muscle, and it has been suggested that magnesium may have a generalized effect on all smooth muscle, including the peripheral vasculature and uterus.[6] In addition, magnesium sulfate may have an effect on the cerebral endothelium to limit vasogenic edema by decreasing stress fiber contraction and paracellular permeability via calcium-dependent second messenger systems such as myosin light chain kinase (MLC kinase).[7] Alternatively, any effects of magnesium sulfate on control of eclamptic convulsions may be wholly or partially, through its role as a blocker of N-methyl-D-aspartate (NMDA) receptors in the brain.[8] These NMDA receptors are activated in response to asphyxia, leading to calcium influx in the neurons, which causes cell injury. It is suggested that magnesium may block these receptors, so reducing calcium influx and protecting the neurons from damage.
Dose-related toxicity of magnesium sulfate is a major concern.[9,10] Potential hazards include maternal hypotension, respiratory depression, respiratory arrest and rarely cardiac arrest, decreased tendon reflexes and reduced urinary output.[11,12] A significant percentage of perinatal and early neonatal morbidity and mortality, is attributed to magnesium toxicity.[13] Magnesium toxicity leads to increased still birth and early neonatal death, birth asphyxia, bradycardia, hypotonia and hyporeflexia, gastrointestinal hypomotility and meconium plug syndrome.[14]
With intravenous administration, the onset of anticonvulsant action of magnesium sulfate is immediate and lasts about 30 min. Following intramuscular administration of magnesium sulfate onset of action occurs in about 1 h and persists for about 3 to 4 h.
Previously, no dose adjustments of magnesium sulfate were made for maternal weight, even though maternal weight is much higher in high- than in low-income countries (65 kg vs. 45 kg).[9,10,15] Various low-dose magnesium sulfate regimens have been described principally because of small size of Indian women and concern about toxicity in circumstances where facility for measurement of serum level of magnesium is not available.[16,17,18,19,20,21]
In this study we have used (Bankura regimen) of low-dose magnesium sulfate for administration, because study with this regimen showed efficacy similar to standard Pritchard regimen with reduced maternal and perinatal mortality.[17] However, neonatal outcome has rarely been studied with low-dose magnesium sulfate regimen. So, we have tried to find the safety of same.
Normal serum concentrations of magnesium for adults are 0.75–1.25 mmol/L. Total magnesium serum concentrations advocated for the treatment of eclamptic convulsions are 1.5–3.5 mmol/L.
The primary outcome measures for this study were assessment of neonatal well-being in terms of Apgar score at 1, 5 min of birth, neonatal complications – birth asphyxia, requirement of intubation in delivery room, bradycardia, hypotonia, hyporeflexia; Neonatal Intensive Care Unit (NICU) care requirement, requirement of significant respiratory support in NICU, time to first stool, time to first void, number of episodes of feeding intolerance, number of still births, and early neonatal deaths.
The secondary outcome measures for this study were – estimation of maternal serum magnesium level and cord blood magnesium level; correlation of cord blood magnesium level with maternal serum magnesium level (at the time of delivery) and correlation of neonatal outcome with cord blood magnesium level. Prior studies did not include all the parameters which we have mentioned in the present study and also did not consider correlation with serum magnesium levels.
Materials and Methods
Study Design
This is an observational study of patients with eclampsia who received low-dose magnesium sulfate regimen, and their neonates. The study was registered retrospectively with Clinical Trial Registry, India (CTRI/2014/08/004892).
Materials and Place of Study
The study was carried out in the Department of Obstetrics and Gynaecology, Burdwan Medical College and Hospital, Burdwan, from January 2011 to September 2012.
Ethics
The study was approved by the institutional ethics committee. Written informed consent was obtained from every patient's relative, at the start of treatment; written informed consent was also obtained from the patient, once her clinical state was normalized.
Inclusion Criteria
Following patients were included in the study:
Eclampsia, was diagnosed if there was history of generalized tonic clonic convulsions with elevated blood pressure (BP) (>140/90 mm Hg) and proteinuria (≥ 1+by dipstick method), in the absence of any underlying seizure disorders, after 20 weeks of gestational age[22]
All cases of antepartum, intrapartum eclampsia, presenting in obstetric emergency, labor rooms, and/or wards
Those who gave informed consent to participate in the study.
Exclusion Criteria
Following patients were excluded from the study:
Eclampsia patients with complications such as renal failure, severe pulmonary edema with respiratory failure, cerebrovascular accident, and disseminated intravascular coagulation (DIC)
Patients who received magnesium sulfate before coming to our hospital
Patients with known seizure disorder
Neonates of multiple pregnancies, those with congenital malformations and neonates of birth weight <1000 g.
Methodology
In all cases detailed history regarding last last menstrual period (LMP), high BP recordings and medications taken, headache, blurring of vision, epigastric pain, number of convulsions, history of preeclampsia/eclampsia in previous and present pregnancy were noted. General physical examination included assessing patient's Glasgow Coma score, vital signs, BP recording, pallor, edema, cyanosis. Systemic examination included respiratory, cardiovascular, neurological and fundus examination. Obstetrical, pelvic examination were conducted, bishop scores were assigned. Obstetrical examination was conducted by attending obstetric and gynecology personnel. Laboratory investigations performed were blood group and Rh type, haemogram, platelet count, liver function tests, renal function tests, serum electrolytes, coagulation profile, and urinary protein concentration.
After taking informed consent, 109 patients with eclampsia were included in this study. They were administered low-dose magnesium sulfate regimen. Magnesium Sulfate Injection IP 50% w/v: (Ciron Drugs & Pharmaceuticals Pvt., Ltd.) (20,550 mmol/L), (2 ml ampoule) was used. Low-dose regimen protocol includes loading dose – 3 g of magnesium sulfate (20% solution) (8,220 mmol/L) prepared from (3 ampoules), 6 ml of 50% solution diluted with 5% dextrose solution and made up to 15 ml, given intravenously slowly (15 min) at the rate of 1 ml/min plus 2.5 g of magnesium sulfate (50% solution) (2.5 ampoules), 5 ml, given intramuscularly in each buttock (for a total of 8 g of magnesium sulfate).
Subsequently magnesium sulfate maintenance dose of 2.5 g (50% solution) (2.5 ampoules), 5 ml, given every 4 hourly intramuscularly in alternate buttock.
If there was a seizure recurrence, after administering the loading dose, then additional dose of 1 g magnesium sulfate (20% solution), prepared from (1 ampoule), 2 ml of 50% solution diluted with 5% dextrose solution and made up to 5 ml, was administered i.v. slowly, at the rate of 1 ml/min, after each recurring seizure and previous dose schedule was continued.
All patients with eclampsia (133) were administered low-dose magnesium sulfate regimen. Those with complications were managed with drugs and supportives; patients with pulmonary edema were administered i.v. furosemide, patients with heart failure were administered i.v. furosemide, digitalis, treated in intensive treatment unit with ventilatory support, patients with renal failure were managed with i.v. fluids, i.v. diuretics and haemodialysis. Patients with DIC were managed with fresh whole blood transfusion and fresh frozen plasma (FFP) transfusion. If diastolic BP was higher than 120 mmHg i.v. labetalol was used.
After stabilization of the patient, artificial rupture of membrane plus oxytocin administration was used to induce labor, (in patients who did not have a spontaneous onset of labor), with dinoprostone gel used only occasionally. In this low-resource setting, vaginal delivery was preferred to avoid the anesthetic and operative hazards of caesarean delivery. Caesarean section was performed for obstetrical indications. All obstetrical interventions were at the discretion of attending obstetric personnel.
Neonatal birth weight, Apgar score at 1, 5 min were recorded. A maximum score of 10 and minimum score of 0 was assigned to the neonates. Presence of respiratory distress, requirement of intubation in delivery room, presence of bradycardia, hypotonia, hyporeflexia, NICU care requirement, requirement of significant respiratory support in NICU, time to first stool, time to first void, number of episodes of feeding intolerance were recorded. Still births and early neonatal deaths were also noted. Neonatal birth weight was recorded by nurses. Apgar score and other parameters of uncomplicated deliveries were assessed by the researchers. In all other cases assessment was by attending pediatric faculty and neonatology personnel, who were routinely present for all high risk deliveries. The event recording limit was set up at 20 s for apnea/respiratory depression, and at < 90 beats/min for bradycardia > 5 s in duration. Neonatal hypotonia was diagnosed if neonate was limp or flaccid and exhibited reduced activity. Time to first stool and first void were said to be delayed if these occurred beyond 24 h and 48 h respectively. Feeding intolerance was said to be present if there were ≥ 3 episodes of feeding intolerance in a day. Neonatal resuscitation in the labor room and NICU was at the discretion of attending pediatric and neonatology personnel. Neonatal resuscitation was as per standardized protocol.
All the adverse events were reported to the pharmacovigilance unit of the institution in Central Drugs Standard Control Organization (CDSCO) reporting form.
Maternal blood samples were collected at 0 h (before administering magnesium sulfate) and at the time of delivery. Cord blood samples were also collected at the time of delivery.
Serum magnesium levels were estimated by dye binding assay using Randox commercial kit adopted to Beckman Coulter Synchron Cx5 random access clinical chemistry analyzer. Magnesium ion reacted with xylidyl blue in an alkaline medium to form a water soluble purple-red chelate, the color intensity of which was proportional to the concentration of magnesium in the sample. Interference of calcium was excluded from the reaction by complexing with ethylene glycol tetraacetic acid (EGTA). Cord blood magnesium levels were correlated with maternal serum magnesium levels, at the time of delivery. Clinical findings of the neonates were correlated with cord blood magnesium levels.
Maternal height and weight were recorded on the 3rd or 4th day after delivery, when the patient was ambulatory.
Statistics
Values are expressed as mean ± standard deviation/frequency (percent). The Pearson Correlation Coefficient (parametric) was used to determine correlation between cord blood magnesium level and maternal serum magnesium level (at the time of delivery). Pearson Correlation Coefficient was also used to determine correlation between Apgar score and cord blood magnesium level. Unpaired t-test (parametric) was used for comparison of maternal serum magnesium level (at the time of delivery) between the groups (received 8 g and > 8 g magnesium sulfate, uptil delivery). Unpaired t-test was also used to compare cord blood magnesium level between the groups. Unpaired t-test was used for comparison of gestational age, birth weight, Apgar score between the groups. The Chi-square/Fisher's exact test (for categorical data) was used to determine whether there was statistically significant difference between the groups with respect to primary study parameters. P ≤ 0.05 were considered significant.
Results
There were total 133 eclampsia cases admitted to the hospital during the study period and after exclusion only 109 cases met the inclusion criteria. Of them, seven unbooked mothers had multiple pregnancy, and were excluded from the study. Also two neonates were of birth weight <1000 g, and were excluded from the study.
Mean age of study population was 22.11 ± 1.67 years. Most (80%) were primigravidas from rural communities who had received little or no antenatal care. The number of convulsions before admission varied from 4 to 8. Seizures on the way to the hospital were very common, but medical help was seldom available during transportation. Most women were of small stature, with a mean height of 151 ± 5.0 cm, a mean weight of 42.7 ± 5.2 kg, and a mean body mass index (calculated as weight in kilograms divided by the square of height in meters) of 20.55 ± 1.02.
The mean maternal serum magnesium level (0 h) was 1 mmol/L. Mean number of doses of magnesium sulfate given (uptil delivery) was 4.56 ± 1.38. Mean total dose of magnesium sulfate administered till delivery was 8.57 ± 2.45 g. Eighty (80%) patients required 8 g and 20 (20%) patients required > 8 g of magnesium sulfate. Eclamptic mothers who had received longer duration of therapy (> 4 h, i.e., one or more maintenance doses) and also eclamptic mothers who had received additional doses of magnesium sulfate due to recurrent convulsions, had received > 8 g of magnesium sulfate (uptil delivery); rest of the patients had received 8 g magnesium sulfate (i.e., loading dose only), uptil delivery. Of the patients, (90%) had a vaginal delivery, which was forceps assisted in (12%) cases. There was no maternal mortality observed.
Serum Magnesium Levels
The mean maternal serum magnesium level was 2.3 mmol/L for patients received 8 g magnesium sulfate, and 3.46 mmol/L for patients received > 8 g magnesium sulfate (difference of means 1.16, 95% confidence interval 1.08 to1.38) [Figure 1]. The mean cord blood magnesium level was 2.2 mmol/L and 3.36 mmol/L, for the groups respectively (difference of means 1.16, 95% confidence interval 1.08 to1.38) [Figure 2]. Cord blood magnesium level was found to increase significantly with increase in maternal serum magnesium level (at the time of delivery), (R = 1.000, P = 0.000) [Figure 3].
Figure 1.
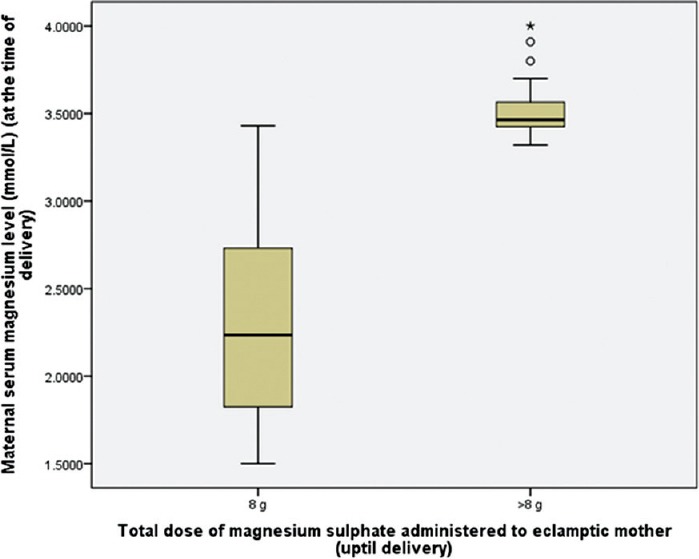
Box plot: Comparison of maternal serum magnesium level (at the time of delivery), between the groups (received 8 g and >8 g magnesium sulfate, uptil delivery). Unpaired t-test, t(97.779)=16.287, P=0.000 (P is significant)
Figure 2.
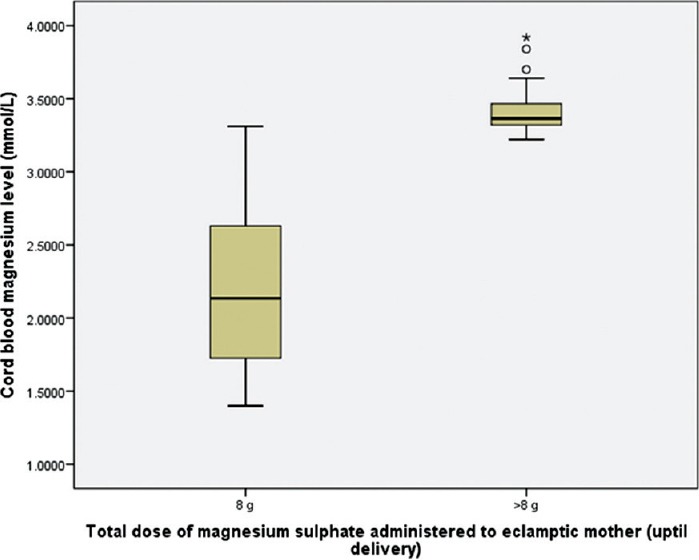
Box plot: Comparison of neonatal cord blood magnesium level between the groups received 8 g and >8 g magnesium sulfate, uptil delivery. Unpaired t-test, t(97.405)=16.259, P=0.000 (P is significant)
Figure 3.

Scatter/Dot: Correlation of cord blood magnesium level with maternal serum magnesium level (at the time of delivery). Pearson Correlation Coefficient, R=1.000, P=0.000 (P is significant)
Apgar Score and Neonatal Outcome
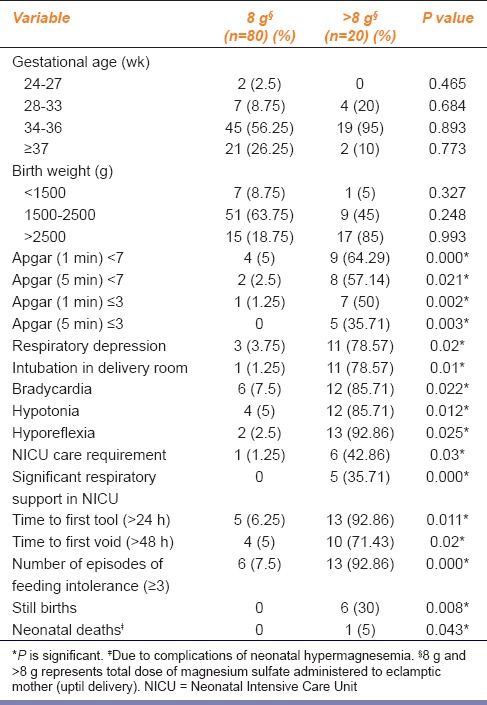
Gestational age at delivery and neonatal birth weight did not vary significantly between the groups [Tables 1 and 2]. Apgar score was significantly lower in neonates of patients who had received > 8 g magnesium sulfate (P ≤ 0.05) [Table 1]. Neonatal outcome was significantly poorer, in neonates of patients who had received > 8 g magnesium sulfate (P ≤ 0.05) [Table 2].
Table 1.
Gestational age, Birth weight, Apgar score in relation to total dose of magnesium sulfate administered to eclamptic mother (uptil delivery) with low-dose magnesium sulfate regimen
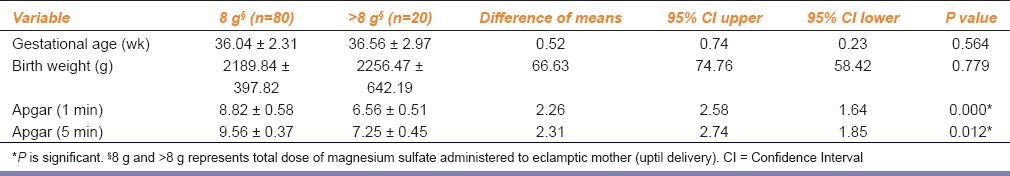
Table 2.
Neonatal outcome in relation to total dose of magnesium sulfate administered to eclamptic mother (uptil delivery) with low-dose magnesium sulfate regimen
Correlation of Neonatal Outcome Parameters with Serum Magnesium Level
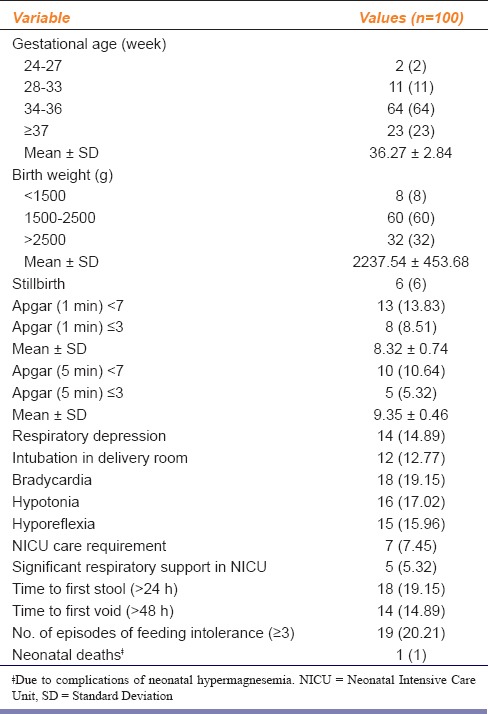
Apgar scores decreased significantly with increase in cord blood magnesium levels [Table 3]. Cord blood magnesium level of severely depressed neonates (Apgar score ≤ 3), was in the range of (3.3–3.5 mmol/L). Cord blood magnesium level of neonates with hypotonia, hyporeflexia was of the order of (3.2–3.4 mmol/L). Cord blood magnesium level of neonates with bradycardia, respiratory depression, and delayed time to first stool was (3.3–3.4 mmol/L). Six (6%) still births were recorded; neonatal cord blood magnesium level was 3.5-3.8 mmol/L [Table 4]. One case of neonatal death, due to birth asphyxia, was noted; cord blood magnesium level was 3.7 mmol/L [Table 2].
Table 3.
Correlation of Apgar score with cord blood magnesium level

Table 4.
Perinatal outcome following maternal treatment with low-dose magnesium sulfate for eclampsia
Discussion
The perinatal mortality rate was 8% in the present study. There were 6 (6%) stillbirths and early neonatal deaths were 2 (2%). Birth asphyxia led to 1% neonatal death, (1% neonatal death was due to cause other than complications related to neonatal hypermagnesemia). In the present study, birth asphyxia was observed in 14 (14.89%) newborns. Similar results were observed by researchers, using low-dose magnesium sulfate regimen, for the treatment of eclamptic mothers.[17,23] Studies using standard dose magnesium sulfate regimen (Pritchard regimen) in our hospital, reported perinatal death 30.07% - still birth rate 22.7% and neonatal death 7.67%.[24] Collaborative Eclampsia Trial using standard dose magnesium sulfate regimen, found perinatal mortality rate of 24-26% and birth asphyxia of 44-48% in eclampsia.[9]
Our analysis indicates that several neonatal outcomes are significantly related to increasing concentrations of magnesium ion in the maternal circulation. Apgar scores were decreased, and hypotonia, intubation in the delivery room, and admission to NICU were all increased as the maternal magnesium level increased from 1.5 to 3.5 mmol/L or greater. The great preponderance of maternal magnesium levels were in the desired therapeutic range of 2.0 to 3.5 mmol/L; only 2 (2%) women had levels exceeding 3.8 mmol/L and the highest level was 4.0 mmol/L. That is, the neonatal effects of magnesium ion that we observed occurred primarily within the therapeutic range and were not solely attributable to excessive levels of magnesium in the maternal circulation. Similar results have been reported by other studies.[14,25,26,27,28,29,30,31]
The great preponderance of cord blood magnesium levels were in the range of 1.2 to 3.4 mmol/L. Cord blood magnesium level was 80–96% of maternal serum magnesium level. Similar results have been reported by other studies.[32] Only 2 (2%) neonates had levels exceeding 3.6 mmol/L, and the highest level was 3.8 mmol/L. All adverse neonatal effects of magnesium sulfate, including neonatal death and serious morbidities such as need for significant respiratory support in NICU were statistically related to higher cord blood magnesium levels. However, we believe that larger prospective trials would be necessary to more completely evaluate the potential adverse effects of neonatal hypermagnesemia.
Low-dose magnesium sulfate regimen used in the treatment of eclampsia are Dhaka regimen and Bankura regimen.[16,17] Dose of magnesium sulfate is 40% lower than in the Collaborative Eclampsia Trial, using standard Pritchard regimen - (40 g vs. 25.9 g; P < 0.001) in Dhaka regimen and (40 g vs. 23.9 g; P < 0.001) in Bankura regimen.[9,16,17] The results are clinically relevant to physicians from low-income countries, where maternal height and weight are almost always low. The study results have various implications:First, following the regimen virtually eliminated the risk of magnesium toxicity to mothers and newborns and thus increased the safety of the drug. Second, with a lesser toxicity, magnesium sulfate treatment is likely to become acceptable at peripheral health centers. Recent evidence from Bangladesh suggests that administering the drug at peripheral health centres significantly reduces the recurrence of seizures, maternal deaths and improves perinatal outcome.[33] In the present study, very few patients had received magnesium sulfate before admission to Burdwan Medical College and Hospital.
Limitations of the Study
Facilities for continuous electrocardiographic monitoring of all the newborns was not available in our set up; so bradycardia and subtle ECG changes due to neonatal hypermagnesemia, could not be monitored.
Conclusion
The increasing, almost ubiquitous use of magnesium sulfate in obstetrics has not occurred without concerns as to the safety of magnesium sulfate. More studies are required for documentation of maternal adverse drug events with magnesium sulfate. Our results, showing neonatal effects and confirming largely forgotten observations made more than 40 years ago, lead us to conclude that administration of magnesium sulfate to pregnant women has discernible effects on the neonate.
Financial Support and Sponsorship
Burdwan Medical College Research fund.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation International. Collaborative Study of Hypertensive Disorders of pregnancy. Geographic Variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1958;158:80–3. [PubMed] [Google Scholar]
- 3.Dutta DC. Hypertensive Disorders in Pregnancy. In: Konar H, editor. DC Dutta's Text Book of Obstetrics. 8th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2015. p. 268. [Google Scholar]
- 4.Knight M. UKOSS. Eclampsia in the United Kingdom 2005. BJOG. 2007;114:1072–8. doi: 10.1111/j.1471-0528.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 5.Dutta DC. Hypertensive Disorders in Pregnancy. In: Konar H, editor. DC Dutta's Text Book of Obstetrics. 8th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2015. p. 271. [Google Scholar]
- 6.Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg 2+-Ca 2+ interaction in contractility of vascular smooth muscle: Mg 2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987;65:729–45. doi: 10.1139/y87-120. [DOI] [PubMed] [Google Scholar]
- 7.Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93:254–61. doi: 10.1113/expphysiol.2007.039966. [DOI] [PubMed] [Google Scholar]
- 8.Hallak M, Berman RF, Irtenkauf SM, Janusz CA, Cotton DB. J Soc Gynecol Investig. 1994. Magnesium sulfate treatment decreases N-methyl-D-aspartate receptor binding in the rat brain: An autoradiographic study; pp. 5–30. [DOI] [PubMed] [Google Scholar]
- 9.Duley L. The Eclampsia Trial collaborative Group. Which Anticonvulsant for women with Eclampsia?. Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–63. [PubMed] [Google Scholar]
- 10.Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: Evaluation of 245 cases. Am J Obstet Gynecol. 1984;148:951–63. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 11.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate?. The magpie trial: A randomised placebo-controlled trial. Lancet. 2002;359:1877–90. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 12.Idama TO, Lindow SW. Magnesium sulphate: A review of clinical pharmacology applied to obstetrics. Br J Obstet Gynaecol. 1998;105:260–8. doi: 10.1111/j.1471-0528.1998.tb10084.x. [DOI] [PubMed] [Google Scholar]
- 13.Nassar AH, Sakhel K, Maarouf H, Naassan GR, Usta IM. Adverse maternal and neonatal outcome of prolonged course of magnesium sulfate tocolysis. Acta Obstet Gynecol Scand. 2006;85:1099–103. doi: 10.1080/00016340600756896. [DOI] [PubMed] [Google Scholar]
- 14.Abbassi-Ghanavati M, Alexander JM, McIntire DD, Savani RC, Leveno KJ. Neonatal effects of magnesium sulfate given to the mother. Am J Perinatol. 2012;29:795–9. doi: 10.1055/s-0032-1316440. [DOI] [PubMed] [Google Scholar]
- 15.Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study: Introduction. Bull World Health Organ. 1995;73(Suppl):1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Begum R, Begum A, Johanson R, Ali MN, Akhter S. A low dose (“Dhaka”) magnesium sulphate regime for eclampsia. Acta Obstet Gynecol Scand. 2001;80:998–1002. [PubMed] [Google Scholar]
- 17.Jana N, Dasgupta S, Das AK, Santra D, Samanta B. Experience of a low-dose magnesium sulfate regimen for the management of eclampsia over a decade. Int J Gynaecol Obstet. 2013;122:13–7. doi: 10.1016/j.ijgo.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Sardesai S, Maira S, Patil A. Low dose Magnesium Sulphate therapy for Eclampsia and imminent Eclampsia ─ regimen tailored for Indian women. J Obstet Gynecol India. 2003;53:546–50. [Google Scholar]
- 19.Shilva, Saha SC, Kalra J, Prasad R. Safety and efficacy of low-dose MgSO4 in the treatment of eclampsia. Int J Gynaecol Obstet. 2007;97:150–1. doi: 10.1016/j.ijgo.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Bangal V, Kwatra A, Raghav S, Jadhav S. Low dose magnesium sulphate regime for Eclampsia. Pravara Med Rev. 2009;4:13–5. [Google Scholar]
- 21.Mahajan NN, Thomas A, Soni RN, Gaikwad NL, Jain SM. ‘Padhar regime’ – A low-dose magnesium sulphate treatment for eclampsia. Gynecol Obstet Invest. 2009;67:20–4. doi: 10.1159/000158647. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al., editors. Williams Obstetrics. 24th ed. New York: McGraw–Hill Education; 2014. Hypertensive Disorders; pp. 728–9. [Google Scholar]
- 23.Sahu L, Singh S, Tempe A, Koner BC. A randomized comparative study between low-dose magnesium sulphate and standard dose regimen for management of eclampsia. Int J Reprod Contracept Obstet Gynecol. 2014;3:79–86. [Google Scholar]
- 24.Pal A, Bhattacharyya R, Adhikari S, Roy A, Chakrabarty D, Ghosh P, et al. Eclampsia-scenario in a hospital – a ten years study. Bangladesh Med Res Counc Bull. 2011;37:66–70. doi: 10.3329/bmrcb.v37i2.8437. [DOI] [PubMed] [Google Scholar]
- 25.Lipsitz PJ. The clinical and biochemical effects of excess magnesium in the newborn. Pediatrics. 1971;47:501–9. [PubMed] [Google Scholar]
- 26.Stone SR, Pritchard JA. Effect of maternally administered magnesium sulfate on the neonate. Obstet Gynecol. 1970;35:574–7. [PubMed] [Google Scholar]
- 27.Donovan EF, Tsang RC, Steichen JJ, Strub RJ, Chen IW, Chen M. Neonatal hypermagnesemia: Effect on parathyroid hormone and calcium homeostasis. J Pediatr. 1980;96:305–10. doi: 10.1016/s0022-3476(80)80835-3. [DOI] [PubMed] [Google Scholar]
- 28.Lipsitz PJ, English IC. Hypermagnesemia in the newborn infant. Pediatrics. 1967;40:856–62. [PubMed] [Google Scholar]
- 29.Rasch DK, Huber PA, Richardson CJ, L’Hommedieu CS, Nelson TE, Reddi R. Neurobehavioral effects of neonatal hypermagnesemia. J Pediatr. 1982;100:272–6. doi: 10.1016/s0022-3476(82)80654-9. [DOI] [PubMed] [Google Scholar]
- 30.Green KW, Key TC, Coen R, Resnik R. The effects of maternally administered magnesium sulfate on the neonate. Am J Obstet Gynecol. 1983;146:29–33. doi: 10.1016/0002-9378(83)90922-5. [DOI] [PubMed] [Google Scholar]
- 31.Riaz M, Porat R, Brodsky NL, Hurt H. The effects of maternal magnesium sulfate treatment on newborns: A prospective controlled study. J Perinatol. 1998;18:449–54. [PubMed] [Google Scholar]
- 32.Chesley LC. New York: Appleton-Century-Crofts; 1978. Hypertensive Disorders in Pregnancy. [Google Scholar]
- 33.Shamsuddin L, Nahar K, Nasrin B, Nahar S, Tamanna S, Kabir RM, et al. Use of parenteral magnesium sulphate in eclampsia and severe pre-eclampsia cases in a rural set up of Bangladesh. Bangladesh Med Res Counc Bull. 2005;31:75–82. [PubMed] [Google Scholar]


