Abstract
Objective:
To investigate the antidiabetic and antihyperlipidemic activities of polyherbal formulation (PHF) containing hydroalcoholic extracts of four plants namely Salacia oblonga, Salacia roxbhurgii, Garcinia indica and Lagerstroemia parviflora in streptozotocin (STZ)-induced diabetic rats by administering oral doses (200 and 400 mg/kg body weight).
Materials and Methods:
Animals were divided into diabetic and nondiabetic groups. Rats were fed with a high-fat diet (HFD) and induced with a single low dose of STZ (35 mg/kg) i.p. Diabetic rats were treated with formulation (200 and 400 mg/kg) and metformin 250 mg/kg. Blood glucose levels were measured using blood glucose test strips with ACCU CHEK glucometer. Lipid profile and gluconeogenic enzymes were determined in normal and STZ-induced diabetic rats after oral administration of the PHF for 28 days. Histopathological changes in diabetic rat organs (pancreas, liver, and kidney) were also observed after PHF treatment.
Results:
Treatment of diabetic rats with PHF and metformin decreased plasma glucose and lipid profile levels. Blood glucose level showed significant reduction after 28 days of treatment with formulation at 200 and 400 mg/kg and in metformin. Formulation treated rats showed significant (P < 0.001) decrease in the activities of gluconeogenic enzymes. Histological examination of various organ tissues of normal control, diabetic control, and drug-treated rats revealed significant results. Treatment with PHF reverses the most blood and tissue changes toward the normal level.
Conclusion:
These findings suggested the antihyperglycemic and antihyperlipidemic properties of the PHF and thus help in preventing future complications of diabetes.
KEY WORDS: Metformin, polyherbal formulation, type 2 diabetes
Introduction
Type 2 diabetes mellitus is a metabolic disorder characterized by hyperglycemia due to either insulin resistance or insufficient insulin secretion by β-cells of the pancreas. According to WHO, it has been recently projected that a total number of patients diagnosed with type II diabetes will be more than 300 million before 2025. The increasing rate of mortality and morbidity due to diabetes is mainly because of the microvascular and macrovascular disease associated with diabetes, making it as one of the five leading cause of death in the world.[1,2] Thus, preventive measures are necessary for diabetic patients from developing several complications such as heart attack, nephropathy, retinopathy, and neuropathy.[3]
Although, the prevalence of diabetes is increasing day by day but an effective treatment is still lacking. Considering the various side effects and disadvantages of modern medicine an alternative management for type 2 diabetes mellitus is increasing worldwide.
Herbs and phytochemicals play a major role in the discovery of new therapeutic agents and have received attention as sources of antioxidants, hypoglycemic, and antihyperlipidemic agents. There are numerous traditional plants mentioned in Siddha and Ayurvedic system of medicine which are used as antidiabetic agents. Sharangdhar Samhita, an Ayurvedic literature from 1300AD has stressed the importance of polyherbalism.[4] Polyherbal formulations (PHFs) enhance the therapeutic action and reduce the concentrations of single herbs, thereby reducing the adverse events. Compared to the single herb, the PHF has better and multi-targeted therapeutic potential.
In the Ayurvedic system of medicine, several plants have been advocated for their hypoglycemic effects and are still in practice. Taking the lead from ancient literature four plants Salacia roxburghii, Salacia oblonga, Garcinia indica, and Lagerstroemia parviflora were selected out of various screened plants carried out for this purpose. In a traditional system, the plants of Salacia species are being used for anti-inflammatory, antidiabetic, leprosy, skin disease, dyspepsia,[5] etc. G. indica called as “kokum” is widely used a fruit juice during summer. This plant has been widely used as an herbal supplement as an antiobesity agent. Also, they are known for its antimicrobial activity,[6] antioxidant,[7] anti-inflammatory agent,[8], etc., extracts of Lagerestroemia species have been proved for its antibacterial[9] and antitussive activity.[10] Since, another species of Lagerstroemia (L. speciosa) have been extensively studied for its antidiabetic activity,[11] in our study we have selected L. parviflora. The formulation is IPR protected and is already formulated. This study is to evaluate the antidiabetic and antihyperlipidemic activities of the PHF.
Materials and Methods
Chemicals
Streptozotocin (STZ) was procured from Sigma-Aldrich (USA). All other chemicals used in this study were of analytical grade.
Composition of Polyherbal Formulation
The PHF was formulated into the capsule as per the patent information of the effective doses by the M/s Varanasi Bio Research Pvt Ltd., Varanasi, India. Each capsule contains hydroalcoholic extracts of Salacia roxbhurgii (112.5 mg), S. oblonga (162.5 mg), G. indica (87.5 mg), and L. parviflora (112.5 mg) and excipients 25 mg. 200mg and 400mg of the PHF powder were weighed and dissolved in 0.5% CMC and used for animal studies.
Toxicity Studies
Toxicity studies of the formulation were carried out as per Organization for Economic Co-operation and Development guidelines, and it was found that there was not toxic effect up to the dose of 2000 mg/kg. The dose of one-tenth of the maximum dose was selected for the study.
Experimental Animals
Animals
Adult Sprague–Dawley rats (150–170g) of either sex were obtained from Central Animal House, Institute of Medical Sciences, Banaras Hindu University. The animals were maintained in a well-ventilated room with 12:12 h light/dark cycle in polypropylene cages. Standard pellet feed and drinking water were provided ad libitum till beginning of the experiment. Animals were acclimatized to laboratory conditions 1-week prior to initiation of experiments. Ethical Committee clearance was obtained from Institutional Animal Ethics Committee - AIMSR/MC/ESTT/07/2K8/796 of Committee for the Purpose of Control and Supervision of Experiments on Animals.
Experimental Induction of Diabetes
After 1-week of acclimatization, the six rats were treated with normal pellet diet and the rest were given as per the grouping. The composition and preparation of high-fat diet (HFD) was as per Srinivasan et al.[12] After 4 weeks of the HFD the rats (N = 24) were fasted overnight, and the animals were rendered diabetic by a single intraperitoneal injection of STZ (35 mg/kg BW). STZ (Sigma USA) at a dose of 35 mg/kg was prepared in cold citrate buffer (pH 4.4, 0.1 M) and administered. The STZ-injected animals exhibited hyperglycemia after 72 h. Blood samples were taken by tail vein puncture and fasting blood glucose levels were monitored using glucometer (ACCU-CHEK). Rats with fasting blood glucose level ≥11.1 mM/L[13,14] were considered diabetic and were used in the study. The diabetic rats were randomly divided into five groups each consisting each of six rats and the study was continued up to 28 days.
Experimental Design
Group I: Normal control rat group were fed basal diet throughout the experiment
Group II: STZ-induced diabetic rats were treated with water
Group III: Diabetic rats treated with an oral dose of PHF 200 mg/kg b.w
Group IV: Diabetic rats treated with an oral dose of PHF 400 mg/kg b.w
Group V: Diabetic rats treated with an oral dose of metformin 250 mg/kg b.w.
The drugs (metformin and PHF) were given once daily.
Biochemical Estimations
At the end of the experimental period, the rats were deprived of food overnight and sacrificed by cervical decapitation. The blood samples were collected on ethylenediaminetetraacetic acid containing tubes and serum was separated immediately. Fasting and postprandial glucose levels (FBS and PPBS), serum lipid profiles were measured by clinical chemistry analyzer (ERBA), and insulin level was measured using enzyme-linked immunosorbent assay kit.
Tissue Preparation
Liver, pancreas and kidney was immediately dissected, washed in ice-cold saline to remove the blood and stored at − 80°C for assay of carbohydrate metabolic enzymes.
Tissues were sliced separately into pieces and homogenized (Glass–Teflonpotter homogenizer) with buffer (0.025 M Tris–HCl buffer of pH 7.5). The homogenate was centrifuged at 10,000 rpm for10 min at 41°C. The supernatant was separated and used for various antioxidant enzyme estimations. On day 28th day when the animals were sacrificed, histopathological examinations of the pancreas, liver, and kidney were carried out. For histopathological examination, kidney, liver, and pancreas from each treatment group were fixed in formalin solution (10% v/v) for 24 h, bisected longitudinally and embedded in paraffin. Sections of 4–6 µm thickness were cut stained by aqueous hematoxylin and alcoholic eosin and were examined by bright field microscopy (Olympus, India).
Estimation of Gluconeogenic Enzymes
Activities of glucose-6-phosphatase and fructose-1, 6-bisphosphatase were assayed according to the methods of King,[15] and Gancedo, and Gancedo,[16] respectively.
Statistical Analysis
Data were statistically evaluated by one-way ANOVA, followed by Dunnett's multiple comparison test. The values were considered significant when P < 0.0001.
Results
Effect of Formulation on Bodyweight
Body weight increased significantly [Figure 1] and all animals except diabetic rat. All animals ingested normal amounts of food and water during the study period.
Figure 1.
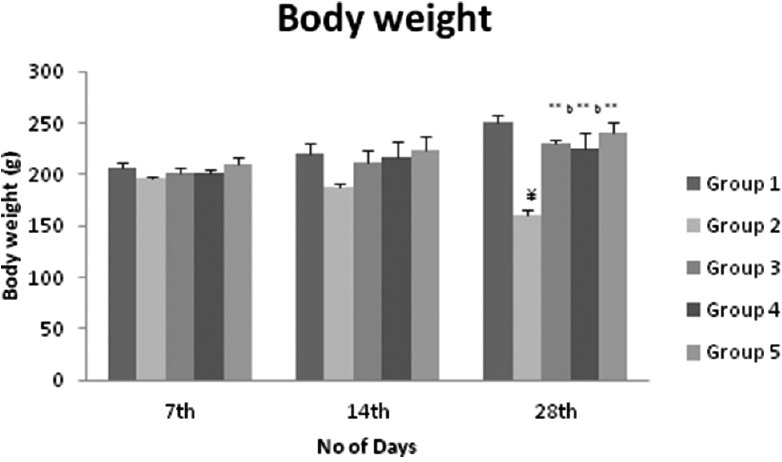
Effect of formulation on body weight in streptozotocin induced diabetic rats statistical analysis was by a one-way ANOVA with Dunnett's multiple comparison test. Values are expressed as mean ± standard deviation and n = 6 for all groups. ***P < 0.0001, **P < 0.001, *P < 0.01 when group 3, 4, and 5 are compared with group 2 (diabetic control), a = 0.0001, b = 0.001, c = 0.05 when group 3 and 4 compared with group 5
Effect of Polyherbal Formulation on Glucose and Insulin Levels
Lowering blood glucose results after treatment of 28 days showed significance with the treatment of formulation of both doses to the diabetic animal group in comparison to the metformin-treated group. Diabetic rats showed decreased levels of plasma insulin and increased level of blood glucose [Table 1], whereas oral administration of formulation (200 mg/kg and 400 mg/kg) to diabetic rats depicted significant (P< 0.001 and P < 0.0001) decrease in the level of blood glucose and increase in the level of insulin.
Table 1.
Effect of formulation on blood glucose of STZ induced rats after 28 days

Effect of Polyherbal Formulation on Lipid Profile
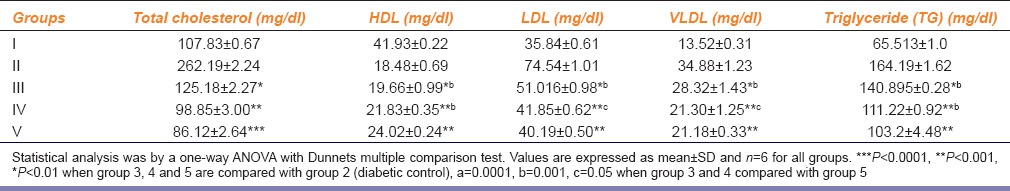
The result of the serum lipid profile (triglycerides [TG], total-cholesterol [TC], low-density lipoprotein cholesterol [LDLC], and high-density lipoprotein cholesterol [HDLC]) revealed that there was elevation in the levels of serum TG, TC, and LDLC compared to the NC rat group [Table 2]. However, the levels of serum TG, TC, and LDLC were significantly (P< 0.001 and P < 0.0001) reduced in groups treated with metformin and PHF (both doses) treated type 2 diabetic rat groups. Furthermore, plasma HDLC was reduced in type 2 diabetic control rat group when compared with the NC rat group. However, the level of HDLC was significantly (P< 0.001) elevated in PHF treated type 2 diabetic rat groups when compared with type 2 diabetic control.
Table 2.
Effect of polyherbal formulation on lipid profile of STZ induced rats after 28 days
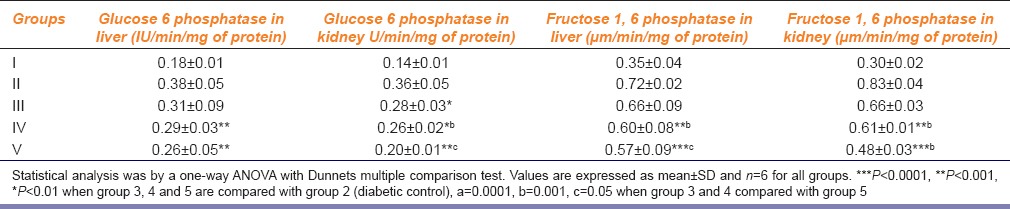
Effect of Polyherbal Formulation on Gluconeogenic Enzymes
Diabetic rats showed increased gluconeogenic enzyme activities [Table 3] but after the treatment with metformin and PHF diabetic rats showed decreased activities of gluconeogenic enzymes.
Table 3.
Effect of polyherbal formulation on gluconeogenic enzymes of STZ induced rats
Histopathological Studies
Diabetic rats showed dilation in both central veins in the hepatic parenchyma and the portal veins and the bile ducts in the portal area. There were diffuse mononuclear leukocytes inflammatory cells infiltration and Kupper cell proliferation in between the degenerated and fatty changed hepatocytes [Figure 2a]. These alterations were normalized in rats treated with PHF.
Figure 2.
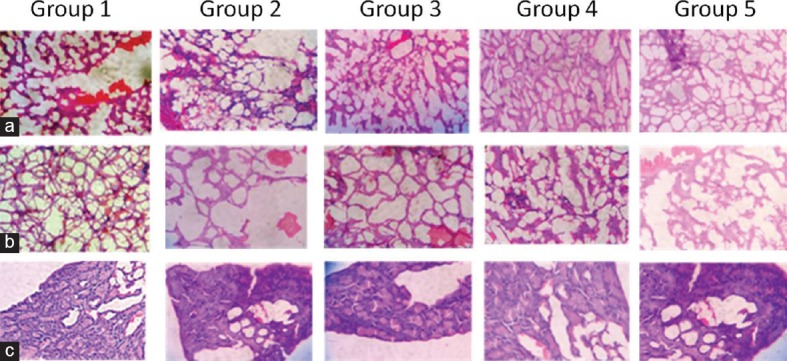
Effect of polyherbal formulation in rat liver (a), kidney (b), and pancreatic (c) tissues (Group I) Normal rat kidney. (Group II) Diabetic rat. (Group III) Diabetic rat treated with formulation (200 mg/kg) (Group IV) Diabetic rat treated with formulation (400 mg/kg) (Group V) Diabetic rats treated with metformin (250 mg/kg). Treatment with polyherbal formulation has reverted the pathological changes toward normal
Histological study of the normal kidney revealed normal glomerulus surrounded by the Bowman's capsule, proximal, and distal convoluted tubules without any inflammatory changes. Kidneys of untreated diabetic rats showed degenerated glomeruli infiltrated by the inflammatory cells and thickening of the basement membrane [Figure 2b]. The groups that were treated with PHF showed features of healing that is normal glomerulus, the absence of inflammatory cells, normal basement membrane and capillaries, decrease in the mucopolysaccharide, and hyaline deposit, respectively.
Histopathology of the pancreas [Figure 2c] in control animals showed normal pancreatic parenchyma cells and islet cell. In diabetic control, pancreas section showed moderate hyperplasia of islet cells, severe congestion in the pancreatic parenchyma and mild infiltration of inflammatory cells. In diabetic animals treated with PHF, pancreas showed mild hyperplasia of islet cells and congestion of pancreatic parenchyma.
Discussion
This is the first study to combine a novel formulation using S. roxbhurgii and L. parviflora. The administration of a dose of STZ (35 mg/kg) after 2 weeks of a dietary regimen of HFD to rats is based on an earlier report by Srinivasan et al.[12] HFD rats may be susceptible to insulin resistance because the receptor cells are blocked by fat deposits, and the diabetic action of a low dose of STZ is enhanced. Hence, it has been established by several authors that the combinatorial effect of HFD and a low dose of STZ may reflect the development of an ideal model for noninsulin dependent diabetes mellitus.
The significant elevation of body weight and blood glucose level in type 2 diabetic rats when compared to NC rats [Table 1] could be attributed to the dietary regimen of HFD and STZ administration. However, the hyperglycemic state in type 2 diabetic control rats were gradually suppressed in groups treated with metformin and PHF. The observed decrease in blood glucose levels after treatment with formulation may suggest the antihyperglycemic properties in vivo. The intestinal enzymes α-glucosidase and α-amylase break down starches, dextrins, maltose, and sucrose into absorbable monosaccharides, thus, it could be suggested that the antidiabetic property of Salacia is partially attributed to intestinal α-glucosidase inhibitory activity[17] and glucose uptake activity by tannins[11] present in Lagerstroemia species.
Furthermore, inhibition of above enzymes delays glucose absorption into the blood and suppresses postprandial hyperglycemia, resulting in improved glycemic control.[18] In addition, mangiferin, a major active compound of Salacia can activate proliferator-activated receptors (PPAR)-α luciferase activity in human embryonic kidney 293 cells and enhances PPAR-α-dependent lipoprotein lipase expression and activity in the THP-1 derived macrophage cell line.[19] This compound could also inhibit aldose reductase activity, thereby delaying the onset or progression of diabetic complications.[20]
Reduction in lipid profile shows the antihyperlipidemic activity of the PHF, which may be due to the presence of hydroxycitric acid. Various investigators that both in vitro and in vivo that hydroxycitric acid in animals not only inhibited the actions of citrate cleavage enzyme and suppressed de novo fatty acid synthesis,[21] but also increased rates of hepatic glycogen synthesis.[22]
A partial or total deficiency of insulin causes changes in carbohydrate metabolism. Glucose-6-phosphatase and fructose 1, 6-bisphophatase are gluconeogenic enzymes which play an important role in glucose homeostasis. These enzymes are activated in the state of insulin deficiency and availability of surplus gluconeogenic substrates because, under normal conditions, insulin functions as a suppressor of gluconeogenic enzymes.[23] In our study, administration of PHF to the diabetic rats resulted in a significant decrease in the activities of glucose-6-phosphatase and fructose-1,6-bisphophatase. The reduction in the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase can lead to decreased gluconeogenesis and thereby reducing the endogenous production of glucose.
Conclusion
The treatment of PHF on high-fat - STZ induced diabetic animals showed significant increase in serum HDL cholesterol levels, whereas significant decrease in low-density lipoprotein-cholesterol, very low-density lipoprotein L-cholesterol, gluconeogenic enzymes thus may play a role in the prevention and management of vascular complications associated with diabetes. Further studies should be carried out to check the inflammatory markers in PHF treated rats.
Financial Support and Sponsorship
The authors would like to thank the Department of Science and Technology, Government of India for their support in the study.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Vats V, Yadav SP, Grover JK. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J Ethnopharmacol. 2004;90:155–60. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Kumar GP, Arulselvan P, Kumar S, Subramanian SP. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J Health Sci. 2006;52:283–91. [Google Scholar]
- 3.Prasad DS, Kabir Z, Dash AK, Das BC. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: A clinico epidemiological evidence summary. J Cardiovasc Dis Res. 2011;2:199–205. doi: 10.4103/0975-3583.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava S, Lal VK, Pant KK. Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacology. 2012;2:1–15. [Google Scholar]
- 5.Iyer R. Hyderabad: Orient Longman Ltd; 1996. Indian Medicinal Plants – A Compendium of 500 Species; p. 47. [Google Scholar]
- 6.Chatterjee A, Yasmin T, Bagchi D, Stohs SJ. The bactericidal effects of Lactobacillus acidophilus, garcinol and Protykin compared to clarithromycin, on Helicobacter pylori. Mol Cell Biochem. 2003;243:29–35. doi: 10.1023/a:1021649427988. [DOI] [PubMed] [Google Scholar]
- 7.Mishra A, Bapat MM, Tilak JC, Devasagayam TP. Antioxidant activity of Garcinia indica (kokam) and its syrup. Curr Sci. 2006;91:90–3. [Google Scholar]
- 8.Hong J, Sang S, Park HJ, Kwon SJ, Suh N, Huang MT, et al. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis. 2006;27:278–86. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 9.Mazumder A, Saha BP, Basu SP, Mazumder R. Antibacterial activity of methonolic extract of leaves of Lagerstroemia parviflora. Indian J Nat Prod. 2003;19:20–3. [Google Scholar]
- 10.Mazumder A, Saha BP, Basu SP, Mazumder R, Boominathan R, Devi BP, et al. Evaluation of antitussive activity of Lagerstroemia parviflora leaf extract. Phytother Res. 2004;18:780–2. doi: 10.1002/ptr.1571. [DOI] [PubMed] [Google Scholar]
- 11.Klein G, Kim J, Himmeldirk K, Cao Y, Chen X. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Evid Based Complement Alternat Med. 2007;4:401–7. doi: 10.1093/ecam/nem013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Liu X, Jia L, Liu Y, Yang H, Wang G, et al. Insulin therapy restores impaired function and expression of P-glycoprotein in blood-brain barrier of experimental diabetes. Biochem Pharmacol. 2008;75:1649–58. doi: 10.1016/j.bcp.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Deng Y, Yu S, Lu S, Xie L, Liu X. Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats. Pharmazie. 2008;63:384–8. [PubMed] [Google Scholar]
- 15.King J. London: Dvan Nostrand Co Ltd; 1965. Practical Clinical Enzymology; p. 208. [Google Scholar]
- 16.Gancedo JM, Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76:132–8. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Huang TH, Yamahara J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci. 2008;82:1045–9. doi: 10.1016/j.lfs.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Heacock PM, Hertzler SR, Williams JA, Wolf BW. Effects of a medical food containing an herbal alpha-glucosidase inhibitor on postprandial glycemia and insulinemia in healthy adults. J Am Diet Assoc. 2005;105:65–71. doi: 10.1016/j.jada.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Huang TH, Yang Q, Harada M, Uberai J, Radford J, Li GQ, et al. Salacia oblonga root improves cardiac lipid metabolism in Zucker diabetic fatty rats: Modulation of cardiac PPAR-alpha-mediated transcription of fatty acid metabolic genes. Toxicol Appl Pharmacol. 2006;210:78–85. doi: 10.1016/j.taap.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa M, Shimoda H, Nishida N, Takada M, Matsuda H. Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lipolytic activities have mild antiobesity effects in rats. J Nutr. 2002;132:1819–24. doi: 10.1093/jn/132.7.1819. [DOI] [PubMed] [Google Scholar]
- 21.Lowenstein JM. Effect of (-)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J Biol Chem. 1971;246:629–32. [PubMed] [Google Scholar]
- 22.Sullivan AC, Triscari J, Neal MO. The influence of (-) hydroxycitrate on in vivo rates of hepatic glycogenesis: Lipogenesis and cholesterol-genesis. Fed Proc. 1974;33:65. [Google Scholar]
- 23.Baquer NZ, Gupta D, Raju J. Regulation of metabolic pathways in liver and kidney during experimental diabetes: Effects of antidiabetic compounds. Indian J Clin Biochem. 1998;13:63–80. doi: 10.1007/BF02867866. [DOI] [PMC free article] [PubMed] [Google Scholar]


