Abstract
Objective:
The seeds of Trigonella foenum-graecum (TFG) (family: Leguminosae) are widely consumed both as a spice in food and Traditional Medicine in India. The present study was undertaken to evaluate the inhibitory effect of standardized extract of TFG and its major constituent trigonelline (TG) on rat liver microsome (RLM) and cytochrome P450 (CYP450) drug metabolizing isozymes (CYP3A4 and CYP2D6), which may indicate the possibility of a probable unwanted interaction.
Materials and Methods:
Reverse phase-high performance liquid chromatography method was developed to standardize the hydroalcoholic seed extract with standard TG. The inhibitory potential of the extract and TG was evaluated on RLM and CYP isozymes using CYP450-carbon monoxide (CYP450-CO) complex assay and fluorescence assay, respectively.
Results:
The content of TG in TFG was found to be 3.38% (w/w). The CYP-CO complex assay showed 23.32% inhibition on RLM. Fluorescence study revealed that the extract and the biomarker had some inhibition on CYP450 isozymes e.g. CYP3A4 and CYP2D6 (IC50 values of the extract: 102.65 ± 2.63–142.23 ± 2.61 µg/ml and TG: 168.73 ± 4.03–180.90 ± 2.49 µg/ml) which was very less compared to positive controls ketoconazole and quinidine. Inhibition potential of TFG was little higher than TG but very less compared to positive controls.
Conclusions:
From the present study, we may conclude that the TFG or TG has very less potential to inhibit the CYP isozymes (CYP3A4, CYP2D6), so administration of this plant extract or its biomarker TG may be safe.
KEY WORDS: Cytochrome enzyme, interaction potential, reverse phase-high performance liquid chromatography, Trigonella foenum graceum, trigonelline
Introduction
The seeds of Trigonella foenum-graecum (TFG) (commonly known as Fenugreek, family: Leguminosae) are widely consumed in India due to its strong flavor and aroma as a spice and flavoring agent in food and beverages and also as a traditional medicine. There are several reports concerning the antiulcer,[1] antidiabetic,[2] wound healing,[3] immunomodulatory,[4] central nervous system stimulant,[5] antioxidant,[6] anticancer,[7] anti-inflammatory, and antipyretic[8] effects of TFG extract. The TFG seed extract contains various bioactive compounds such as trigonelline (TG), protodioscin, trigoneoside, diosgenin, yamogenin, 4-hydroxyisoluecine, and galactomannans.[9] Among them TG, an alkaloid is the major compound. TG has both antidiabetic and neuroregenerative activity associated with memory improvement in Alzheimer's disease.[10]
Majority of drugs are metabolized through cytochrome P450 (CYP450) enzymes, which consists of the superfamily of hemoproteins that catalyze the oxidation of an enormous number of endogenous (steroids, fatty acids, and prostaglandins) and exogenous chemicals including drugs.[11] Inhibition of CYP450 may increase plasma levels of simultaneously administered drug thus increasing the incident of drug-induced toxicity.[12] CYP3A4, CYP2D6, CYP2C9, and other isozymes are responsible for drug metabolism. Among all CYP isozymes, more than 80% of the drugs are metabolized through CYP3A4 and CYP2D6 isozymes.[13,14] Report on cytochrome inhibition potential of several Indian medicinal plants have been reported from our laboratory.[11,15,16] It is important to standardize the extract to maintain the quality and safety because the pharmacological activity of the extract depends on biomarkers present in it, and a higher amount of this biomarker may cause toxicity.[17]
The common people use both phytomedicine and synthetic medicine together without consulting with the doctor as they believe that the use of phytomedicine is safe.[18] Multidrug therapy is now very common for treatment of different diseases.[19] People are unaware about the fact of cytochrome inhibition and thus faces some unwanted clinically significant adverse effects.[20] Nowadays awareness is growing about the phytomedicines and isolated compounds. Phytomedicine may severely affect the disposition of conventional pharmaceuticals by inhibiting the activity of drug metabolizing enzymes (DMEs).[21,22] The literature shows that Allium sativum, Silybum marianum, and Ginkgo biloba have some potential effect on DME such as CYPs in the form of inhibition or induction.[23]
Based on the above context on wide use of plants and their interaction with CYP isozymes responsible for drug metabolism, the present work was designed to study the effect of TFG and its bioactive component TG on pooled rat liver microsome (RLM), CYP3A4 and CYP2D6 isozymes.
Materials and Methods
Chemicals and Reagents
TG was purchased from Sigma-Aldrich. Vivid® CYP450 screening kit and Vivid® substrates (7-benzyloxymethyloxy-3 cyanocoumarin [Cat. No. P2861], ethoxymethyloxy- 3-cyanocoumarin [Cat. No. P3024]) were purchased from Invitrogen Drug Discovery Solutions, USA. CYP3A4 (Cat. No. P2858) and CYP2D6 (Cat. No. P2972) blue screening kit included baculosome (respective isozymes and NADPH-P450 reductase); regeneration system (glucose-6-phosphate, glucose-6-phosphate dehydrogenase) and NADP+ were used for the study. 96-well black-microplate was obtained from NUNC (Roskilde, Denmark). Ketoconazole and quinidine were obtained from Merck (Mumbai, India).
Plant Material
TFG seeds were purchased from the local vendor of Kolkata, India and authenticated by Dr. S. Rajan, Botanist, department of AYUSH, Tamil Nadu, India. A voucher specimen (SNPS-JU/1034) was deposited at School of Natural Product Studies, Jadavpur University, Kolkata. Plant material (seeds) was washed with running water and dried. TFG seeds were grounded into coarse powder and extracted with 70% methanol by cold maceration method. The hydroalcoholic extract was evaporated to dryness in rotary vacuum evaporator at 45°C to produce a semi-solid residue. Then it was lyophilized and kept in a desiccator for further experiment.
Standardization of Trigonella foenum-graecum Through Reverse Phase-high Performance Liquid Chromatography
Extract of TFG was standardized with respective biomarker TG by using reverse phase-high performance liquid chromatography (RP-HPLC). RP-HPLC system (Shimadzu Prominence, Kyoto, Japan) equipped with two Shimadzu LC-20 AD UFLC reciprocating pumps, a variable Shimadzu SPD-M20A Prominence PDA detector and a Rheodyne manual injector with a loop size of 20 µl was used. The peak area was calculated with LC solution software. The analysis was carried out in isocratic condition using a C18 reverse phase column having dimension of 250 mm (length) ×4.6 mm (width) with a particle size of 5 μm (Phenomenex-Luna C18, Torrance, CA, USA). Samples were filtered through Whatmman NYL 0.45 μm syringe filter and an aliquot of 20 µl of each sample was injected into injector port. Elution was carried out with methanol: Water containing 0.5% acetic acid (60:40) at a flow rate of 1 ml/min and eluate was monitored at 254 nm. TG (1 mg/ml) solution was prepared in methanol as a stock solution. Calibration curve was plotted by diluting the stock solution in the concentration range of 200–1000 μg/ml. TG present in TFG extract were identified by comparing with the retention time (Rt) in chromatographic peaks of standard with that of the extract. Percentage of TG present in TFG was determined by constructing a calibration curve.
Sample Preparation for the Study
The extract of TFG was solubilized in both ethanol and dimethyl sulphoxide (DMSO) solvent separately to make a concentration of 1 mg/ml. TG (0.1 mg/ml) was solubilized in both DMSO and ethanol solvent. We used ketoconazole and quinidine as a positive control. The first one was used for CYP-carbon monoxide (CYP-CO) assay and both were used for fluorescence assay. Extract without microsome were used as the negative control, and appropriate solvent controls were used for the study.
Cytochrome P450 Inhibition Study
Preparation of rat liver microsomes
Three male Swiss Wistar rats (~200 g) were used for isolation of liver microsome. This was performed based on the guidelines of the Animal Ethical Committee (Institutional Animal Ethical Committee approval no: 344). Microsome was isolated by following the method described by Ponnusankar et al.[14] Initially, rats were anesthetized with diethyl ether and the livers were removed rapidly. The livers were perfused with 1.15% potassium chloride (KCl) solution. It was homogenized with four volumes (w/v) of ice-cold 1.15% KCl solution. Twenty percentage of the homogenate (w/v) was centrifuged at 9000 × g for 20 min, and the supernatant was collected (Beckman Coulter 64R ALLEGRA). It was subjected to ultracentrifugation at 105,000 × g for 1 h at 4°C. The microsomal fraction pellets were collected from the homogenates (SORVALL RC100) and resuspended in 1.15% KCl solution and stored at −80°C for further use. Bovine serum albumin was used as a standard to estimate the protein concentration. Modified biuret method and the instrument photo analyzer (Roma, Italy) were used for protein estimation.
Cytochrome P450-carbon Monoxide Complex Assay
CYP450-CO complex assay was performed with pooled liver microsome in 96-well microplate followed the method described by Pandit et al.[19] CYPP450 concentration was determined using a formula that incorporates the change in absorbance at 450 nm relative to 490 nm, and a millimolar difference extinction coefficient of 91. Isolated microsomes were diluted with a phosphoglycerol buffer (10 mM potassium phosphate, pH 7.4, 20% glycerol). The reaction between extract (dissolved in ethanol and DMSO) and microsome was initiated by adding NADPH-generating system (4.20 mg/ml of NADP+ in solution of 100 mM glucose-6-phosphate, 100 mM MgCl2 and 100 U/ml glucose-6-phosphate dehydrogenase) and incubated for 10 min at 37°C. One microplate (P) was sealed with tape and kept outside the CO chamber and the other microplate (PC) was incubated in the CO chamber for 15 min. Then 5–10 µl of 0.5 M sodium hydrosulfite was added to reduce all the samples. Ketoconazole was used as positive control. The absorbance of the samples was measured with a microplate reader (Bio-Rad, Model 680XR CA, USA), and the absorbance difference was monitored.
Fluorometric Assays
Inhibition of CYP3A4 and CYP2D6 isozymes activity was assayed by using high throughput CYP450 inhibition kit protocol provided by Invitrogen Drug Discovery Solutions (USA). NADPH-cofactor mixture (1.3 mM NADP+, 66 mM MgCl2 and 66 mM glucose 6-phosphate) was prepared and 144 µl was added to the first row of the microwell (96) plates. Solvent mixture and 100 µl cofactors (ethanol, DMSO) were added to the remaining well except blank. Six microliters test sample was added to each row of first well. Two-fold serial dilutions were performed by transferring 50 µl of extract and NADPH cofactor mixture from 1st to 8th column. Both ethanolic and DMSO solution of ketoconazole (10 µM) and quinidine (1 µM) were used as a positive control for CYP3A4 and CYP2D6, respectively. The plate was preincubated for 10 min at 37°C. After preincubation, 100 µl enzyme-substrate solutions were added to initiate the reaction. Respective substrates for CYP3A4 and CYP2D6 isozymes were used to make an enzyme-substrate mixture. After 20 min of incubation at 37°C, the reaction was stopped by using 0.5 M tris base. Fluorescence intensity was measured in a microplate fluorescence reader (BioTek FLx 800T) by using an emission and excitation wavelength of 530 nm, 409 nm for CYP3A4 and 460 nm, 390 nm for CYP2D6, respectively. Percentage of inhibition was calculated according to the formula described by Ponnusankar et al.[23] and IC50 values were calculated according to the following formula:
Percentage of inhibition = 100 – (Signal of well – Blank) × 100/(No inhibition well signal – Blank)
IC50= ([50% – LP] × [HC – LC] + LC)/(HP – LP)
Where, LP = low percentage of inhibition; HP = high percentage of inhibition; LC = low concentration; HC = high concentration.
Statistical Analysis
All the tests were conducted in triplicate and results were expressed as a mean ± standard error of the mean. The statistical analysis was performed using the Graph-Pad Prism (5.0) (GraphPad Software, Inc., USA). The results were subjected to one-way analysis of variance followed by the Bonferroni test for the statistical analysis. The difference between the means was considered significant when P < 0.05 and above.
Results
Quantitative Analysis of Trigonelline Through Reverse Phase-high Performance Liquid Chromatography
Standard TG exhibited good linearity in the range from 200 to 1000 μg/ml in the calibration curve. A good correlation between concentration and peak area was obtained with the correlation coefficient (r2) value 0.997. The chromatogram of TG and TFG obtained from RP-HPLC analysis has been shown in Figure 1a and b. The peak of TG in the extract was identified by comparing with the Rt of the standard of TG (Rt = 4 min). These results were considered satisfactory and acceptable for subsequent quantitative analysis. The content of TG was found to be 3.38% (w/w) in the TFG.
Figure 1.
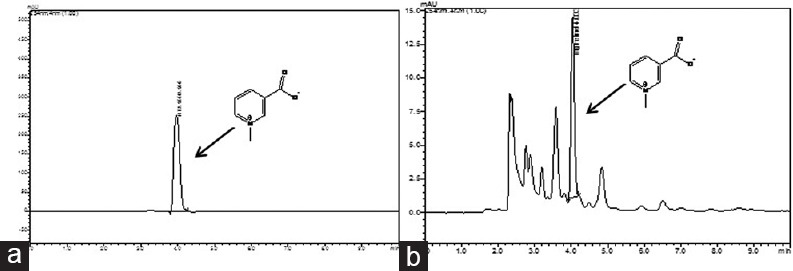
(a) High performance liquid chromatography chromatogram of standard trigonelline and (b) high performance liquid chromatography chromatogram of Trigonella foenum graceum (eluent; methanol: Water containing 0.5% acetic acid [60:40 v/v])
Cytochrome Inhibition Study
Cytochrome P450-carbon monoxide complex assay
Inhibitory potential of TFG and TG was predicted by this method. The protein concentration in the isolated microsomes was found to be 8.10 mg/ml. The content of CYP450 in RLM was found to be 0.401 nmol/mg protein. The concentration of CYPs was determined by incubating microsome with test sample and standard. Percentage inhibition of the TFG and TG with respect to positive control has been shown in Figure 2a. Extract and TG dissolved in ethanol and DMSO showed a concentration-dependent inhibition on microsome as shown in Figure 2b. Results indicated that plant extract and TG showed significantly less inhibition than ketoconazole. TFG extract dissolved in DMSO showed the highest percentage of inhibition (24.32 ± 1.46%) and the TG showed lowest inhibition (13.25 ± 0.76%) in ethanol. The present results showed that the interaction of TFG extract with pooled microsome was more than TG.
Figure 2.
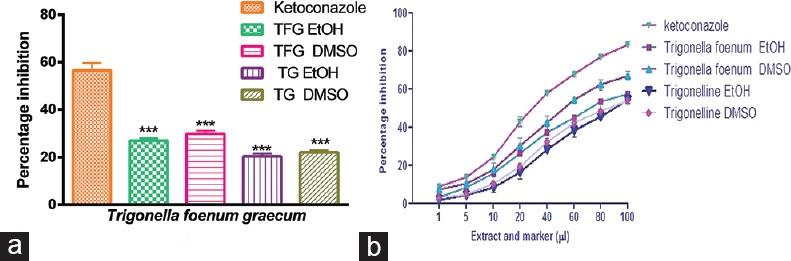
(a) Cytochrome P450-carbon monoxide complex assay. Percentage inhibition of Trigonella foenum graecum extract and trigonelline and (b) concentration dependent percentage inhibition curve of Trigonella foenum graecum and trigonelline (values are mean ± standard error of mean; n = 3. ***P < 0.001 vs. positive control ketoconazole. TFG = Trigonella foenum graceum, TFG EtOH = Trigonella foenum graecum dissolved in ethanol, TFG DMSO = Trigonella foenum graecum dissolved in dimethyl sulphoxide, trigonelline EtOH = trigonelline dissolved in ethanol, trigonelline DMSO = trigonelline dissolved in dimethyl sulphoxide)
Fluorogenic Assays
This assay was performed to characterize the inhibition potential of TFG extract, TG with two CYP isozymes at the concentration range of 1.5–200 µg/ml. All samples were taken in triplicate. Inhibition potential was expressed in terms of IC50 values which have been represented in Table 1. Higher IC50 values mean interaction is lower. All samples showed the concentration-dependent inhibitory effect on CYP3A4, CYP2D6 isozymes. Figure 3 showed the percentage inhibitory effect of TFG and TG. The results indicated that the TFG extract and TG had less inhibition potential on the tested isozymes compared to respective positive controls. Results also showed that the extract had little higher inhibition potential comparing to TG.
Table 1.
IC50 (μg/ml) values for the Trigonella foenum graceum, trigonelline and positive inhibitors on drug metabolizing isozymes such as CYP3A4 and CYP2D6 (values are mean±SEM, n=3)
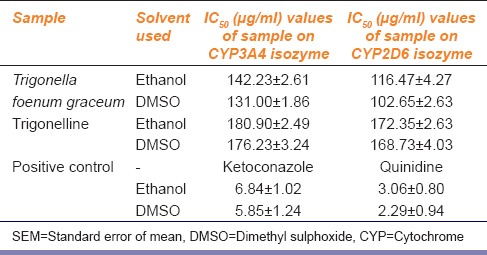
Figure 3.
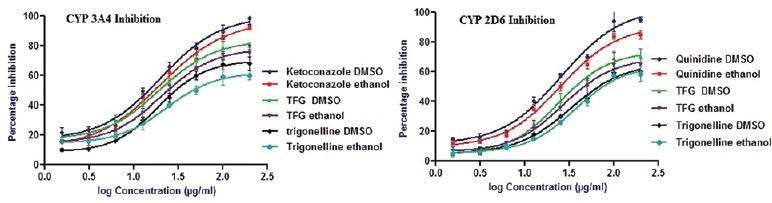
Fluorescence assay. Percentage inhibitory effect of Trigonella foenum graecum extract, trigonelline and positive controls on drug metabolizing enzymes cytochrome P3A4 and cytochrome P2D6 (values are mean ± standard error of the mean; n = 3). Ketoconazole dimethyl sulphoxide = ketoconazole dissolved in dimethyl sulphoxide, ketoconazole ethanol = ketoconazole dissolved in ethanol, TFG DMSO = Trigonella foenum graecum dissolved in dimethyl sulphoxide, TFG ethanol = Trigonella foenum graecum dissolved in ethanol, trigonelline DMSO = trigonelline dissolved in dimethyl sulphoxide, trigonelline ethanol = trigonelline dissolved in ethanol, quinidine DMSO = quinidine dissolved in dimethyl sulphoxide, quinidine ethanol = quinidine dissolved in ethanol
Discussion
The interaction of phytomolecules leading to induction or inhibition of DMEs may cause severe adverse effect or drug induced toxicity. Hence, it is essential to evaluate the safety issue on herbs used therapeutically.[24] TG is the major biomarker in TFG, which has been reported for several biological activities, so TFG was standardized in respect of TG.[10] The RLM and recombinant human liver microsome are frequently used for cytochrome inhibition study at the initial stage of drug development to assess the safety of herbal drugs.[25] To evaluate the interaction study, we took RLM, CYP3A4, and CYP2D6 isozymes with TFG and TG. In CYP-CO complex assay ketoconazole only was used as positive control. This complex assay showed that the TFG extract and TG had dose-dependent enzyme inhibition. It is essential to compare the effect of the extract with positive control. In the fluorescence study, both ketoconazole and quinidine was used as positive control for CYP3A4 and CYP2D6 isozymes as they are the potent inhibitor of those isozymes.[11] In this study, different concentration of extract and biomarker showed good linearity over the dose-dependent inhibition. The IC50 values of extract and TG were higher compared to positive controls thus showing less inhibition potential. The respective positive control for each isozyme was used for confirmation of assay precision. Testing with biomarker explained whether the activity was due to either the biomarker or caused by other phytoconstituents present in the extract. Two percent DMSO was used for solubilization as DMSO (>5%) itself inhibit the cytochrome.[11] The results indicated that TFG extract and TG solubilized in DMSO has higher enzyme inhibion than the TFG extract and TG solubilized in ethanol. It may be due to synergistic effect of other molecules present in the DMSO extract which are absent in ethanol extract. The inhition exhibited by extract and TG was very less compared to positive controls.
The inhibition potential of TFG seed extract and TG with cytochrome isozymes was very less. The IC50 values of TG were little more than the TFG extract, which may be due to other components present in the extract. IC50 values of the extract were much higher than positive controls ketoconazole and quinidine. This suggest that TFG extract has less inhibitory potential with the DMEs (CYP450 enzymes), and its traditional use may be safe.
Financial Support and Sponsorship
National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India, New Delhi, India for providing project grant (F.No.Z.18017/187/CSS/R and D/WB.01/2009.10.NMPB) to the School of Natural Product Studies, Jadavpur University, Kolkata.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Indian Council of Medical Research for providing research associateship to Dr. Kakali Mukherjee. We gratefully acknowledge the financial support received from the National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India, New Delhi, India for providing project grant (F. No.Z.18017/187/CSS/R and D/WB-01/2009-10-NMPB) to the School of Natural Product Studies, Jadavpur University, Kolkata. National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India, New Delhi, India for providing project grant (F. No.Z.18017/187/CSS/R and D/WB-01/2009-10-NMPB) to the School of Natural Product Studies, Jadavpur University, Kolkata.
References
- 1.Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002;81:393–7. doi: 10.1016/s0378-8741(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: A double blind placebo controlled study. J Assoc Physicians India. 2001;49:1057–61. [PubMed] [Google Scholar]
- 3.Taranalli AD, Kuppast IJ. Study of wound healing activity of seeds of Trigonella foenum graecum in rats. Indian J Pharm Sci. 1996;58:117–9. [Google Scholar]
- 4.Bin-Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–65. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 5.Natrajan B, Muralidharan A, Satish R, Dhananjayan R. Neuropharmacologicial activity of Trigonella foenum graecum Linn. Seeds. J Nat Remedies. 2007;7:160–5. [Google Scholar]
- 6.Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV, Priyadarsini KI. In-vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007;103:31–7. [Google Scholar]
- 7.Shabbeer S, Sobolewski M, Anchoori RK, Kachhap S, Hidalgo M, Jimeno A, et al. Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol Ther. 2009;8:272–8. doi: 10.4161/cbt.8.3.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadiani A, Javan M, Semnanian S, Barat E, Kamalinejad M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J Ethnopharmacol. 2001;75:283–6. doi: 10.1016/s0378-8741(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 9.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8. [PubMed] [Google Scholar]
- 10.Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine. 2010;17:292–5. doi: 10.1016/j.phymed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Pandit S, Mukherjee PK, Mukherjee K, Gajbhiye R, Venkatesh M, Ponnusankar S, et al. Cytochrome P450 inhibitory potential of selected Indian spices – Possible food drug interaction. Food Res Int. 2012;45:69–74. [Google Scholar]
- 12.Iwata H, Tezuka Y, Kadota S, Hiratsuka A, Watabe T. Mechanism-based inactivation of human liver microsomal CYP3A4 by rutaecarpine and limonin from Evodia fruit extract. Drug Metab Pharmacokinet. 2005;20:34–45. doi: 10.2133/dmpk.20.34. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003;35:35–98. doi: 10.1081/dmr-120018248. [DOI] [PubMed] [Google Scholar]
- 14.Ponnusankar S, Pandit S, Babu R, Bandyopadhyay A, Mukherjee PK. Cytochrome P450 inhibitory potential of Triphala – A Rasayana from Ayurveda. J Ethnopharmacol. 2011;133:120–5. doi: 10.1016/j.jep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee PK, Ponnusankar S, Pandit S, Hazam PK, Ahmmed M, Mukherjee K. Botanicals as medicinal food and their effects on drug metabolizing enzymes. Food Chem Toxicol. 2011;49:3142–53. doi: 10.1016/j.fct.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich M, Modarai M, Kortenkamp A. Herbal extracts used for upper respiratory tract infections: Are there clinically relevant interactions with the cytochrome P450 enzyme system? Planta Med. 2008;74:657–60. doi: 10.1055/s-2008-1034292. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK. 3rd ed. New Delhi (India): Business Horizon; 2002. Quality Control of Herbal Drugs – An Approach to Evaluation of Botanicals. [Google Scholar]
- 18.Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Pandit S, Mukherjee PK, Ponnusankar S, Venkatesh M, Srikanth N. Metabolism mediated interaction of a-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 2011;82:369–74. doi: 10.1016/j.fitote.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–82. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 21.Strandell J, Neil A, Carlin G. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomedicine. 2004;11:98–104. doi: 10.1078/0944-7113-00379. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Choi HK, Jeong TC, Jahng Y, Kim DH, Lee SH, et al. Selective inhibitory effects of mollugin on CYP1A2 in human liver microsomes. Food Chem Toxicol. 2013;51:33–7. doi: 10.1016/j.fct.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Ponnusankar S, Pandit S, Venkatesh M, Bandyopadhyay A, Mukherjee PK. Cytochrome P450 inhibition assay for standardized extract of Terminalia chebula Retz. Phytother Res. 2011;25:151–4. doi: 10.1002/ptr.2993. [DOI] [PubMed] [Google Scholar]
- 24.Pandit S, Ponnusankar S, Bandyopadhyay A, Ota S, Mukherjee PK. Exploring the possible metabolism mediated interaction of Glycyrrhiza glabra extract with CYP3A4 and CYP2D6. Phytother Res. 2011;25:1429–34. doi: 10.1002/ptr.3426. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz JS, DeVane CL, Boulton DW, Carson SW, Nahas Z, Risch SC. Effect of St. John's wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Life Sci. 2000;66:133–9. doi: 10.1016/s0024-3205(99)00659-1. [DOI] [PubMed] [Google Scholar]


