Abstract
Objectives:
The present study investigated the effects of aqueous extract of Hibiscus sabdariffa (HS) on the three basic components of renin-angiotensin-aldosterone system: Plasma renin, serum angiotensin-converting enzyme (ACE), and plasma aldosterone (PA) in mild to moderate essential hypertensive Nigerians and compared with that of lisinopril, an ACE inhibitor.
Materials and Methods:
A double-blind controlled randomized clinical study was used. Seventy-eight newly diagnosed but untreated mild to moderate hypertensive subjects attending Medical Outpatients Clinic of Enugu State University Teaching Hospital, Enugu were recruited for the study. Those in Group A received placebo (150 mg/kg/day), Group B were given lisinopril (10 mg once daily) while those in Group C received aqueous extract of HS (150 mg/kg/day). After 4 weeks of treatment, the levels of plasma renin, serum ACE, and PA were determined.
Results:
HS and lisinopril significantly (P < 0.001) reduced PA compared to placebo by 32.06% and 30.01%, respectively. Their effects on serum ACE and plasma renin activity (PRA) were not significant compared to placebo; they reduced ACE by 6.63% and 5.67% but increased plasma PRA by 2.77% and 5.36%, respectively.
Conclusion:
HS reduced serum ACE and PA in mild to moderate hypertensive Nigerians with equal efficacy as lisinopril. These actions are possibly due to the presence of anthocyanins in the extract.
KEY WORDS: Aldosterone and essential hypertension, angiotensin-converting enzyme, Hibiscus sabdariffa, lisinopril, renin
Introduction
Essential hypertension remains the most common cause of cardiovascular disease among black Africans, and it is a significant cause of adult morbidity and mortality.[1] The excess mortality in blacks due to heart disease, renal failure, and stroke is directly related to the excess burden of hypertension.[2] Renin-angiotensin-aldosterone system (RAAS) is among the homeostatic mechanisms that regulate blood pressure (BP) even in hypertensive subjects[3] and dysregulation of some components of RAAS, such as angiotensin-converting enzyme (ACE) in essential hypertension, have been reported.[4] ACE inhibitors have been shown to lower BP in hypertensive patients.[5] The pathway for RAAS is initiated by the regulated secretion of renin, the rate-limiting processing enzyme;[6] and inhibition of plasma renin activity has been reported to reduce BP.[7]
Although the benefit of antihypertensive therapy is well-established, the cost and side effects have triggered the search, especially in less developed and poor countries, for pharmacologically active antihypertensive agents that are effective, cheaper with fewer side effects. Among these agents, Hibiscus sabdariffa (HS) has been widely researched and reported to possess antihypertensive actions.[8,9,10] The speculation, that aqueous preparation of HS (called “Zobo”) has antihypertensive action, has greatly increased its consumption both at home and at social gatherings in Nigeria by both hypertensives and nonhypertensives.[11] Several antihypertensive mechanisms of action of HS have been reported. Its diuretic[10] and vasodilatory activities[8] were reported. Its inhibition of Ca2+ influx was also reported.[3] Few human studies have reported that HS possess ACE inhibitory activity[12] and ACE inhibitors have been shown to decrease aldosterone secretion[13] None of these human studies has been carried out in blacks. The present study investigated the effect of aqueous extract of HS on the three basic components of RAAS (plasma renin, serum ACE, and plasma aldosterone [PA]) in mild to moderate hypertensive Nigerian and compared it with that of lisinopril, a known ACE inhibitor.
Materials and Methods
Plant Collection
Dried calyces of HS were purchased from Ogbete Main Market, Enugu. They were authenticated by Mr. A. Ozioko of the Herbarium Section of Botany Department, University of Nigeria, Nsukka and a specimen voucher number UNH/314b was assigned to it.
Subjects
Seventy-eight mild to moderate hypertensive subjects (aged 31–70 years) attending Medical Outpatient Clinic of Enugu State University Teaching Hospital, Parklane, Enugu were recruited for the study, but only 75 completed it. The study was carried out in line with the guidelines of the Helsinki Declaration for human studies (as amended) and approved by the Institutional Ethics Committee (ESUTTH/EC/11002).
Inclusion Criteria
Newly diagnosed but untreated mild to moderate hypertension, using the World Health Organization-International Society of Hypertension[14] classification of hypertension
The subjects were properly briefed about the study and informed consent obtained
All participants were prohibited from participating in any other clinical studies for the duration of this study.
Exclusion Criteria
Patients with diabetes, nephropathy, cardiopathy, hepatic disease, and cancer were excluded from this study
Pregnant women, individuals with evidence of secondary hypertension, chronic smokers, and alcoholics were also excluded
Those who did not complete the study were also excluded.
Subjects who met the inclusion criteria were randomly divided into three groups (A, B, and C):
Group A: Subjects were given with equivalent dose (150 mg/kg) in volume of placebo taken orally once daily before breakfast for 4 weeks. Ribena fruit drink ((blackcurrant) by GlaxoSmithKline, UK) was used as placebo.
Group B: Subjects were given with 10 mg lisinopril (Zestril® by Reals Pharmaceuticals) orally once daily for 4 weeks.[15]
Group C: Subjects took orally 150 mg/kg of HS infusion once daily before breakfast for 4 weeks.
All the subjects were given weekly appointments and a week worth of infusion/medication. Also those in group A were monitored daily via phone calls. Plasma renin, serum ACE, and PA concentrations were measured before (baseline) and at weekly intervals during treatment. Clinical evaluation and treatment adherence were evaluated weekly; the subjects as well as the clinicians do not know the groups assigned to them.
Preparation of Hibiscus sabdariffa Infusion
The method of Herrera-Arellano et al.[12] was used with two modifications. Twenty grams of dry calyces were weighed and ground in electric mill to obtain particles <2 mm. It was used to make an infusion by adding 1 L of boiling clean bottle water (Aquafina bottled water by Seven UP bottling company) and allowed to stand for 30 min.
The solution was filtered using Whatman No. 1 filter paper. The filtrates were stored in clean plastic containers at room temperature.
The following modifications were made:
Infusions were prepared and given to subjects
Time allowed for extraction was extended from 10 to 30 min.
Placebo Preparation
Blackcurrant (Glaxosmithkline, UK) was diluted with clean bottle water (Aquafina) to obtain approximately the same color as HS infusion.
Hibiscus sabdariffa Dosage Calculation
Daily dose = 150 mg/kg.
1 kg = 150 mg.
Weight of subject = W kg.
W kg = 150 × W mg = 0.15 × Wg.
From extraction,
Thus,
0.15×W g=(0.15×W/20×1)L=(0.0075×W)L
150 mg/kg was selected because it is far below the LD50 of HS (>5000 mg/kg), and similar dose has been used in a previous human study;[12] this dose also produced approximately the same color as the locally brewed HS (“Zobo”) drink.
Hibiscus sabdariffa Extract Standardization
The total anthocyanins concentration was obtained using the formula:[16]
Concentration (mg/ml) = A × MW × FD × 1000/(ɛ × 1).
(A = Absorbance of diluted sample; MW = Molecular weight of anthocyanin; FD = Dilution factor; ɛ = Molar absorptivity).
From this method, it was observed that the total anthocyanin contained in 20 g of HS dissolved in 1 L was 10.04 mg.
Measurements
Blood pressure measurement
BP was measured in sitting position by a physician who was not aware of the treatment group assigned to subjects. The subjects were also not aware of the group they belong to. Two consecutive readings were taken from each subject at 5 min interval, and the average of these was taken as the mean BP reading. Measurement was taken between 8.00 am to 10.00 am.
Hormonal Analysis
High-performance liquid chromatography (HPLC) was used to determine the plasma renin, serum ACE, and PA concentrations. For measurement of plasma renin and aldosterone, the sensitivity and specificity of the HPLC assay were 98% and 92%, respectively. For measurement of ACE, serum was incubated with the synthetic substrate (N-{3-(2-furylacryloyl}L-phenylalanyl glycyl glycine (FAPGG) by Sigma Aldrich) at 10 times concentration and injected into hydrophobic reverse phase column.
Electrolyte Analysis
Electrolyte analysis of placebo (undiluted and diluted), HS solution and water used for dilution, was done using atomic absorption spectrophotometer (Solaar 969 Unicam Series) with air acetylene flame.
Statistical Analyses
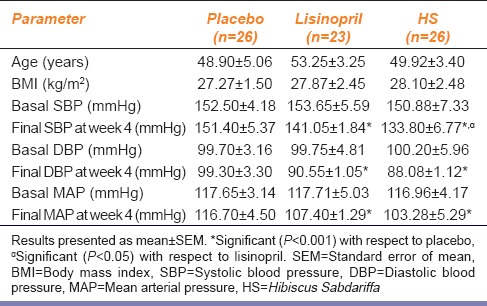
Results are presented as mean ± standard error of mean. Data were analyzed using SPSS version 20 (IBM, USA). One-way analysis of variance with Bonferroni posttest using Prism 5.0 (GraphPad Software, USA) was used to compare differences between groups. P ≤ 0.05 was considered as statistically significant. The clinical characteristics of subjects recruited for the study is shown in Table 1.
Table 1.
Clinical characteristics of hypertensive patients recruited for the study
Results
HS achieved 76% successful reduction in BP to normal level while lisinopril achieved 65%. Three subjects from the lisinopril group developed cough at different points during the study, their medication was changed and they withdrew from the study. BP increased in two subjects in the placebo group; they were withdrawn from the study and placed on antihypertensive drugs. No side effect was recorded in both HS and placebo groups. When compared to placebo, HS significantly decreased systolic BP (SBP) at week 2 (P < 0.01), weeks 3–4 (P < 0.001) while lisinopril significantly (P < 0.001) decreased SBP only at week 4. The effect of HS on SBP when compared to that of lisinopril was significant (P < 0.05) at week 4 [Figure 1]. The effects of HS and lisinopril on diastolic BP were significant (P < 0.001) at weeks 3–4 compared to placebo but not significant when compared to each other throughout the duration of the study [Figure 2]. HS produced a significant reduction in mean arterial pressure (MAP) from week 2 whereas lisinopril did from week 3. When both active treatments were compared to each other, their effect on MAP was not significant [Figure 3].
Figure 1.
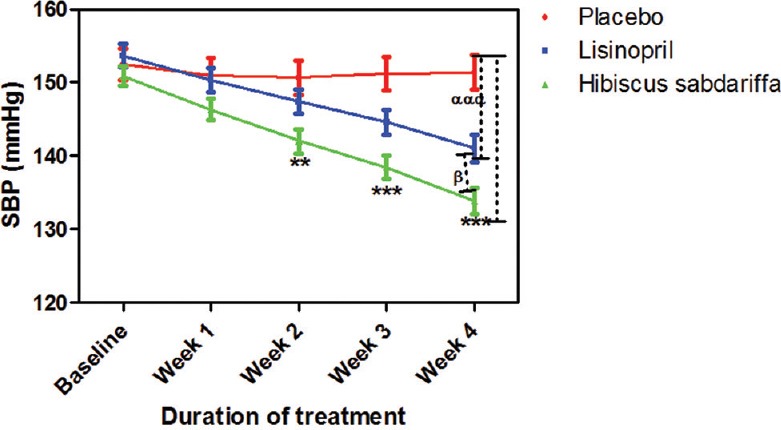
Effect of drug treatments on systolic blood pressure in mild to moderate hypertensive subjects. Each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean. **P < 0.01, ***P < 0.001 (placebo vs. Hibiscus sabdariffa), αααP < 0.001 (placebo vs. lisinopril) and βP < 0.05 (Hibiscus sabdariffa vs. lisinopril) two-way analysis of variance with Bonferroni posttest
Figure 2.
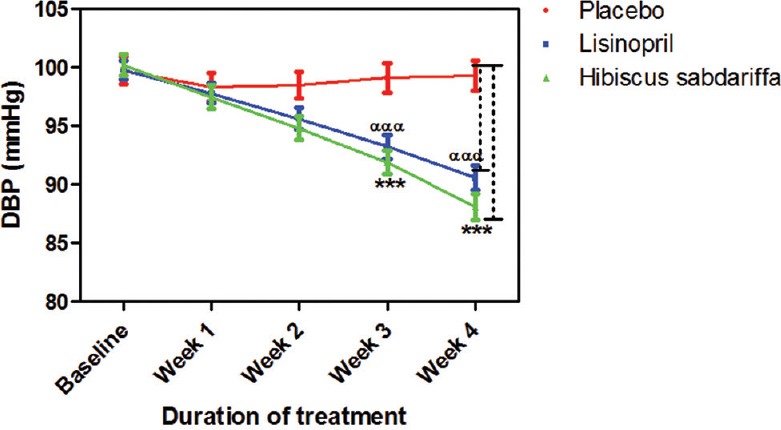
Effect of drug treatments on diastolic blood pressure each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean; ***P < 0.001 (placebo vs. Hibiscus sabdariffa). αααP < 0.001 (placebo vs. lisinopril) two-way analysis of variance with Bonferroni posttest
Figure 3.
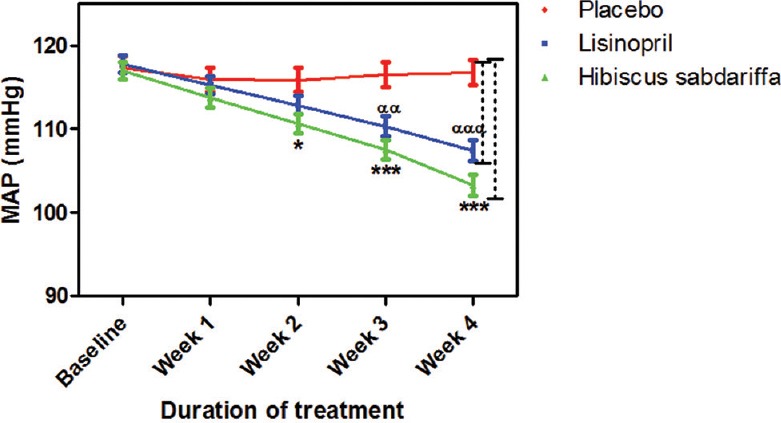
Mean arterial pressure measurements following drug treatments. Each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean. *P< 0.05, ***P < 0.001 (placebo vs. Hibiscus sabdariffa) and ααP < 0.01, αααP < 0.001 (placebo vs. lisinopril) two-way analysis of variance with Bonferroni posttest
Plasma renin increased in both lisinopril and HS groups, but the increase was not significant neither compared to placebo nor each other [Figure 4].
Figure 4.
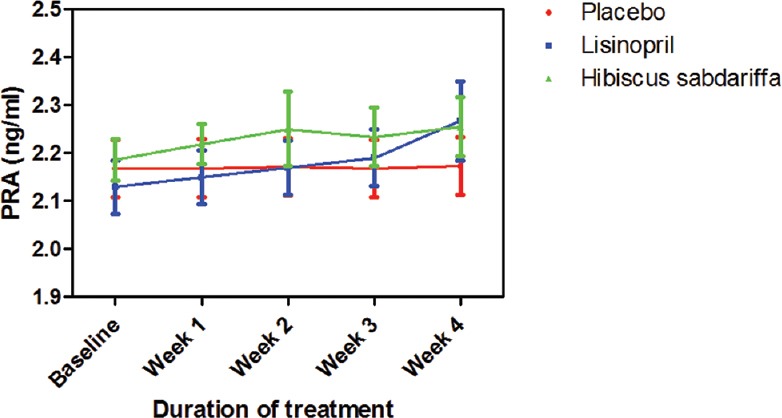
Plasma renin activity following drug treatments in mild to medium hypertensive subjects. Each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean
Both lisinopril and HS reduced serum ACE by 5.6% and 6.6%, respectively, but their effects were not significant neither compared to placebo nor compared to each other [Figure 5].
Figure 5.
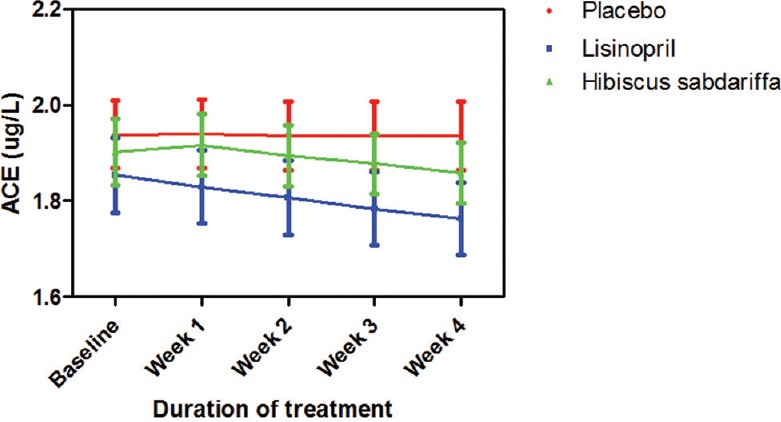
Serum angiotensin-converting enzyme measurements following the administration of test drugs in mild to medium hypertensive subjects. Each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean
After 4 weeks of treatment, lisinopril and HS reduced PA by 30.1% and 32.1%, respectively. The effects of the two active treatments on PA were significant (P < 0.001) when compared to placebo but when compared to each other, there was no significant difference between them throughout the period of study [Figure 6].
Figure 6.
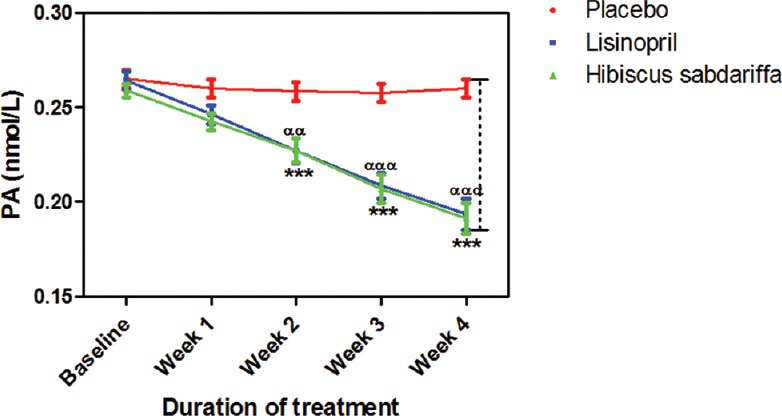
Plasma aldosterone measurements following the administration of placebo (control), lisinopril and Hibiscus sabdariffa on mild to medium hypertensive subjects. Each point on the graph represents the average of at least 25 independent measurements. Error bars are standard error of mean. *P < 0.001 (placebo vs. Hibiscus sabdariffa) and ααP < 0.01, αααP < 0.001 (placebo vs. lisinopril) two-way analysis of variance with Bonferroni posttest
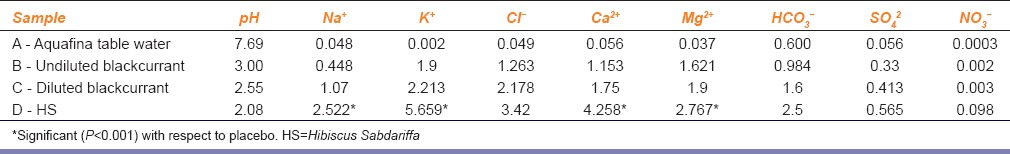
Electrolyte analysis of placebo and HS solution showed that the Mg2+ concentration in HS was twice that of placebo [Table 2].
Table 2.
Electrolyte concentrations of placebo, HS and water used (mmol/L)
Discussion
The antihypertensive actions of HS have been reported in both animal[9] and human studies.[12] These workers suggested several mechanisms of action; the antihypertensive action of HS may be due to direct vasorelaxant effect which may be mediated through cholinergic and/or histaminergic mechanisms which were produced by membrane stabilization and stimulation of vascular Na+–K+ ATPase activity and inhibition of Ca2+ release from intracellular stores[9] and HS induced relaxation of vascular smooth muscle which occurred via endothelium-dependent and -independent mechanisms.[10] The endothelium-dependent vasodilator effect was reported to be due to activation of endothelium-derived nitric oxide/current good manufacturing practice-relaxant pathway and that the endothelium-independent effect was possibly due to inhibition of Ca2+ influx by the action of quercetin and eugenol present in the HS.[6]
Renin-angiotensin-aldosterone hormonal cascade begins with the biosynthesis of renin by the juxtaglomerular cells that line the afferent (and occasionally efferent) arteriole of the renal glomerulus. In addition to this regulated pathway, it appears that the kidney also releases unprocessed prorenin via a constitutive pathway.[17] Control of renin secretion is a key determinant of the activity of the RAAS. Renin regulates the initial, rate-limiting step of the RAAS. Results of the present study showed that both HS and lisinopril increased plasma renin by 2.29% and 2.27%, respectively, which agree with earlier reports that angiotensin-converting enzyme (ACE) inhibitors therapy is associated with a decrease in angiotensin II and aldosterone and an increase in renin release.[18] The increase in plasma renin may be a compensatory mechanism resulting from reduced ACE and aldosterone production.
In two separate clinical trials, inhibition of ACE by HS was reported[11,12] and this could be another important antihypertensive mechanism of action. Subsequent studies confirmed that HS has ACE inhibitory action.[19] These workers attributed this action to the presence of anthocyanins which are one of the major groups of compounds present in aqueous extract of HS.[20] They reported that anthocyanins are the bioactive compounds producing different antihypertensive actions such as inhibition of angiotensin I and angiotensin II converting enzymes as earlier suggested.[12] The HS standardization protocol used in the present study[16] confirmed the presence of anthocyanins in the extract (20 g of HS dissolved in 1 L of water yielded 10.04 mg of anthocyanin). In the present study, the decrease in serum ACE caused by HS was comparable to that of lisinopril though none of the treatment was significant compared to placebo. The effects of both active treatments on PA confirmed the similarity between the actions of HS and lisinopril on RAAS; both significantly reduced PA compared to placebo that confirms earlier reports by other workers where ACE inhibitors were found to reduce the secretion of aldosterone.[21] The high percentage reduction in PA suggests that apart from ACE inhibition, other renal antihypertensive mechanisms such as direct blockage of angiotensin II type 1 (AT1) receptor binding to angiotensin II may also be present like anthocyanins have done in other plant species.[22] Similar to ACE inhibitors, angiotensin receptor blockers reduce BP by decreasing systemic vascular resistance. Reduced systemic vascular resistance results from a combination of inhibition of angiotensin II-mediated vasoconstriction, reduced sympathetic nervous system activity, and reduced extracellular volume.[23]
The high Mg2+ content in HS may also contribute to the decreased secretion of aldosterone since Mg2+ has been shown to suppress aldosterone production through intracellular calcium mobilization.[24] This action may also be an additional mechanism through which HS exerts antihypertensive action.
The effect of HS on RAAS was greater though not significant compared to that of lisinopril (a known ACE inhibitor); 6.63% versus 5.6% reduction in ACE and 32.06% versus 30.01% reduction in PA. This finding strengthens the hypothesis that apart from ACE inhibition, HS may have additional inhibitory actions on RAAS such as AT1 receptor blocking and Mg2+-mediated aldosterone inhibition as earlier suggested. The higher percentage satisfactory reduction in BP in HS group compared to lisinopril (76% vs. 65%) confirms earlier reports that apart from RAAS mechanism that HS possess other antihypertensive mechanisms of action. However, further experimental evidence may be required to confirm the AT1 and Mg2+ inhibition mechanisms.
Conclusion
The result of the present study has shown that both aqueous extract of HS and lisinopril exerted similar effects on RAAS in mild to moderate hypertensive Nigerians. Both treatments reduced serum ACE and PA. The action on aldosterone was much higher than that on ACE and this could be due to a combination of several factors such as blockage of AT1 receptors and inhibitory action of Mg2+ present in HS on aldosterone secretion. Further studies are, however, required to investigate these mechanisms.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
We express our appreciation to the Management of Enugu State University Teaching Hospital, Parklane, Enugu for allowing us to use their facility and providing technical support during the study. We also thank Mr. John Ugwuona and Mr. Stanley Ugwubujo of the Physiology Laboratory, University of Nigeria Enugu Campus for their assistance in the weekly preparation of HS infusion.
References
- 1.Akinkugbe OO. Current epidemiology of hypertension in Nigeria. Arch Ib Med. 2003;1:3–5. [Google Scholar]
- 2.Laragh J. Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens. 2001;14:186–94. doi: 10.1016/s0895-7061(00)01317-0. [DOI] [PubMed] [Google Scholar]
- 3.Morgan L, Broughton PF, Kalsheker N. Angiotensinogen: Molecular biology, biochemistry and physiology. Int J Biochem Cell Biol. 1996;28:1211–22. doi: 10.1016/s1357-2725(96)00086-6. [DOI] [PubMed] [Google Scholar]
- 4.Davis JO. Aldosteronism and hypertension. Prog Cardiovasc Dis. 1965;8:129–40. doi: 10.1016/s0033-0620(65)80004-4. [DOI] [PubMed] [Google Scholar]
- 5.Meunier MT, Villié F, Jonadet M, Bastide J, Bastide P. Inhibition of angiotensin I converting enzyme by flavanolic compounds: In vitro and in vivo studies. Planta Med. 1987;53:12–5. doi: 10.1055/s-2006-962606. [DOI] [PubMed] [Google Scholar]
- 6.Staessen JA, Li Y, Richart T. Oral renin inhibitors. Lancet. 2006;368:1449–56. doi: 10.1016/S0140-6736(06)69442-7. [DOI] [PubMed] [Google Scholar]
- 7.Nileeka BB, Vasantha RH. Plant flavonoids as angiotensin converting enzyme inhibitors in the regulation of hypertension. Funct Foods Health Dis. 2011;5:172–88. [Google Scholar]
- 8.Ibrahim MM. RAS inhibition in hypertension. J Hum Hypertens. 2006;20:101–8. doi: 10.1038/sj.jhh.1001960. [DOI] [PubMed] [Google Scholar]
- 9.Adegunloye BJ, Omoniyi JO, Owolabi OA, Ajagbonna OP, Sofola OA, Coker HA. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr J Med Med Sci. 1996;25:235–8. [PubMed] [Google Scholar]
- 10.Obiefuna PC, Owlabi OA, Adegunloye BJ. The petal extract of Hibiscus sabdariffa produces relaxation of isolated rat aorta. Int J Pharmacogn. 1994;32:69–74. [Google Scholar]
- 11.Onyenekwe PC, Ajani EO, Ameh DA, Gamaniel KS. Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem Funct. 1999;17:199–206. doi: 10.1002/(SICI)1099-0844(199909)17:3<199::AID-CBF829>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Arellano A, Flores-Romero S, Chávez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine. 2004;11:375–82. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Ali MB, Salih WM, Mohamed AH, Homeida AM. Investigation of the antispasmodic potential of Hibiscus sabdariffa calyces. J Ethnopharmacol. 1991;31:249–57. doi: 10.1016/0378-8741(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 14.WHO/ISH Statement on hypertension. Management of hypertension. J Hypertens. 2003;17:151–83. [Google Scholar]
- 15.Stimpel M, Koch B, Jansen T, Fox A, Loh I. Moexipril versus captopril in patients with mild to moderate hypertension. J Cardiovasc Pharmacol. 1996;28:769–73. doi: 10.1097/00005344-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Fuleki T, Francis FJ. Quantitative methods of anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci. 1968;33:78–83. [Google Scholar]
- 17.Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst. 2006;7:3–14. doi: 10.3317/jraas.2006.003. [DOI] [PubMed] [Google Scholar]
- 18.Aguwa CN, Ndu OO, Nwanma CC, Udeogaranya PO, Akwara NO. Verification of folkloric diuretic claim of Hibiscus sabdariffa L.Petal extract. Niger J Pharm Res. 2004;3:1–8. [Google Scholar]
- 19.McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140:298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 20.Mungole A, Chaturvedi A. Hibiscus sabdariffa L. A rich source of secondary metabolites. Int J Pharm Sci. 2011;6:83–7. [Google Scholar]
- 21.López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004;25:1454–70. doi: 10.1016/j.ehj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Caballero-George C, Vanderheyden PM, De Bruyne T, Shahat AA, Van den Heuvel H, Solis PN, et al. In vitro inhibition of [3H]-angiotensin II binding on the human AT1 receptor by proanthocyanidins from Guazuma ulmifolia bark. Planta Med. 2002;68:1066–71. doi: 10.1055/s-2002-36344. [DOI] [PubMed] [Google Scholar]
- 23.Weir MR. Effects of renin-angiotensin system inhibition on end-organ protection: Can we do better? Clin Ther. 2007;29:1803–24. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Ichihara A, Suzuki H, Saruta T. Effects of magnesium on the renin-angiotensin-aldosterone system in human subjects. J Lab Clin Med. 1993;122:432–40. [PubMed] [Google Scholar]


