Abstract
The purpose of this study was to estimate the influence of adjuvant radiotherapy for primary breast cancer (BC) on the risk of contralateral BC (CBC) in BRCA1 or BRCA2(BRCA1/2) mutation carriers, with special attention to patients irradiated at age younger than 40 years. Additionally, tendencies in locoregional treatments and rates of contralateral risk-reducing mastectomy over time were explored. In this retrospective cohort study, 691 BRCA1/2-associated BC patients treated between 1980 and 2013 were followed from diagnosis until CBC or censoring event including ipsilateral BC recurrence, distant metastasis, contralateral risk-reducing mastectomy, other invasive cancer diagnosis, death, or loss to follow up. Hazard ratios (HR) for CBC associated with radiotherapy were estimated using Cox regression. Median follow-up time was 8.6 years [range 0.3–34.3 years]. No association between radiotherapy for primary BC and risk of CBC was found, neither in the total population (HR 0.82, 95 % CI 0.45–1.49) nor in the subgroup of patients younger than 40 years at primary diagnosis (HR 1.36, 95 % CI 0.60–3.09). During follow-up, the number of patients at risk decreased substantially since a large proportion of patients were censored after contralateral risk-reducing mastectomy or BC recurrence. Over the years, increasing preference for mastectomy without radiotherapy compared to breast-conserving surgery with radiotherapy was found ranging from less than 30 % in 1995 to almost 50 % after 2010. The rate of contralateral risk-reducing mastectomy increased over the years from less than 40 % in 1995 to more than 60 % after 2010. In this cohort of BRCA1/2-associated BC patients, no association between radiotherapy for primary BC and risk of CBC was observed in the total group, nor in the patients irradiated before the age of 40 years. The number of patients at risk after 10 and 15 years of follow-up, however, was too small to definitively exclude harmful effects of adjuvant radiotherapy.
Keywords: Radiotherapy, BRCA mutation, Contralateral breast cancer, Risk-reducing mastectomy, Breast-conserving surgery
Introduction
Both normal breast tissue and breast cancer cells are sensitive to ionizing radiation. Although adjuvant radiotherapy for early breast cancer (BC) reduces the risk of local recurrence and improves BC-specific survival [1, 2], it also leads to a low-dose scatter radiation to the surrounding healthy tissue with potentially carcinogenic effects. In sporadic BC patients, adjuvant radiotherapy has been associated with an increased risk of contralateral breast cancer (CBC), although only among women younger than 45 years at primary BC diagnosis and after a latency period of at least 10–15 years [3–6].
The vulnerability of cells for ionizing radiation largely depends on the rate of cell proliferation, the total dose of radiation, the fractionation scheme, and the capability of the cells to repair DNA damage [7]. Younger patients have higher breast cell proliferation (in particular during puberty, adolescence, and pregnancy) and thus increased DNA synthesis that might render breast tissue particularly susceptible to the carcinogenic effects of radiation [8, 9]. The capacity to repair DNA damage might substantially differ between BC patients, in particular when considering patients with or without a BRCA1 or BRCA2 (BRCA1/2) mutation.
BRCA1/2-associated BC is characterized by homologous recombination deficiency, leading to inadequate repair of double-strand DNA breaks [10, 11]. Ionizing radiation can cause cell damage by induction of double-strand DNA breaks. This has led to the hypothesis that adjuvant radiotherapy administered for BRCA1/2-associated BC might be more effective than radiotherapy administered for sporadic BC. On the contrary, surrounding healthy breast tissue among BC patients with a BRCA1/2 mutation might be more vulnerable to the deleterious effects of adjuvant radiotherapy, including the development of a CBC, compared to those without a BRCA1/2 mutation.
In unaffected BRCA1/2 mutation carriers, exposure to low cumulative doses of diagnostic radiation (including screening mammography) at young age (<30 years) has been reported to be associated with an increased risk of BC, with a clear dose–effect relationship [12] compared to no exposure to diagnostic radiation. The possible carcinogenic effect of scatter ionizing radiation after adjuvant radiotherapy on the contralateral breast in BRCA1/2-associated BC patients, however, is not clear. Although a number of studies addressed this question, all these studies are compromised by a short duration of follow-up and the lack of subgroup analyses regarding young BC patients. [13–15]. Knowledge about the possibly increased risk of CBC by radiotherapy might be of great importance for optimal shared decision making regarding mastectomy without radiotherapy versus breast-conserving surgery including radiotherapy at primary BC diagnosis.
We therefore studied the impact of radiotherapy on the risk of CBC among BRCA1/2-associated BC patients in a retrospective cohort study, with special attention to patients younger than 40 years at primary BC diagnosis. Since over the years an increasing proportion of BRCA1/2 mutation carriers after developing BC seems to opt for bilateral mastectomy instead of unilateral mastectomy or breast-conserving treatment with radiotherapy [16], we also explored potential tendencies in locoregional treatments and the rates of contralateral risk-reducing mastectomy over the past decades.
Methods
Patient selection
From the Rotterdam Family Cancer Clinic database, we extracted all female patients with early stage BC (n = 2,268). From this population, we selected proven or obligate BRCA1 or BRCA2 mutation carriers, treated at the Erasmus MC Cancer Institute. Patients diagnosed from January 1st 1980, corresponding to the start of linear accelerators use for adjuvant breast radiotherapy at the Erasmus MC, to January 1st 2013 were included (n = 790). Time of observation ended at April 1st 2014. Patients with less than 3 months of follow-up were excluded (n = 52; see statistical analysis). Patients who were treated with breast/chest wall radiotherapy or systemic anticancer therapy because of another invasive malignancy, either prior or synchronous to the primary BC, were excluded (n = 16). Patients who had synchronous bilateral BC and received bilateral radiation therapy or mastectomy (n = 31) were also excluded, leaving a total of 691 patients available for the analyses.
For the eligible patients, data on primary BC and CBC characteristics (type of histology, differentiation grade, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, and stage) and primary BC therapy (surgery, radiotherapy, chemotherapy, and/or endocrine therapy) were retrieved. We also collected data on type of mutation (i.e., BRCA1 or BRCA2), date of birth, primary and contralateral BC diagnoses, dates of and findings at contralateral risk-reducing mastectomy and salpingo-oophorectomy, and dates of disease recurrence and death or date of last follow-up if no event occurred.
Statistical analysis
The primary endpoint was the development of CBC defined as the occurrence of carcinoma in situ or invasive BC in the contralateral breast at least 3 months after primary BC diagnosis and no signs of metastatic disease. CBC diagnosis within 3 months was considered as synchronous bilateral BC and assumed to be unrelated to the delivery of radiotherapy for the first BC [3–5]. For this reason, patients with less than 3 months of follow-up were excluded.
For comparisons of patient, tumor, and treatment characteristics between subgroups, we used Pearson’s χ2 tests. Differences in age at primary BC diagnosis and follow-up time were analyzed using the Wilcoxon rank-sum test (Mann–Whitney).
In the Cox analyses, we applied left truncation of analysis time and so considered outcome data from prospective follow-up only. Hereby, we aimed to correct for potential selection bias, possibly arising due to inclusion of patients undergoing genetic testing after primary BC or CBC diagnosis [17, 18]. Censoring events were ipsilateral BC recurrence for which radiotherapy or systemic therapy was applied, distant metastasis, contralateral risk-reducing mastectomy, other (non-breast) invasive cancer for which radiotherapy or systemic therapy was applied, death, and loss to follow up.
We estimated hazard ratios (HRs) and 95 % confidence intervals (CIs) for radiotherapy (after lumpectomy vs. after mastectomy vs. none), adjuvant chemotherapy (yes vs no), adjuvant endocrine therapy (yes vs. no), salpingo-oophorectomy (treated as time-dependent variable), age at primary BC, and BRCA mutation type (BRCA1 vs. BRCA2) using Cox regression in univariate and multivariate analyses. The cumulative 5-, 10-, and 15-year risks of CBC were calculated using Kaplan–Meier analysis including only patients who underwent DNA testing for BRCA1/2 mutation before the diagnosis of CBC, to correct for potential selection bias.
Analyses were performed for the total group and for patients younger than 40 years at primary BC, as it has been previously reported that younger patients are more susceptible for radiation-induced BC [3–6].
The proportion of patients undergoing different locoregional treatments over time, including breast-conserving treatment and mastectomy with or without radiotherapy, was estimated with a regression line of best fit and 95 % CI based on the proportion per year. The same was performed for the proportion of patients undergoing contralateral prophylactic mastectomy over time. For statistical analysis STATA, version 13.0, was used. For computing the figures, R version 3.2.2 (released on 2015-08-14) and the package GGplot version 1.0.1. were used.
Results
A total of 691 BRCA1/2-associated BC patients, consisting of 517 BRCA1 and 174 BRCA2 mutation carriers, were eligible for data analysis (Tables 1, 2). Median time of follow-up of the entire cohort was 8.6 years with a range from 0.3 to 34.3 years. A total of 439 patients were treated with radiotherapy either after lumpectomy (n = 349) or after mastectomy (n = 85). A total of 325 patients were younger than 40 years at primary BC diagnosis (Table 2). Further details on patient, tumor, and treatment characteristics are presented in Tables 1 and 2.
Table 1.
Characteristics of the patients, radiotherapy vs. no radiotherapy
| Total (n = 691)* | RT after lumpectomy (n = 349) | No RT after mastectomy (n = 252) | RT after mastectomy (n = 85) | p value | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Age at primary BC | |||||
| <30 years | 55 (8.0) | 29 (8.3) | 19 (7.5) | 7 (8.2) | 0.943 |
| 30–34 years | 115 (16.6) | 59 (16.9) | 39 (15.5) | 15 (17.0) | |
| 35–39 years | 155 (22.4) | 78 (22.3) | 57 (22.6) | 20 (23.5) | |
| 40–44 years | 129 (18.7) | 64 (18.3) | 49 (19.4) | 16 (18.8) | |
| 45–50 years | 100 (14.5) | 48 (13.8 | 35 (13.9) | 16 (18.8) | |
| >50 years | 137 (19.8) | 71 (20.3) | 53 (21.0) | 11 (12.9) | |
| Mutation status | |||||
| BRCA1 | 517 (74.8) | 277 (79.4) | 186 (73.8) | 50 (58.8) | <0.001 |
| BRCA2 | 174 (25.2) | 72 (20.6) | 66 (26.2) | 35 (41.2) | |
| Period of primary BC | |||||
| 1980–1989 | 105 (15.2) | 64 (18.3) | 27 (10.7) | 14 (16.5) | 0.017 |
| 1990–1999 | 256 (37.1) | 139 (39.8) | 101 (35.3) | 27 (31.8) | |
| 2000–2013 | 330 (47.8) | 146 (41.8) | 164 (54.0) | 44 (51.8) | |
| Tumor stage | |||||
| Tis | 26 (4.0) | 14 (4.1) | 12 (5.2) | 0 | <0.001 |
| T1 | 364 (56.0) | 209 (61.8) | 130 (56.5) | 25 (30.9) | |
| T2 | 227 (34.9) | 114 (33.7) | 80 (34.8) | 32 (39.5) | |
| T3 | 25 (3.9) | 0 | 7 (3.0) | 18 (22.2) | |
| T4 | 8 (1.2) | 1 (0.3) | 1 (0.4) | 6 (7.4) | |
| Unknown | 41 | 11 | 22 | 4 | |
| Nodal status | |||||
| N0 | 424 (64.3) | 241 (71.9) | 169 (70.1) | 13 (16.0) | <0.001 |
| N1–3 | 235 (35.7) | 94 (28.1) | 72 (29.9) | 68 (84.0) | |
| Unknown | 32 | 14 | 11 | 4 | |
| Histological grade | |||||
| Grade 1 | 17 (3.3) | 8 (3.1) | 7 (3.6) | 2 (3.0) | 0.988 |
| Grade 2 | 106 (20.4) | 54 (21.0) | 37 (19.2) | 14 (20.9) | |
| Grade 3 | 396 (76.3) | 195 (75.9) | 149 (77.2) | 51 (76.1) | |
| Unknown | 172 | 92 | 59 | 18 | |
| Hormone receptor status | |||||
| Positive | 227 (39.5) | 108 (37.8) | 80 (37.9) | 39 (50.0) | 0.124 |
| Negative | 348 (60.5) | 178 (62.2) | 131 (62.1) | 39 (50.0) | |
| Unknown | 116 | 63 | 41 | 7 | |
| HER2 status | |||||
| Positive | 17 (6.7) | 9 (8.1) | 5 (5.2) | 3 (7.5) | 0.646 |
| Negative | 236 (93.3) | 101 (91.8) | 95 (94.8) | 37 (92.5) | |
| Unknown | 438 | 239 | 152 | 45 | |
| (Contralateral) risk-reducing mastectomy | |||||
| No | 424 (64.5) | 243 (73.0) | 127 (51.8) | 54 (68.4) | <0.001 |
| Yes | 233 (35.5) | 90 (27.0) | 118 (46.2) | 25 (31.7) | |
| Unknown | 34 | 16 | 7 | 6 | |
| Salpingo-oophorectomy | |||||
| No | 259 (41.2) | 135 (42.5) | 87 (38.2) | 35 (44.3) | 0.499 |
| Yes | 370 (58.8) | 183 (57.5) | 141 (61.8) | 44 (55.7) | |
| Unknown | 62 | 31 | 24 | 6 | |
| (Neo-) adjuvant chemotherapy | |||||
| No | 319 (46.6) | 176 (51.0) | 109 (43.6) | 30 (35.7) | 0.022 |
| Yes | 365 (53.4) | 169 (49.0) | 141 (56.4) | 54 (64.3) | |
| Unknown | 7 | 4 | 2 | 1 | |
| Adjuvant endocrine therapy | |||||
| No | 555 (81.1) | 300 (87.2) | 203 (81.2) | 48 (56.5) | <0.001 |
| Yes | 129 (18.9) | 44 (12.8) | 47 (18.9) | 37 (43.5) | |
| Unknown | 7 | 5 | 2 | 0 | |
RT radiotherapy; BC breast cancer
* Data on type of surgery (either lumpectomy or mastectomy) were missing in 5 patients who were treated with radiotherapy
Table 2.
Characteristics of the patients with age at primary breast cancer diagnosis <40 years, radiotherapy vs. no radiotherapy
| Total (n = 325)* | RT after lumpectomy (n = 166) | No RT after mastectomy (n = 115) | RT after mastectomy (n = 42) | p value | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Age at primary BC | |||||
| <30 years | 55 (16.9) | 29 (17.5) | 19 (16.5) | 7 (16.7) | 0.996 |
| 30–34 years | 115 (35.4) | 59 (35.5) | 39 (33.9) | 15 (35.7) | |
| 35–39 years | 155 (47.7) | 78 (47.0) | 57 (49.6) | 20 (47.6) | |
| Mutation status | |||||
| BRCA1 | 261 (80.3) | 143 (86.1) | 89 (77.4) | 27 (64.3) | 0.004 |
| BRCA2 | 64 (19.7) | 23 (13.9) | 26 (22.6) | 15 (35.7) | |
| Period of primary BC | |||||
| 1980–1989 | 43 (13.2) | 33 (19.9) | 5 (4.4) | 5 (11.9) | <0.001 |
| 1990–1999 | 114 (35.1) | 68 (41.0) | 35 (30.4) | 10 (23.8) | |
| 2000–2013 | 168 (51.7) | 65 (39.2) | 75 (65.2) | 27 (64.3) | |
| Tumor stage | |||||
| Tis | 9 (2.9) | 4 (2.6) | 5 (4.5) | 0 | <0.001 |
| T1 | 179 (58.5) | 95 (60.5) | 70 (63.6) | 14 (35.9) | |
| T2 | 103 (33.7) | 57 (36.3) | 31 (28.2) | 15 (38.5) | |
| T3 | 8 (2.6) | 0 | 3 (2.7) | 5 (12.8) | |
| T4 | 7 (2.3) | 1 (0.6) | 1 (0.9) | 5 (12.8) | |
| Unknown | 19 | 9 | 5 | 3 | |
| Nodal status | |||||
| N0 | 206 (66.0) | 120 (74.5) | 78 (70.3) | 7 (17.9) | <0.001 |
| N1–3 | 106 (34.0) | 41 (25.5) | 33 (29.7) | 32 (82.1) | |
| Unknown | 13 | 5 | 4 | 3 | |
| Histological grade | |||||
| Grade 1 | 6 (2.5) | 2 (1.7) | 2 (2.1) | 2 (6.5) | 0.561 |
| Grade 2 | 45 (18.4) | 21 (17.7) | 17 (18.1) | 7 (22.6) | |
| Grade 3 | 193 (79.1) | 96 (80.7) | 75 (79.8) | 22 (71.0) | |
| Unknown | 81 | 47 | 21 | 11 | |
| Hormone receptor status | |||||
| Positive | 93 (33.1) | 41 (29.5) | 31 (30.7) | 21 (52.5) | 0.020 |
| Negative | 188 (66.9) | 98 (70.5) | 70 (69.3) | 19 (47.5) | |
| Unknown | 44 | 27 | 14 | 2 | |
| HER2 status | |||||
| Positive | 10 (7.6) | 4 (7.8) | 3 (5.5) | 3 (12.0) | 0.592 |
| Negative | 122 (92.4) | 47 (92.2) | 52 (94.5) | 22 (88.0) | |
| Unknown | 193 | 115 | 60 | 17 | |
| (Neo-) adjuvant chemotherapy | |||||
| No | 125 (38.9) | 75 (45.7) | 33 (28.9) | 16 (39.0) | 0.019 |
| Yes | 196 (61.1) | 89 (54.3) | 81 (71.1) | 25 (61.0) | |
| Unknown | 4 | 2 | 1 | 1 | |
| Adjuvant endocrine therapy | |||||
| No | 262 (81.4) | 148 (90.2) | 90 (78.9) | 22 (52.4) | <0.001 |
| Yes | 60 (18.6) | 16 (9.8) | 24 (21.1) | 20 (47.6) | |
| Unknown | 3 | 0 | 1 | 0 | |
| Contralateral risk-reducing mastectomy | |||||
| No | 174 (55.8) | 105 (66.0) | 46 (41.1) | 23 (56.1) | <0.001 |
| Yes | 138 (44.2) | 54 (34.0) | 66 (58.9) | 18 (43.9) | |
| Unknown | 13 | 7 | 3 | 1 | |
| Salpingo-oophorectomy | |||||
| No | 128 (42.8) | 66 (43.7) | 43 (40.6) | 18 (45.0) | 0.825 |
| Yes | 171 (57.2) | 85 (56.3) | 63 (59.4) | 22 (55.0) | |
| Unknown | 26 | 15 | 9 | 2 | |
RT radiotherapy; BC breast cancer
* Data on type of surgery (either lumpectomy or mastectomy) were missing in 2 patients who were treated with radiotherapy
Of all patients, 161 (23 %) developed CBC, of whom 87 were younger than 40 years at BC onset. The cumulative 5-, 10-, and 15-year risks of CBC for the total cohort were 8, 19, and 32 %, respectively. Among the patients younger than 40 years, the cumulative 5-, 10-, and 15-year CBC risks were 11, 32, and 40 %, respectively. Cumulative risks for age- and BRCA-specific subgroups suggest a higher cumulative risk for BRCA1-associated patients compared to BRCA2-associated patients (Table 3). Median time interval between primary BC and CBC was 4.8 years (range 0.5–29.0) for the entire cohort and 5.5 years (range 0.5–29.0 years) for patients diagnosed before the age of 40.
Table 3.
Cumulative 5-, 10-, and 15-year risks of contralateral breast cancer
| Years after diagnosis | Overall % (n at risk) | BRCA1 mutation % (n at risk) | BRCA2 mutation % (n at risk) | Age < 40 % (n at risk) | Age ≥ 40 % (n at risk) |
|---|---|---|---|---|---|
| 5 | 8 (198) | 9 (140) | 5 (58) | 11 (86) | 6 (112) |
| 10 | 19 (98) | 21 (75) | 15 (23) | 32 (39) | 10 (59) |
| 15 | 32 (47) | 35 (37) | 15 (10) | 40 (17) | 23 (30) |
Cumulative 5-, 10-, and 15-year risks of contralateral breast cancer in different subgroups of breast cancer patients (BRCA1 mutation carriers vs. BRCA2 mutation carriers and age at primary breast cancer <40 vs. ≥40 years). Only those patients who underwent DNA testing for BRCA1/2 mutation before the diagnosis of contralateral breast cancer were included
Left truncation was applied to correct for survival bias that may occur in studies with patient recruitment at a variable time after diagnosis (see statistical analysis). Consequently, a considerable number of patients did not contribute person-time to the prospective follow-up, leaving 418 patients for the main analyses. In univariate analysis, the risk of CBC was increased in patients younger than 40 years compared to those older than 40 years at primary BC (HR 2.42, 95 % CI 1.34–4.38). Furthermore, mutation carriership of BRCA1 was associated with increased risk of CBC as compared to BRCA2 mutation carriership (HR 2.32, 95 % CI 0.98–5.51). Both chemotherapy and endocrine therapy were significantly associated with a decreased risk of CBC (HR 0.45, 95 % CI 0.25–0.81 and HR 0.27, 95 % CI 0.08–0.86, respectively). For salpingo-oophorectomy, no association with CBC risk was found (HR 0.73, 95 % CI 0.37–1.43) (Table 4).
Table 4.
Univariate and multivariate hazard ratios for risk of contralateral breast cancer associated with selected factors
| Overall | Age < 40 years | ||
|---|---|---|---|
| Univariate analyses Number of patients: n = 418 Person years: 1105 years HR (95 % CI) |
Univariate analyses Number of patients: n = 211 Person years: 467 years HR (95 % CI) |
Multivariate analysis* Number of patients: n = 211 Person years: 467 years HR (95 % CI) |
|
| Age at primary breast cancer | |||
| <40 years | 2.42 (1.34–4.38) | ||
| ≥40 years | 1 | ||
| Age at primary breast cancer | |||
| Continuous | 0.94 (0.90–0.97) | 0.93 (0.85–1.01) | 0.96 (0.88–1.06) |
| BRCA mutation | |||
| BRCA1 | 2.32 (0.98–5.51) | 3.52 (0.83–14.99) | 2.33 (0.51–10.73) |
| BRCA2 | 1 | 1 | 1 |
| Chemotherapy | |||
| No | 1 | 1 | 1 |
| Yes | 0.45 (0.25–0.81) | 0.51 (0.24–1.09) | 0.52 (0.24–1.14) |
| Endocrine therapy | |||
| No | 1 | 1 | 1 |
| Yes | 0.27 (0.08–0.86) | 0.24 (0.06–1.02) | 0.25 (0.05–1.23) |
| Salpingo-oophorectomy (time-dependent) | |||
| No | 1 | 1 | |
| Yes | 0.73 (0.37–1.43) | 1.22 (0.53–2.81) | |
| Radiotherapy | |||
| No radiotherapy after mastectomy | 1 | 1 | 1 |
| Radiotherapy after mastectomy | 0.62 (0.17–2.23) | 0.94 (0.18–4.86) | 0.97 (0.41–2.30) |
| Radiotherapy after lumpectomy | 0.84 (0.46–1.55) | 1.41 (0.62–3.23) | 1.53 (0.22–10.51) |
HR Hazard ratio
* The following variables were incorporated in the multivariate model: age at primary breast cancer (continuous variable), type of BRCA mutation (BRCA1 vs. BRCA2), adjuvant chemotherapy (yes vs. no), adjuvant endocrine therapy (yes vs. no), and radiotherapy (no radiotherapy after mastectomy vs. radiotherapy after mastectomy and vs. radiotherapy after lumpectomy)
No deleterious effect of radiotherapy for primary BC, either after lumpectomy or after mastectomy, on CBC risk was found for the entire population (HR 0.84, 95 % CI 0.46–1.55 and HR 0.62, 95 % CI 0.17–2.23, respectively) (Table 4). Adjusting for age, adjuvant chemotherapy, adjuvant endocrine therapy, and type of BRCA mutation in a multivariate analysis still showed no association of radiotherapy on CBC risk (HR 0.74, 95 % CI 0.40–1.37 and HR 0.96, 95 % CI 0.23–3.97, respectively).
Subgroup analyses of patient younger than 40 years at BC onset
Also in the subgroup of patients younger than 40 years at primary BC diagnosis, no effect of radiotherapy for primary BC, either after lumpectomy or after mastectomy, on CBC risk was found in univariate analysis (n = 211; HR 1.41, 95 % CI 0.62–3.23 and HR 0.94, 95 % CI 0.18–4.86, respectively), and this was maintained in multivariate analysis (HR 1.53, 95 % CI 0.22–10.51 and HR 0.97, 95 % CI 0.41–2.30, respectively) (Fig. 1; Table 4). Median time interval between primary BC and CBC diagnoses was not significantly different between those treated with radiotherapy for primary BC compared to those patients not receiving radiotherapy (5.5 vs. 4.9 years, p = 0.88).
Fig. 1.
Kaplan–Meier estimates of the contralateral breast cancer (CBC) risk in BRCA1/2 mutation carriers, younger than 40 years of age at primary BC diagnosis. For this analysis, left truncation of analysis time at the DNA test date was applied, to correct for survival bias. Patients treated with radiotherapy (either after lumpectomy or after mastectomy) were compared to those not treated with radiotherapy at primary BC diagnosis
During follow-up, the number of patients at risk substantially decreased because a large proportion of patients were censored as they underwent a contralateral risk-reducing mastectomy, developed a BC recurrence or a second non-breast malignancy. In the group younger than 40 years at BC onset, 165 of 325 patients (51 %) were censored in the first 10 years of follow-up because of these three reasons (Fig. 2). Furthermore, since a large proportion of patients had less than 10 years of follow-up time, only 29 and 14 patients were available for the prospective analyses after 10 and 15 years of follow-up in this age group, respectively.
Fig. 2.
Cumulative frequency of contralateral breast cancer (CBC) or reasons for censoring event at study start and after 5, 10, 15, and 20 years of follow-up in all included patients who were younger than 40 years of age at primary breast cancer diagnosis. Recurrence includes both ipsilateral recurrence, a second ipsilateral primary tumor, and metastatic disease. (C)RRM = (contralateral) risk-reducing mastectomy. End of FU (follow-up) comprises patients who did not reach the primary endpoint or other censoring event at data cut-off or were lost to follow up
Treatment choices over time
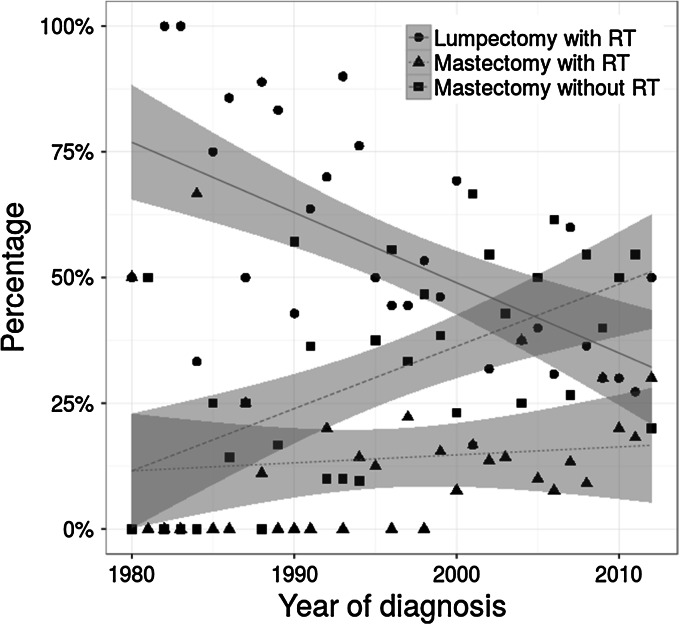
Over the past decades, the proportion of patients at risk for radiation-induced CBC changed substantially as a result of an increased rate of mastectomy without radiotherapy instead of breast-conserving therapy for primary breast cancer, and an increased rate of contralateral risk-reducing mastectomy (Figs. 3, 4). For example, patients aged younger than 40 years at diagnosis more often opted for mastectomy without radiotherapy instead of breast-conserving therapy in 2010 (reaching 50 %), compared to less than 30 % in 1995. The proportion of patients receiving radiotherapy following mastectomy was relatively stable over time being around 10–15 % (Fig. 3). Since 2010, more than 60 % of patients younger than 40 years at primary diagnosis opted for contralateral risk-reducing mastectomy, after primary breast cancer treatment, which was less than 40 % in 1995 (Fig. 4).
Fig. 3.
Distribution of the choice of local therapy at primary breast cancer diagnosis by year of diagnosis among patients younger than 40 years of age with a BRCA1 or BRCA2 mutation. Regression line of best fit and estimate of 95 % confidence interval (gray). RT Radiotherapy
Fig. 4.
Proportion of patients with a BRCA1 or BRCA2 mutation and breast cancer diagnosis below the age of 40 opting for contralateral (or bilateral) risk-reducing mastectomy (either at primary breast cancer treatment or within the years after primary breast cancer) by year of breast cancer diagnosis. Regression line of best fit and estimate of 95 % confidence interval (gray)
Discussion and conclusion
The risk of CBC among BC patients with a BRCA1/2 mutation is high, especially for younger patients. An association between adjuvant radiotherapy and the development of CBC in BRCA1/2-associated BC patients was not observed, neither in the entire cohort, nor in the subgroup of patients younger than 40 years at primary diagnosis. We found in this study that during follow-up the number of patients at risk for developing CBC substantially decreased due to either contralateral risk-reducing mastectomy or BC recurrence (26 and 14 %, respectively, within the first 5 years after primary BC among patients younger than 40 years). As a consequence, the number of patients at risk after 10 and 15 years of follow-up was too small to definitively exclude harmful effects of radiotherapy on the development of CBC among young BRCA1/2 mutation carriers.
A few other studies also reported on CBC risk in BRCA1/2-associated BC patients treated with adjuvant radiotherapy compared to patients not treated with radiotherapy [13–15], and did not find an increased risk of CBC associated with adjuvant radiotherapy either. In the two multi-center retrospective cohort studies of breast cancer patients attending high-risk clinics [13, 14], the numbers of young BRCA1/2 mutation carriers and follow-up periods were comparable to our study (145 out of 655 patients younger than 35 years with a median follow-up of 8 years in the study of Pierce et al. [13], and 357 out of 810 patients younger than 40 years with a median follow-up of 11 years in the study of Metcalfe et al. [14]). However, subgroup analyses among these younger patients were not reported. Bernstein performed a nested case–control study within the WECARE study (Women’s Environmental Cancer and Radiation Epidemiology Study), which is a population-based study of patients with metachronous CBC [15], but again no results of subgroup analysis in younger patients were shown.
The main limitation of our study regarding the impact of radiotherapy on the CBC risk is the small number of patients at risk for CBC after 10–15 years of follow-up, as studies including sporadic patients suggest that a minimal latency period of 10–15 years is needed to develop radiation-induced BC [19, 20]. It is, however, not known whether the latency period between exposure and development of a radiation-induced malignancy is similar for BRCA1/2 mutation carriers compared to sporadic patients. Even, if the latency period in BRCA1/2 mutation carriers is shorter, the number of patients at risk for CBC in our study group was too small to make definitive conclusions, especially since a large proportion of patients were already censored in the first 5 years. Given the number of events in patients younger than 40 years at primary BC diagnosis, our study had 80 % power to find an HR of at least 2.8 for adjuvant radiotherapy to be associated with increased risk of CBC.
In our total cohort, the 10-year cumulative risk of CBC in BRCA1/2 mutation carriers was 19 %, while in the subgroup of patients younger than 40 years at BC onset this risk was 32 %. These risks are comparable to the risks reported in other studies [14, 21, 22]. Furthermore, the CBC risk was higher in BRCA1 compared to BRCA2 mutation carriers. Both the increased risk in younger patients and the increased risk in BRCA1- compared to BRCA2-associated BC patients have been described in other studies [14, 21–23]. Additionally, in our cohort adjuvant systemic therapy for primary BC, applying for both endocrine therapy and chemotherapy, was associated with a decreased risk of CBC. This effect, however, was only significant in the entire cohort and not in the subgroup of younger patients. Since the HRs were similar, this might be due to the lack of statistical power. The risk-reductive effect of adjuvant endocrine therapy on CBC risk in BRCA1/2 mutation carriers has been reported in previous studies [14, 24, 25]. Regarding chemotherapy, three studies have investigated the association between chemotherapy and CBC [14, 23, 26], whereby only Reding et al. found a significant association with a relative risk of 0.5. Although this latter association is biologically not totally clear, further research is certainly warranted. We did not find any impact of salpingo-oophorectomy on CBC risk, which is in contrast with previous reports [27, 28], but is in line with more recent literature [29].
In our cohort, we found a growing preference over time for mastectomy without radiotherapy instead of breast-conserving therapy including radiotherapy. At the same time, the rate of contralateral risk-reducing mastectomy after primary breast cancer treatment has increased. Important reasons for the shift toward ablative breast surgery might be the improvements in and availability of (direct) breast reconstructive options, the increased awareness of the magnitude of the CBC risk and distress of screening, and the wish to avoid another treatment session for a second primary BC. Finally, the important findings of Heemskerk et al. showing that contralateral risk-reducing mastectomy improves survival, mainly in younger patients and those with favorable primary tumor characteristics [30], might lead to an even larger proportion of younger patients opting for mastectomy without radiotherapy and contralateral risk-reducing mastectomy after primary breast cancer diagnosis in the nearby future.
These trends in locoregional treatments eventually decreased the proportion of patients at risk for radiation-induced CBC over the past few decades. Nevertheless, the question whether adjuvant radiotherapy has deleterious effect on CBC risk still remains clinically important for a significant number of patients, who want to conserve their (ipsilateral and) contralateral breast. Moreover, in the nearby future a larger proportion of patients potentially might opt for breast-conserving treatment and abstain from contralateral risk-reducing mastectomy, due to an increased use of endocrine therapy as chemoprevention, improved diagnostic imaging techniques for screening, and improved effectiveness of adjuvant systemic therapy (for example, in combination with PARP inhibitors) [31–33].
In the current study, we could not find an association between radiotherapy for primary BC and risk of CBC in (young) BRCA1/2 mutation carriers compared to sporadic patients; however, the number of patients at risk after 10 and 15 years of follow-up was too small to definitively exclude harmful effects of adjuvant radiotherapy. An increase in the percentage of young patients with BRCA1/2-associated breast cancer choosing for conserving their (ipsilateral and) contralateral breast is not unlikely. Therefore, future research in larger study populations with minimal follow-up of 10 years is needed to achieve a better understanding of the true effect of radiotherapy on the CBC risk in BRCA1/2-associated BC patients. This will only be possible by combining study populations through collaborative efforts on a national or even international level.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, et al. Effects of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X, Fisher SF, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;4:1038–1045. doi: 10.1016/S0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 4.Hooning MJ, Aleman BMP, Hauptmann M, et al. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol. 2008;26:5561–5568. doi: 10.1200/JCO.2007.16.0192. [DOI] [PubMed] [Google Scholar]
- 5.Stovall M, Smith SA, Langholz BM, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer int the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drooger JC, Hooning MJ, Seynaeve CM, et al. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: a critical review of the literature. Cancer Treat Rev. 2015;41:187–196. doi: 10.1016/j.ctrv.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Connell PP, Kron SJ, Weichselbaum RR. Relevance and irrelevance of DNA damage response to radiotherapy. DNA Repair (Amst) 2004;3(8–9):1245–1251. doi: 10.1016/j.dnarep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks JD, Boice JD, Stovall M, Reiner AS, Bernstein L, John EM, et al. Reproductive status at first diagnosis influences risk of radiation-induced second primary contralateral breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2012;84:917–924. doi: 10.1016/j.ijrobp.2012.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21:8981–8993. doi: 10.1038/sj.onc.1206176. [DOI] [PubMed] [Google Scholar]
- 11.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 12.Pijpe A, Andrieu N, Easton DF, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK) BMJ. 2012;6:e5660. doi: 10.1136/bmj.e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce LJ, Phillips K, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121:389–398. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalfe K, Gershman S, Lynch HT, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carries. Br J Cancer. 2011;104:1384–1392. doi: 10.1038/bjc.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein JL, Thomas DC, Shore RE, et al. Contralateral breast cancer after radiotherapy among BRCA1 and BRCA1 mutation carriers: a WECARE study report. Eur J Cancer. 2013;49:2979–2985. doi: 10.1016/j.ejca.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mislowsky A, Domchek S, Stroede C, et al. Breast cancer surgery trend changes since the introduction of BRCA1/2 mutation screening: a retrospective cohort analysis of 158 mutation carriers treated at a single institution. Ann Surg Oncol. 2011;18:745–751. doi: 10.1245/s10434-010-1381-9. [DOI] [PubMed] [Google Scholar]
- 17.Azatto EM, Greenberg D, Shah M, et al. Prevalent cases in observational studies of cancer survival: do they bias hazard ratio estimates? Br J Cancer. 2009;100:1806–1811. doi: 10.1038/sj.bjc.6605062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heemskerk-Gerritsen BAM, Seynaeve CM, van Asperen, CJ, et al. (2015) Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst 107(5):djv033. http://jnci.oxfordjournals.org/content/107/5/djv033.long [DOI] [PubMed]
- 19.Ronckers CM, Doody MM, Lonstein JE, et al. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomark Prev. 2008;17:605–613. doi: 10.1158/1055-9965.EPI-07-2628. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim EM, Abouelkhair KM, Kazkaz GA, et al. Risk of second breast cancer in female Hodgkin’s lymphoma survivors: a meta-analysis. BMC Cancer. 2012;12:197. doi: 10.1186/1471-2407-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brekelmans CTM, Tilanus-Linthorst MMA, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43:867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 23.Menes TS, Terry MB, Goldgar D, et al. Second primary breast cancer in BRCA1 and BRCA2 mutation carriers: 10-year cumulative incidence in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2015;151:653–660. doi: 10.1007/s10549-015-3419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 25.Philips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091–3099. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reding KW, Bernstein JL, Langholz BM, et al. Adjuvant systemic therapy for breast cancer in BRCA1/BRCA2 mutation carriers in a population-based study of risk of contralateral breast cancer. Breast Cancer Res Treat. 2010;123:491–498. doi: 10.1007/s10549-010-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 28.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24:2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 29.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;303:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heemskerk-Gerritsen BAM, Rookus MA, Aalfs CM, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer. 2015;136:668–677. doi: 10.1002/ijc.29032. [DOI] [PubMed] [Google Scholar]
- 31.Boetes C. Update on screening breast MRI in high-risk women. Obstet Gynecol Clin N Am. 2011;38:149–158. doi: 10.1016/j.ogc.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med. 2015;13:188. doi: 10.1186/s12916-015-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimers LL, Sivasubramanian PS, Hershman D, et al. Breast cancer chemoprevention among high-risk women and those with ductal carcinoma in situ. Breast J. 2015;21:377–386. doi: 10.1111/tbj.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]