Abstract
Objectives:
To determine whether the mean platelet volume (MPV) and MPV/platelet (PLT) values can be used in the study of sepsis and systemic inflammatory response syndrome (SIRS).
Methods:
In this retrospective case-controlled study, 69 sepsis, 69 SIRS patients, and 72 control group who were treated in the years 2012-2013 were reviewed, and both the MPV and MPV/PLT rates were evaluated in all groups at Kahramanmaras Sutcu Imam University Intensive Care Unit, Kahramanmaras, Turkey.
Results:
Statistically significant difference was found between sepsis, SIRS, and control groups when comparing the MPV and MPV/PLT ratio (p<0.05), and no significant difference was found between sepsis and SIRS groups in terms of MPV and MPV/PLT ratio (p>0.05). Mean platelet volume values for sepsis and control groups was 10.07/8.731 femtoliter (fL) (p=0.000), and 9.45/8.731 fL (p=0.000) for SIRS and control groups. In the group of sepsis patients, the MPV was found to be at cut-off 8.915, sensitivity 71%, and specificity 63.9%. In the group of patients with SIRS, MPV was found to be at cut-off 8.85, sensitivity 69.6%, and specificity 62.5%. For the MPV/PLT values, the specificity and sensitivity were found to be insignificant.
Conclusion:
This study shows that although there was no significant reduction in the PLT values between the sepsis and SIRS patients, the MPV and MPV/PLT ratio values were found to have significant differences. However, the specificity and sensitivity of the values were not reliable standard to be used as a test.
Sepsis is a life-threatening condition that occurs when the body’s response to an infection injures its own tissues and organs.1 The pathogenesis of sepsis involves a series of complex regulatory interactions, with concomitant and often antagonistic processes, resulting in a dysregulated host response with both exaggerated inflammation and immune suppression. The pro-inflammatory response to sepsis leads to activation of the coagulation system with concurrent inhibition of anticoagulant mechanisms and fibrinolysis.1 Consequently, fibrinolytic and fibrinogen products are consumed, clot forms, and bleeding shows itself in the form of disseminated intravascular coagulation (DIC). Disseminated intravascular coagulation results as increased platelet destruction.2 Mean platelet volume (MPV) is a measurement of the average size of platelets found in the blood. There are high MPV levels in destructive thrombocytopenia and low MPV levels in hypoproliferative thrombocytopenia.3 A rapid and reliable test for the discrimination of SIRS and sepsis in the ICU would therefore be very useful. In this retrospective study, we’ve researched whether the MPV and MPV/platelets (PLT) values could be used in the study of sepsis and systemic inflammatory response syndrome (SIRS).
Methods
Patients and study design
In this study, to determine whether the MPV and MPV/PLT values could be used in the study of sepsis and SIRS. The results of 69 sepsis, 69 SIRS patients, and 72 control patients from the Kahramanmaras Sutcu Imam University intensive care unit, Kahramanmaras, Turkey who were treated in the years 2012-2013 were retrospectively reviewed. The local ethics committee approved the study. Inclusion criteria; all consecutive patients who exhibited sepsis or SIRS and stayed for >72 hours in the intensive care unit (ICU) were considered for inclusion in this study. According to our standard definition, patients were defined as having SIRS if they exhibited at least 2 of the following 4 criteria: 1) fever (>38°C) or hypothermia (<36°C); 2) tachypnoea (>20/minute); 3) tachycardia (>90/minute); or 4) leucopenia (<4.0_109/L), leukocytosis (>12.0_109/L), or a leftward shift (>10% immature granulocytes). If SIRS was proved to be accompanied by bacterial infection by cultures or on clinical grounds, the patient was accepted as having sepsis. The severity of sepsis was evaluated using the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definition. Sepsis was defined as infection plus systemic manifestations of infection.4 Sepsis plus Sepsis-induced organ dysfunction, or tissue hypoperfusion was defined as severe sepsis.4 Exclusion criteria; for ages less than 16 years old, incapability to give informed legal consent and evidence of a clearly different diagnosis, terminal stage of disease (malignancies, bleeding, and coagulation defects) and the patient or relatives did not consent to inclusion. Control group/patients were selected from consecutive patients who were admitted to the outpatient clinic; No infectious disease diagnosis, Normal C-reactive protein (CRP) levels and leukocyte count in laboratory examination, No SIRS criteria in his/her medical records. Were age less than 16 years old, incapability to give informed legal consent and evidence of a clearly different diagnosis, terminal stage of disease (malignancies, bleeding, and coagulation defects), and the patient or relatives did not consent to inclusion.
Data collection
Patient medical records and the electronic patient data monitoring system were examined retrospectively. Subject data including name, age, gender, past medical history, and vital signs were recorded at enrolment. Laboratory examinations, culture results including whole blood leukocyte counts, blood gas analysis, blood biochemistry, x-ray scans, and others were carried out within 24 hours.
Laboratory analyses
The day that a clinical diagnosis of sepsis was made was considered day one and venous blood sampling was performed within the first 12 hours after first presentation. From each sample obtained, determination of white blood cell count, MPV, CRP were performed immediately. Blood for complete blood counts were obtained either by venipuncture, arterial puncture, or through a central catheter. Procalcitonin and MPV determinations were performed using ADVIA 2120i (Siemens, Erlangen, Germany). Mean platelet volume values were measured in femtoliter (fL). Serum concentrations of CRP were measured by Nefrolometer assay on a BN II System-DADE Behning (Siemens, Erlangen, Germany) according to manufacturer’s instruction.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, median, and the range (minimum-maximum) continuous data were assessed with Mann Whitney U test. Mean platelet volume/PLT ratio and MPV values were found to be statistically different between groups, and they were analysed for their diagnostic value in sepsis using receiver operating characteristic (ROC) analysis. The cut-off values were determined. A 2-tailed p-value<0.05 was considered statistically significant. Statistical analyses were performed using Statistical Package for Social Sciences version 17 (SPSS Inc., Chicago, IL, USA).
Results
There were no statistically significant difference between patients with sepsis (67.6±10.3 [16-85]), SIRS (65.4±13.1 [16-85]), and healthy controls (62±14.1 [16-85]) with respect to age (p>0.05). Gender of the patients are shown in Table 1. As the results were not normally distributed, the comparisons between the groups were made using the Mann Whitney U test. There was no significant difference in the MPV averages between the sepsis and SIRS patients (p>0.05). In comparison with the control group, it was found that there was a significant difference in the MPV and MPV/PLT values for the sepsis and the SIRS patients (p<0.05). There was no difference in the platelet count between the sepsis and SIRS patients (p>0.05). Comparison of MPV, platelet count, and MPV/PLT ratio values between all groups are shown in Table 2. To determine whether the comparison between the MPV are an appropriate test for the SIRS and sepsis patients, a ROC curve was drawn and the specificity and sensitivity were calculated. The highest cut-off values for the sensitivity and the specificity are shown on Table 3. To determine whether the MPV/PLT value is appropriate in diagnosing SIRS and sepsis, it was compared with the control groups and a ROC curve was drawn and the specificity and sensitivity values were calculated. The highest cut-off points for the sensitivity and specificity were determined (Figure 1 & 2).
Table 1.
Comparison of the groups regarding demographic characteristics.
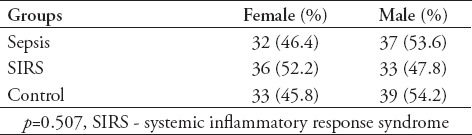
Table 2.
Comparison of mean platelet volume (MPV), platelet count, and MPV/PLT ratio values between all groups.
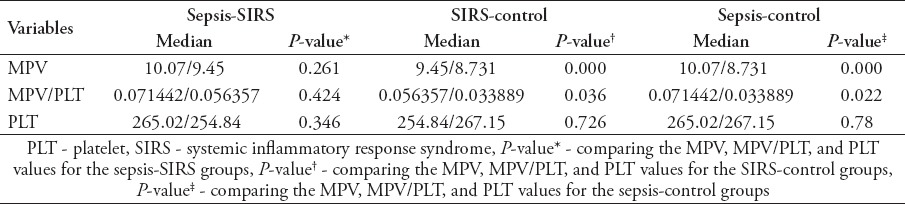
Table 3.
The result of receiver operating characteristic analysis for mean platelet volume (MPV).
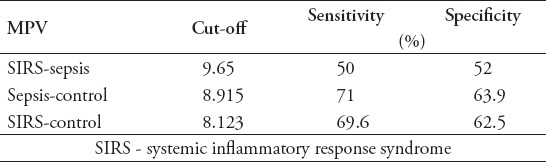
Figure 1.
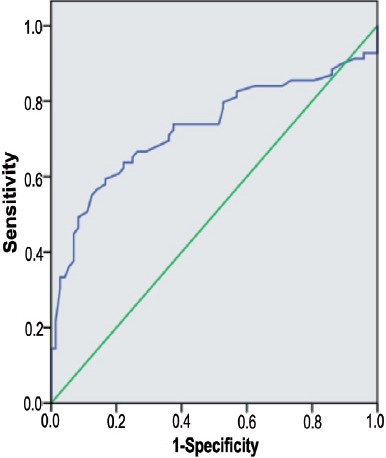
Receiver operating characteristic curve for mean platelet volume value in sepsis. Diagonal segments are produced by ties. Area under the curve 0.728, cut-off 8.915, sensitivity 63.9%, specificity 71%.
Figure 2.
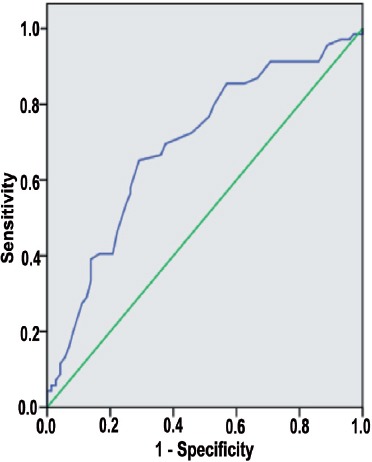
Receiver operating characteristic curve for mean platelet volume value in systemic inflammatory response syndrome. Diagonal segments are produced by ties. Area under the curve 0.698, cut-off 8.123, sensitivity 69.6%, specificity 62.5%.
Discussion
The easily accessible, cheap, and common laboratory tests are very important in determining the severity of the disease in sepsis patients. Mean platelet volume is universally available with routine blood counts by automated hemograms and a simple and easy method of assessing platelet function. To achieve a larger surface, platelets go through changes in structure during activation. Their shape changes from discoid to spherical and pseudopodia are also formed. Platelet vertical diameter is important in measuring platelet volume, which is achieved by a hematology analyser, using deformation of electrical field, based on impedance technology. Volume is determined by measuring the cross diameter of the platelet cell using analyzers with laser optical technology. Consequently, activated platelets seem larger in surface area independently of the principle of measurement.5 In our study we have researched whether the MPV and MPV/PLT values could be used in diagnosing sepsis and SIRS. In recent studies6-9 it has been reported that MPV is associated with respiratory distress syndrome, necrotizing enterocolitis, bronchopulmonary dysplasia, intraventricular hemorrhage, acute appendicitis, and sepsis in newborns. There have been previous studies10,11 that have looked at the relationship between the MPV and sepsis. However, it is been the first time where the MPV/PLT values were used to study patients with sepsis and SIRS. According to our study there is no difference in PLT values in sepsis and SIRS patients, with an increase in MPV. In the comparison of sepsis and SIRS patients with the control group, both the MPV and the MPV/PLT showed significant differences. To determine whether the MPV could be a useful parameter to be used in determining sepsis from SIRS, a roc curve was used. The sensitivity and specificity values obtained showed that it was insignificant at determining sepsis from SIRS. Although the sensitivity and specificity values in the sepsis-control groups were significant (cut-off 8.915 sensitivity 71%, specificity 63.9%), it was not at a required standard to be used as a screening test. Similarly, in the SIRS-control groups the sensitivity and specificity values were also not at a required standard to be used as a screening test. In Kitazawa et al12 study, the difference in MPV is going to be a prognostic marker for blood infections. It was suggested in Kim et al’s11 study that constant measurement of MPV could be useful in determining mortality risk in patients with sepsis and sepsis shock. In Becchi et al’s13 study, it’s suggested that screening of MPV could be useful in the early development of sepsis. In Zampieri et al’s14 study, it was discovered that an increase of MPV in patients with sepsis had a correlation with mortality.13,14 The findings of our study support these statements as it proves an increase in MPV in patients with sepsis and SIRS. Guclu et al15 reported low platelet count and higher MPV in patients with severe sepsis compared with other control patients. In the same study,15 MPV sensitivity was found to be 87.4% and specificity 53.47%. In our study, although there were no major decrease in PLT values, MPV’s sensitivity (71%), and specificity (63.9%) were found similar.
The platelet count is nearly below 80.000 mm3 in 40% patients with severe sepsis.16 While higher MPV levels are observed in the destructive thrombocytopenia, the low MPV levels are determined in hypoproliferative thrombocytopenia.17 In our study we observed low platelet count, but there was no statically significant difference, and we thought that it may be due to early diagnosis of sepsis. The importance of platelet disorders in patients with sepsis remains unclear. The MPV/PLT values have not previously been used in sepsis patients, found to be low in patients with infective endocarditis and found to be high in patients suffering from deep vein thrombosis (deep vein thrombosis MPV/PLT cut-off value of 0.0363 fL/(109/l) showed 60% sensitivity and 73% specificity.)18,19 In our study, although the MPV/PLT value was significantly different in patient’s with sepsis and SIRS compared with the control group, it was found to be not at a standard to be used as a screening test. However, this could also be due to the low number of patients observed. Diabetes, cardiovascular diseases, type 2 diabetes mellitus, prediabetes, smoking, hypertension, hypercholesterolemia, obesity, coronary heart disease, and metabolic syndrome could all affect MPV.20 Not accounting for these factors during our study is a limitation.
In conclusion, from this study, we have gathered that compared with the control groups, in patients with sepsis and SIRS there was no significant fall in PLT values. It is been the first time where the MPV/PLT values were used to study patients with sepsis and SIRS. However, MPV and MPV/PLT ratios showed significant differences. Although these proved significantly different, the sensitivity and specificity of the MPV values were not at a required standard to be used as screening tests. After reducing the factors that limit this study, removing the predisposing factors, and with further research, the MPV and MPV/PLT values could be a fast and reliable marker used for sepsis.
Footnotes
Related Articles.
Hou SY, Feng XH, Lin CL, Tan YF. Efficacy of Xuebijing for coagulopathy in patients with sepsis. Saudi Med J 2015; 36: 164-169.
Kesici S, Turkmen UA, Kesici U, Altan A, Polat E. Effects of enteral and parenteral glutamine on intestinal mucosa and on levels of blood glutamine, tumor necrosis factor-alpha, and interleukin-10 in an experimental sepsis model. Saudi Med J 2012; 33: 262-271.
Bamaga MS, Sobahy TM, Attar AA. Quantitative DNA analysis of very low-level hepatitis B viremic patients reporting to the gastroenterology clinic. Saudi Med J 2011; 32: 135-140.
References
- 1.de Stoppelaar SF, van’t Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112:666–677. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 2.Acikgoz S, Akduman D, Eskici ZM, Can M, Mungan G, Guven B. Thrombocyte and Erythrocyte Indices in Sepsis and Disseminated Intravascular Coagulation. Journal of Medical Biochemistry. 2012;31:60–64. [Google Scholar]
- 3.Farias MG, Schunck EG, Dal Bo S, de Castro SM. Definition of reference ranges for the platelet distribution width (PDW): a local need. Clin Chem Lab Med. 2010;48:255–257. doi: 10.1515/CCLM.2010.035. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Canpolat FE, Yurdakok M, Armangil D, Yigit S. Mean platelet volume in neonatal respiratory distress syndrome. Pediatr Int. 2009;51:314–316. doi: 10.1111/j.1442-200X.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 7.Oncel MY, Ozdemir R, Yurttutan S, Canpolat FE, Erdeve O, Oguz SS, et al. Mean platelet volume in neonatal sepsis. J Clin Lab Anal. 2012;26:493–496. doi: 10.1002/jcla.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cekmez F, Tanju IA, Canpolat FE, Aydinoz S, Aydemir G, Karademir F, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci. 2013;17:134–137. [PubMed] [Google Scholar]
- 9.Erdem H, Aktimur R, Cetinkunar S, Reyhan E, Gokler C, Irkorucu O, et al. Evaluation of mean platelet volume as a diagnostic biomarker in acute appendicitis. Int J Clin Exp Med. 2015;8:1291–1295. [PMC free article] [PubMed] [Google Scholar]
- 10.Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13:333–338. doi: 10.4314/ahs.v13i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim YL, Park KS, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One. 2015;10:e0119437. doi: 10.1371/journal.pone.0119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitazawa T, Yoshino Y, Tatsuno K, Ota Y, Yotsuyanagi H. Changes in the mean platelet volume levels after bloodstream infection have prognostic value. Intern Med. 2013;52:1487–1493. doi: 10.2169/internalmedicine.52.9555. [DOI] [PubMed] [Google Scholar]
- 13.Becchi C, Al Malyan M, Fabbri LP, Marsili M, Boddi V, Boncinelli S. [Mean platelet volume trend in sepsis: is it a useful parameter?] Minerva Anestesiol. 2006;72:749–756. [PubMed] [Google Scholar]
- 14.Zampieri FG, Ranzani OT, Sabatoski V, de Souza HP, Barbeiro H, da Neto LM, et al. An increase in mean platelet volume after admission is associated with higher mortality in critically ill patients. Ann Intensive Care. 2014;4:20. doi: 10.1186/s13613-014-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13:333–338. doi: 10.4314/ahs.v13i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munford RS, Suffredini AF. Sepsis, severe sepsis, and septic shock. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia (PA): Churchill Livingstone; 2010. pp. 987–1010. [Google Scholar]
- 17.Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol. 2009;146:585–596. doi: 10.1111/j.1365-2141.2009.07717.x. [DOI] [PubMed] [Google Scholar]
- 18.Cho SY, Jeon YL, Kim W, Kim WS, Lee HJ, Lee WI, et al. Mean platelet volume and mean platelet volume/platelet count ratio in infective endocarditis. Platelets. 2014;25:559–561. doi: 10.3109/09537104.2013.857394. [DOI] [PubMed] [Google Scholar]
- 19.Han JS, Park TS, Cho SY, Joh JH, Ahn HJ. Increased mean platelet volume and mean platelet volume/platelet count ratio in Korean patients with deep vein thrombosis. Platelets. 2013;24:590–593. doi: 10.3109/09537104.2012.748187. [DOI] [PubMed] [Google Scholar]
- 20.Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–1515. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]


