A conserved tryptophan uniquely present in the voltage-sensing domain of voltage-gated proton channels is surprisingly crucial to four channel-defining properties.
Abstract
Part of the “signature sequence” that defines the voltage-gated proton channel (HV1) is a tryptophan residue adjacent to the second Arg in the S4 transmembrane helix: RxWRxxR, which is perfectly conserved in all high confidence HV1 genes. Replacing Trp207 in human HV1 (hHV1) with Ala, Ser, or Phe facilitated gating, accelerating channel opening by 100-fold, and closing by 30-fold. Mutant channels opened at more negative voltages than wild-type (WT) channels, indicating that in WT channels, Trp favors a closed state. The Arrhenius activation energy, Ea, for channel opening decreased to 22 kcal/mol from 30–38 kcal/mol for WT, confirming that Trp207 establishes the major energy barrier between closed and open hHV1. Cation–π interaction between Trp207 and Arg211 evidently latches the channel closed. Trp207 mutants lost proton selectivity at pHo >8.0. Finally, gating that depends on the transmembrane pH gradient (ΔpH-dependent gating), a universal feature of HV1 that is essential to its biological functions, was compromised. In the WT hHV1, ΔpH-dependent gating is shown to saturate above pHi or pHo 8, consistent with a single pH sensor with alternating access to internal and external solutions. However, saturation occurred independently of ΔpH, indicating the existence of distinct internal and external pH sensors. In Trp207 mutants, ΔpH-dependent gating saturated at lower pHo but not at lower pHi. That Trp207 mutation selectively alters pHo sensing further supports the existence of distinct internal and external pH sensors. Analogous mutations in HV1 from the unicellular species Karlodinium veneficum and Emiliania huxleyi produced generally similar consequences. Saturation of ΔpH-dependent gating occurred at the same pHo and pHi in HV1 of all three species, suggesting that the same or similar group(s) is involved in pH sensing. Therefore, Trp enables four characteristic properties: slow channel opening, highly temperature-dependent gating kinetics, proton selectivity, and ΔpH-dependent gating.
INTRODUCTION
Voltage-gated proton channels (HV1) exist in diverse organisms ranging from unicellular marine species (Smith et al., 2011; Taylor et al., 2011) to humans (Ramsey et al., 2006). Their functions are equally diverse: conversion of CO2 to calcite in coccolithophores (Taylor et al., 2011), triggering the bioluminescent flash in dinoflagellates (Smith et al., 2011), and in humans participating in innate immunity (DeCoursey, 2010), B cell signaling (Capasso et al., 2010), airway acid secretion (Iovannisci et al., 2010), histamine secretion (Musset et al., 2008b), sperm motility (Musset et al., 2012) and capacitation (Lishko et al., 2010), brain damage in ischemic stroke (Wu et al., 2012), breast cancer (Wang et al., 2012), and chronic lymphocytic leukemia (Hondares et al., 2014). All known and suspected HV1 to date, even in species with just 15–18% sequence identity to the human HV1 (hHV1), share a perfectly conserved tryptophan (Trp207 in hHV1) adjacent to the second of three Arg residues in the S4 transmembrane segment (Fig. S1) (DeCoursey, 2013). This Trp is part of the proposed signature sequence of the proton channel RxWRxxR (Smith et al., 2011), and is present even in several unconfirmed HV1-like sequences in fungi in which the third Arg in S4 is replaced by Lys (e.g., Fusarium oxysporum, Ophiostoma piceae, and Metarhizium anisopliae). Among molecules that contain voltage-sensing domains (VSDs), only HV1 and c15orf27 (whose function is unknown) contain Trp in this location (Smith et al., 2011). Here, we ask why this Trp has been conserved. We find that replacing Trp modifies four characteristic properties of hHV1, revealing that it is central to the unique defining properties and functions of HV1. Trp mutants opened and closed 30–100 times faster than WT, with gating kinetics less profoundly sensitive to temperature; they lost proton selectivity at high pHo; and the unique ΔpH dependence of gating was compromised. The striking diversity of the effects of Trp mutation indicates that this residue plays a pivotal role in the HV1 protein.
Tryptophan is the rarest amino acid in proteins, and in membrane proteins, it is often found close to lipid head groups, preferring the interfacial environment (Killian and von Heijne, 2000; MacCallum et al., 2008). Thus, the absolute conservation of a Trp in the middle of the S4 transmembrane segment of HV1 requires some explanation. Three other Trp residues in hHV1 are all in the intracellular N terminus. Perhaps because both Trp and Arg residues share ambivalence by exhibiting hydrophobic mixed with polar characteristics, they interact strongly with each other (Santiveri and Jiménez, 2010). In β-hairpin peptides and in other proteins, the guanidinium group of Arg stacks against the aromatic ring of Trp via cation–π (Gallivan and Dougherty, 1999) and van der Waals interactions, stabilizing the protein structure (Tatko and Waters, 2003; Santiveri and Jiménez, 2010). The proximity of Trp203 and R3 (the third Arg in the S4 segment, Arg207 in mouse) in the closed structure of the mouse HV1 (mHV1; Takeshita et al., 2014) appears to be consistent with this type of interaction, with amino-aromatic distances <6.0 Å (Burley and Petsko, 1986). In the structure identified as a closed conformation of mHV1 (Takeshita et al., 2014), the indole side chain of Trp203 is directed away from the pore toward the interior of the lipid bilayer, pointing downward, partially shielding the R3 side chain, which is directed down and between S4 and S3 but also has some lipid exposure. In all open-state models (Ramsey et al., 2010; Wood et al., 2012; Kulleperuma et al., 2013; Chamberlin et al., 2014), all three Arg residues of the S4 segment face the pore, but Trp still faces away from the pore. We propose that Trp stabilizes the closed hHV1 through cation–π interaction with Arg211, and that loss of this stabilization contributes to the consequences of its mutation. A striking result was that whether Trp was replaced by Ala (hydrophobic), Ser (hydrophilic), or Phe (aromatic), HV1 properties were changed by a quantitatively indistinguishable extent. This result suggests that the heterocyclic aromatic side chain of Trp uniquely anchors the S4 segment in the membrane.
MATERIALS AND METHODS
Gene expression
Site-directed mutants were created using the QuikChange (Agilent Technologies) procedure according to the manufacturer’s instructions. Transfection was done as described previously (Kulleperuma et al., 2013). Both HEK-293 cells and COS-7 cells were used as expression systems. We showed previously that the properties of HV1 expressed in both cell lines were indistinguishable (Musset et al., 2008a). No other voltage- or time-dependent conductances were observed under the conditions of this study. Although most mutations on hHV1 were introduced into a Zn2+-insensitive background (H140A/H193A), which we have done previously as a control for endogenous HV1 (Musset et al., 2011), the level of expression of all mutants studied here was sufficiently high that contamination by native HV1 was negligible.
Electrophysiology
In most experiments, cells expressing green fluorescent protein (GFP)-tagged proton channels were identified using inverted microscopes (Nikon) with fluorescence capability. For constructs that lacked the GFP tag, GFP was cotransfected. Conventional patch-clamp techniques were used (Kulleperuma et al., 2013) at room temperature (20–26°C). Bath and pipette solutions contained 60–100 mM buffer, 1–2 mM CaCl2 or MgCl2 (intracellular solutions were Ca2+ free), 1–2 mM EGTA, and TMAMeSO3 to adjust the osmolality to ∼300 mOsm, titrated with TMAOH. Buffers used were Homo-PIPES (Research Organics) at pH 4.5–5.0, Mes at pH 5.5–6.0, BisTris at pH 6.5, PIPES at pH 7.0, HEPES at pH 7.5, tricine at pH 8.0, CHES at pH 9.0, and CAPS at pH 10.0. Currents are shown without leak correction. To minimize pHi changes caused by large H+ fluxes, pulses for large depolarizations in pulse families were sometimes shortened.
The reversal potential (Vrev) was determined by two methods, depending on the relative positions of Vrev and the threshold voltage for activation of the gH, Vthreshold. For constructs in which Vthreshold was positive to Vrev, the latter was determined by examining tail currents. Because hHV1 currents were the only time-dependent conductance present, Vrev was established by the amplitude and direction of current decay during deactivation. By using this procedure, time-independent leak or other extraneous conductances do not affect Vrev (Morgan and DeCoursey, 2014). Tail currents were not observed in nontransfected cells. For mutants in which Vthreshold was negative to Vrev, it was possible to observe directly the reversal of currents activated during pulse families.
Proton current amplitude (IH) was usually determined by fitting the rising current with a single exponential and extrapolating to infinite time. Proton conductance (gH) was calculated from IH and Vrev measured in each solution: gH = IH/(V −Vrev). In some cases where current activation kinetics was difficult to evaluate, gH was calculated from tail current amplitudes instead of IH.
To evaluate ΔpH dependence, it is necessary to establish the position of the gH–V relationship. For this purpose, we have adopted the voltage at which the gH is 10% of its maximal value as a function of pH (VgH,max/10), in preference to other parameters that have been used, such as the midpoint of a Boltzmann distribution or the threshold for activating detectable H+ current, Vthreshold. Because the gH–V relationship is steepest at low voltages, fairly large errors in estimating the maximum gH (gH,max) produce only small errors in VgH,max/10. This parameter choice avoids the necessity of arbitrarily forcing nonsigmoidal gH–V data to fit a Boltzmann function (Musset et al., 2008a) or, alternatively, the need to identify the elusive threshold of channel activation, Vthreshold, which is subjective and can be difficult when it occurs near EH. Nevertheless, Vthreshold remains useful as a supplemental parameter because its measurement requires minimal current flow and consequently produces negligible pH changes.
Online supplemental material
Fig. S1 shows the sequence of the S4 segment in hHV1, kHV1, and EhHV1, illustrating the conserved Trp in the signature sequence that defines HV1, RxWRxxR. Fig. S2 shows saturation of the ΔpH dependence of WT kHV1 (from the dinoflagellate Karlodinium veneficum) and of the W176F mutant of kHV1. Fig. S3 shows the saturation of the ΔpH dependence of WT EhHV1 (from the coccolithophore Emiliania huxleyi) and of W278X mutants of EhHV1. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201511456/DC1.
RESULTS
The ΔpH dependence of gating in hHV1 saturates above pH 8
Perhaps the most remarkable property of HV1 is the phenomenon of ΔpH-dependent gating. We define ΔpH, the transmembrane pH gradient, as pHo − pHi. Like other voltage-gated ion channels, HV1 opens upon depolarization, but the position of the gH–V relationship is strongly and equally modulated by both pHo and pHi, shifting 40 mV for a unit change in either (Cherny et al., 1995). The set point of this relationship is positioned so that the human channel under normal conditions opens only when the electrochemical gradient is outwards. The practical consequence is that channel opening extrudes acid from the cell, which is essential to most of the functions of HV1.
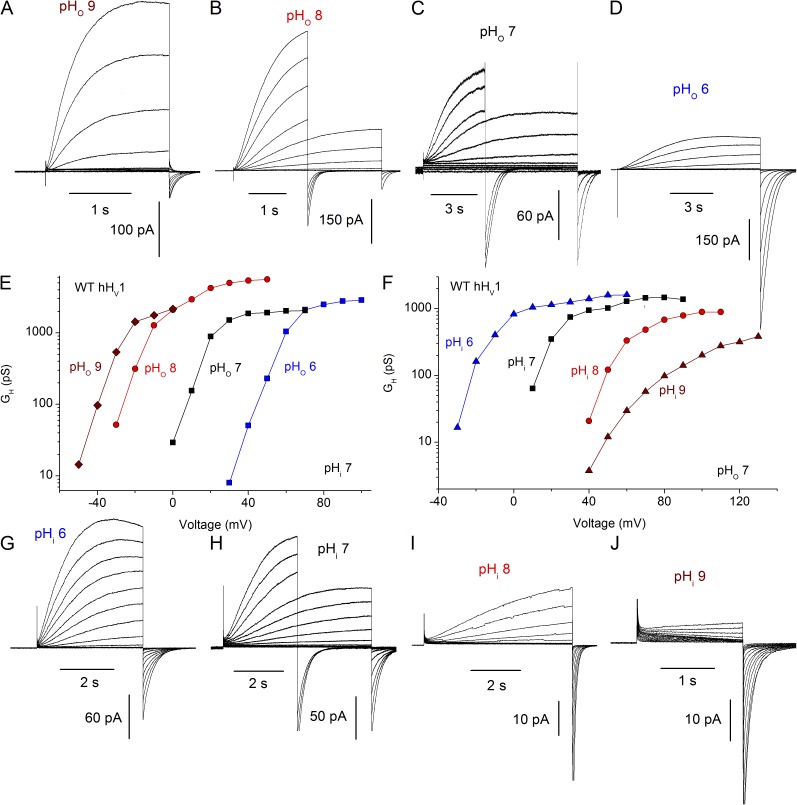
Fig. 1 illustrates ΔpH-dependent gating of the WT hHV1. Families of currents recorded in the same cell at four pHo with pHi 7 are shown in Fig. 1 (A–D). Channel opening is characteristically slow, especially at lower pHo. Examination of the corresponding gH–V (proton conductance–voltage) relationships derived from these currents reveals a −40-mV/U pH shift as pHo increases (Fig. 1 E). However, the shift between pHo 8 and pHo 9 is decidedly less than −40 mV. Saturation of the shift of the gH–V relationship has not previously been identified in WT HV1 at either high or low pHi or pHo. A <40-mV shift between pHo 8 and 9 was noted previously and proposed to reflect the approach of pHo to the pKa of a site that senses pHo (Cherny et al., 1995). This interpretation was later questioned when Vrev was found to deviate substantially from EH at extreme pHo values (pHo 9–10), and speculatively reinterpreted as reflecting loss of pHi control at high pH, perhaps because of OH– transport in rat alveolar epithelial cells (DeCoursey and Cherny, 1997). In the present study, we will show that the attenuation of ΔpH-dependent gating constitutes genuine saturation, because it occurs at a pH where the channel is unequivocally proton selective and pHi is well established (Fig. 4).
Figure 1.
The ΔpH dependence of WT hHV1 saturates at high pHo or pHi. (A–D) Families of currents at several pHo are illustrated in a COS-7 cell expressing WT hHV1 with pHi 7 at holding potentials of −80 (A), −60 (B), or −40 mV (C and D). In some families, shorter pulses were applied for large depolarizations to minimize proton depletion. (E) The gH–V relationships are plotted, showing that the shift between pHo 8 and 9 is distinctly <40 mV. Measured Vrev in A–D was −95, −53, 5, and 57 mV, respectively. (G–J) Current families in an inside-out patch are shown, with pipette pHo 7 and holding potentials of −60 (G), −40 mV (H and I), and −20 mV (J), with gh–V relationships plotted in F. Measured Vrev in G–J was −54, 0, 60, and 115 mV, respectively. For gH calculation, the current amplitude IH was obtained by fitting rising currents to a single-exponential function, with the exception of J, where because of the difficulty of fitting the slowly activating inward and outward currents, the tail current amplitude was used.
Figure 4.
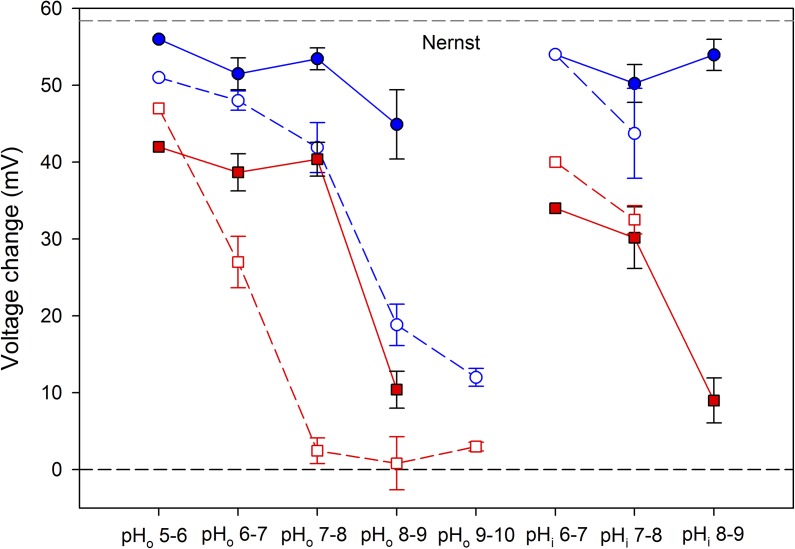
Saturation of ΔpH-dependent gating occurs independently of loss of proton selectivity of Trp207 mutants. The mean ± SEM (error bars) change in Vrev for a 1-U change in pH is plotted for WT hHV1 (closed blue circles) and for W207X (open blue circles). The shift in VgH,max/10 in the same cells is also plotted for WT hHV1 (closed red squares) and for W207X (open red squares). Numbers of cells for both parameters are one, six, eight, and five for increasing pHo in WT; one, six, and eight for increasing pHi in WT; one, five, 11, six, and three for increasing pHo in W207X; and one and four for increasing pHi in W207X. The difference in Vrev in W207X versus WT was significant at pHo 7–8 (P < 0.02) and 8–9 (P < 0.001). The difference in VgH,max/10 in W207X versus WT was significant at pHo 6–7 (P < 0.02) and 7–8 (P < 0.0001).
Fig. 1 (G–J) shows WT hHV1 currents at several pHi measured in an inside-out patch of membrane, a configuration that allows changing pHi. Again, activation (channel opening) is slow, increasingly so at high pHi where the currents become smaller, presumably reflecting the rarity of permeant ions (1 nM H+ at pH 9). Corresponding gH–V relationships plotted in Fig. 1 F exhibit a 40-mV/U shift as pHi increases, but also reveal that the shift appears to saturate between pHi 8 and pHi 9. Our standard procedure is to extrapolate single-exponential fits to obtain “steady-state” current amplitudes. Because the currents in this patch at pHi 9 were small and activation was exceptionally slow, the gH was derived from tail currents, which underestimates the gH. However, Vthreshold was the same at pHi 9 as for pHi 8, indicating a small gH–V shift. Quantitative values for these shifts are presented later (Figs. 3 and 4). Thus, saturation of ΔpH-dependent gating occurs in WT hHV1, and it occurs at roughly the same absolute pH whether internal or external pH is varied. The simplest mechanistic explanation is that ΔpH dependence is caused by titration of a site with pKa of >8 that is accessible from either side of the membrane. In the first (and only) model proposed to explain ΔpH-dependent gating (Cherny et al., 1995), the same group(s) sensed pHi and pHo but was (were) required to be accessible only to one side of the membrane at a time. The data on WT hHV1 up to this point are consistent with this idea, which invokes a single pH-sensing site that has alternating access.
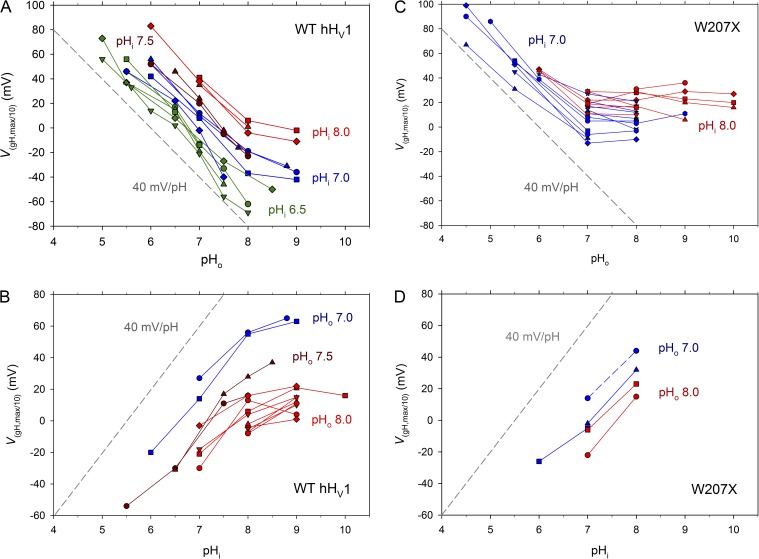
Figure 3.
Saturation of the ΔpH dependence of WT hHV1 (A and B) and of W207X mutants of hHV1 (C and D). The voltage at which gH is 10% maximal (VgH,max/10) is plotted as a function of pHo (A and C) or pHi (B and D), with lines connecting measurements in the same cell. In whole-cell measurements, pHi is color coded, as indicated. In inside-out patches, pHo is color coded, as indicated. For reference, the dashed gray line in each graph shows the slope of the ubiquitous 40-mV/U ΔpH shift in the gH–V relationship (Cherny et al., 1995; DeCoursey, 2003); the horizontal position of this line is arbitrary. (C) Data from 13 W207A, eight W207F, and two W207S cells. (D) Data from three W207A, one W207F, and one W207S patch. No differences were detected among the Trp replacements.
Mutations to Trp207 in hHV1 compromise ΔpH-dependent gating
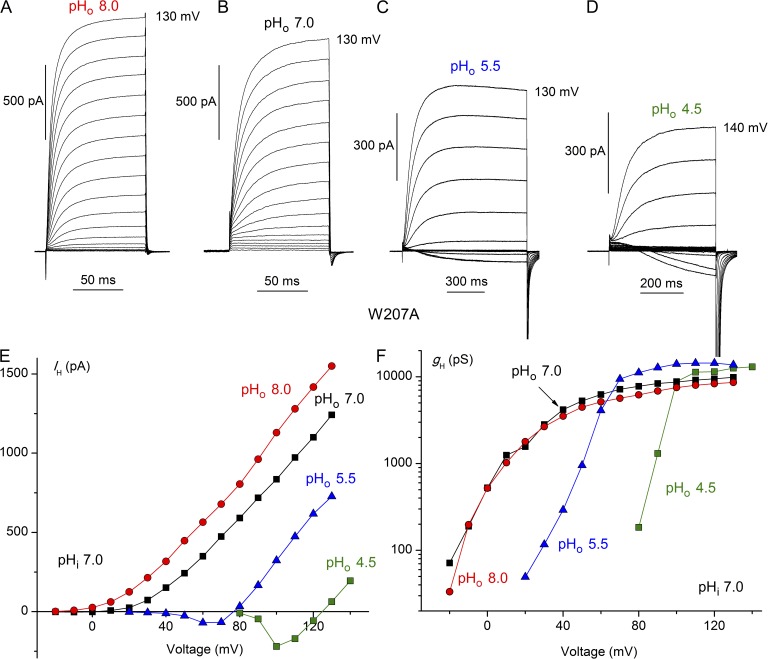
We replaced the bulky Trp207 in the human proton channel, hHV1, with Ala, Phe, or Ser, designating the mutants W207A, W207F, and W207S, respectively. All mutants generated similar voltage- and time-dependent currents. Because we could not distinguish among the properties of these three mutants, their data are combined in data summaries and termed “W207X.” Families of currents recorded in the W207A mutant at four pHo in Fig. 2 (A–D) reveal several noteworthy differences from WT hHV1. Most prominently, channel opening was two orders of magnitude faster, as will be described below (Fig. 5). Also evident is that the absolute position of the gH–V relationship tended to be more negative, resulting in pronounced inward currents at lower pHo (Fig. 2, C and D), also evident in the current–voltage relationships (Fig. 2 E). The voltage at which the gH was 10% of its maximal value, gH,max, in whole-cells and inside-out patches at symmetrical pH 7.0 was variable but averaged 9.8 ± 2.6 mV (mean ± SEM; n = 14) in WT hHV1 and −8.1 ± 3.3 mV (n = 20) in W207X mutants (P < 0.001).
Figure 2.
Modified pHo sensitivity of W207A mutant hHV1. Families of proton currents at several pHo in a cell with pHi 7.0 are illustrated (A–D). Pulses were applied in 10-mV increments up to the voltage indicated, from a holding potential of −60 (A) or −40 mV (B–D). (E) Proton current amplitudes (IH) from the cell in A–D were obtained by fitting the rising current with a single exponential and extrapolating to infinite time. (F) Proton conductance (gH) was calculated from the currents in E using Vrev measured in each solution.
Figure 5.
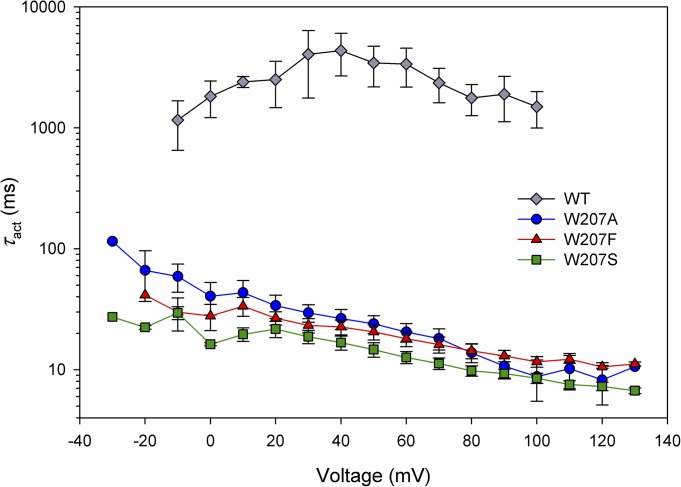
Replacement of Trp207 greatly accelerates hHV1 opening. The activation time constant, τact, was obtained by fitting rising currents to a single exponential. All measurements were done at symmetrical pH 7.0 but include both whole-cell and excised inside-out patch data. Error bars represent mean ± SEM, with n = 7, 9, and 8 for W207F, W207S, and W207A, respectively; WT includes five cells and seven inside-out patches.
More subtly, although voltage-dependent gating was shifted negatively by increases in pHo (Fig. 2, E and F), as in all known HV1, closer inspection reveals that the hallmark ΔpH dependence is altered in Trp207 mutants. When pHo was increased from 4.5 to 5.5 to 7.0, the ubiquitous 40-mV/U shift in the gH–V relationship (Cherny et al., 1995; DeCoursey, 2003) occurred (Fig. 2 F). However, above pHo 7.0, there was no further shift; thus, saturation of the shift occurred at ∼1.5 U lower pHo than in WT (Fig. 1 E). If the mechanism by which ΔpH-dependent gating occurs involves one or more titratable groups, as has been proposed (Cherny et al., 1995), then replacement of Trp207 apparently lowers the effective pKa of this group(s).
To provide a more concise and quantitative way to evaluate ΔpH dependence, in Fig. 3 we plot the voltage at which the gH is 10% of its maximal value (VgH,max/10) as a function of pH (discussed in Materials and methods). Fig. 3 (A and B) reiterates the observation from Fig. 1 that pHo and pHi dependence of gating both change with a slope of 40 mV/U (dashed reference lines in all figures) over a wide range of pH, and both saturate between pH 8 and 9 in WT hHV1. An important additional result is that Fig. 3 (A and B) indicates that the saturation with pHo or pHi occurs independently of pHi or pHo, respectively. Thus, for example, in Fig. 3 B saturation occurred similarly above pHi 8 at either pHo 7 or 8. Evidently, saturation occurs at a particular absolute pHo or pHi, rather than at a particular ΔpH. This result is consistent with the titration of one or more specific protonation sites that sense pH on only one side of the membrane. Hence, this result contradicts the simplest mechanism of a single site with alternating access to both sides of the membrane.
Analogous plots for the Trp207 mutants (Fig. 3 C) show that their pHo dependence is fully saturated at pHo ≥7.0, at least 1 U lower than in WT, with no further shift of the gH–V relationship up to pHo 10, confirming the impression from Fig. 2. Notably, the pHi dependence did not saturate up to pHi 8 (Fig. 3 D), and thus in contrast to WT, the saturating pH differs for pHo and pHi in the Trp207 mutants. This result also speaks against the idea that the same group might sense pH on both sides of the membrane (with alternating access), because in the Trp207 mutants, the pKa of the site(s) that sense pHo and pHi differ. An alternative interpretation that cannot be formally ruled out is that moving a single group to a different local environment might itself alter its pKa. In any event, the presence or absence of Trp207 evidently modulates either the accessibility of the pH-sensing site to the external solution or the effective pKa of the site(s), or both.
A different analysis of the data is shown in Fig. 4. Solid red squares show that the change in VgH,max/10 for a 1-U change in pHo or pHi in WT is roughly 40 mV, but it drops precipitously to ∼10 mV at pHo or pHi 8–9. For W207X (open red squares), the shift is already depressed at pHo 6–7 and is abolished (drops to ∼0 mV) at higher pHo (7–8, 8–9, and 9–10). In contrast, the W207X mutants exhibit no loss of ΔpH dependence up to pHi 7–8, emphasizing that the W207X mutation appears to selectively alter pHo but not pHi sensing. This result supports the idea of distinct external and internal pH sensors.
Mutations to Trp207 in hHV1 facilitate channel opening
Another distinctive consequence of replacing Trp207 was faster channel opening, evident in Fig. 2. The turn-on of current during depolarizing pulses reflects the time course of channels opening. The rising currents were fitted with a single exponential to obtain τact, the time constant of activation (channel opening). Mean τact values plotted in Fig. 5 show that channel opening was ∼100 times faster than WT for each of the Trp207 mutants. WT kinetics was more variable than that of any of the mutants, perhaps reflecting the stronger temperature sensitivity of WT HV1 (DeCoursey and Cherny, 1998; Kuno et al., 2009) or variable proton depletion during the much longer pulses required to determine WT kinetics. Surprisingly, replacement of Trp with an aliphatic hydrophobic residue (Ala), a polar hydrophilic residue (Ser), or an aromatic residue (Phe) produced identically profound acceleration of activation. The kinetic consequences of Trp at position 207 appear to be unique and unrelated to such generic qualities as hydrophobicity, polarity, or aromaticity.
Channel-closing kinetics was examined by measuring τtail, the time constant of tail current decay (deactivation). Measured at symmetrical pH 7.0 at −40 mV, τtail was 227 ± 22 ms (n = 16) in WT hHV1 and 29 times faster in the three Trp207 mutants (7.8 ± 0.8 ms; n = 17).
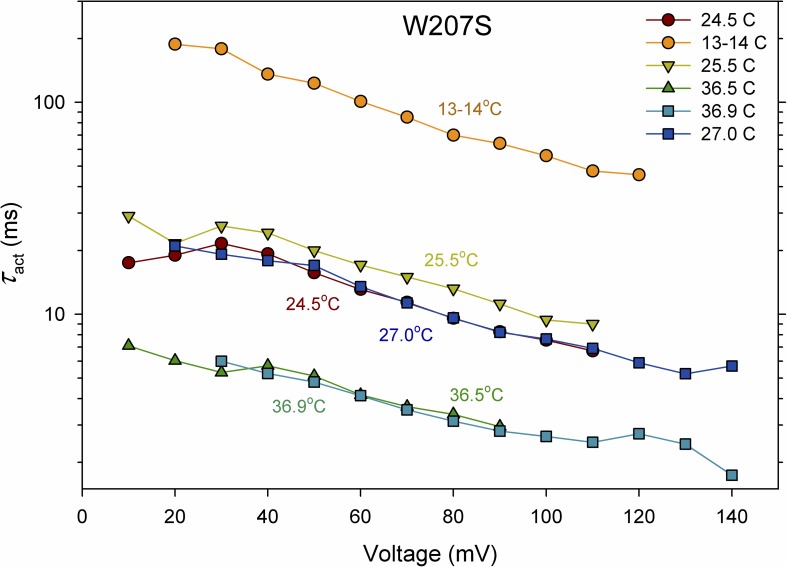
If the slowing of gating by Trp207 were rate determining in WT channels, then the activation energy, Ea for gating should be lower in mutants than the 30–38 kcal/mol in WT channels (DeCoursey and Cherny, 1998). Fig. 6 illustrates that this was the case. The time constant of channel opening (τact) was determined by fitting rising currents with a single-exponential function in current families recorded at several temperatures. The Arrhenius activation energy, Ea, was calculated from Ea = RT1T2/(T2 − T1) ln(τact,1/τact,2), where R is the gas constant (1.9872 cal K−1 mol−1) and T1 and T2 are the lower and higher temperatures (in K) (DeCoursey and Cherny, 1998). In three experiments (two whole cell and one inside-out patch), Ea determined over the entire temperature range from 11–13 to 35–37°C averaged ∼22 kcal/mol. Therefore, the process involved in slow WT activation that is regulated by Trp207 is rate limiting.
Figure 6.
Replacement of Trp207 decreases the Arrhenius activation energy for hHV1 opening. The activation time constant, τact, was determined in one cell during families of pulses at various temperatures at symmetrical pH 7. The sequence is indicated in the inset. The Q10 was somewhat higher at lower voltages (e.g., 4.3 at 20 mV and 3.7 at 100 mV, for the entire temperature range). As reported previously for native HV1 (DeCoursey and Cherny, 1998), Ea also increased at lower temperatures. Temperature drift during families of pulses was on the order of 1°C.
Mutations to Trp207 in hHV1 compromise proton selectivity
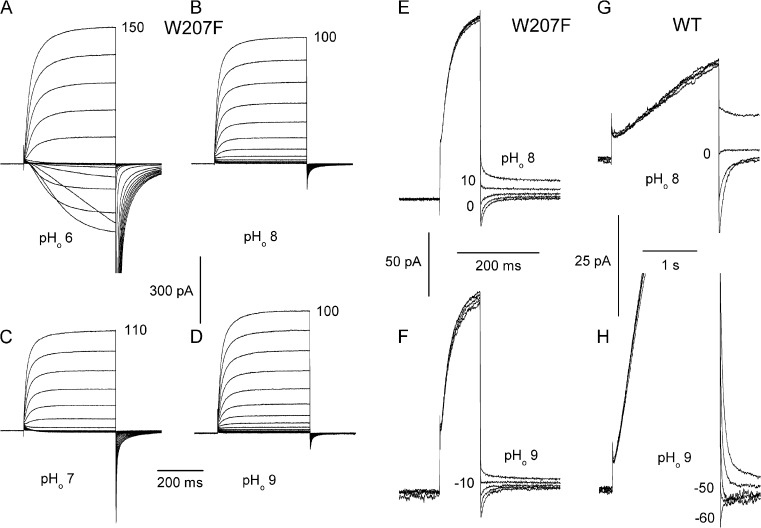
Central to performing all of the functions of HV1 is its perfect proton selectivity. Proton selectivity was evaluated by measuring the reversal potential, Vrev, at various pH. Because replacing Trp207 shifted the gH–V relationship negatively, at some ΔpH, Vrev could be observed directly as reversal of the current during families of pulses, as illustrated in Fig. 7 (A and C). Alternatively, Vrev can be estimated in the usual manner using tail currents (Fig. 7, E and F). Surprisingly, there was only a small shift in Vrev (<20 mV) between pHo 8 and 9 in the W207F mutant (Fig. 7, E and F). In comparison, the same pHo change produced a nearly Nernstian (58-mV) shift in the WT hHV1 (Fig. 7, G and H). The replacement of Trp207 compromised proton selectivity.
Figure 7.
Measurement of Vrev in W207F and in WT hHV1. (A–D) Families of currents in a cell expressing W207F channels were elicited by depolarizing pulses applied in 10-mV increments up to the voltage shown. When it is positive to Vthreshold, Vrev is easily obtained by interpolating between inward and outward currents, as in families at pHo 6 (A) or 7 (C). In the same cell at pHo 8 (B and E) and 9 (D and F), Vrev was extracted from the reversal of tail currents. In this cell, Vrev at pHo 6, 7, 8, and 9 was 102, 52, 7, and −10 mV; EH was 117, 58, 0, and −58 mV, respectively. Loss of proton selectivity is indicated by the large deviation of Vrev from EH at high pHo. In WT hHV1, the shift in Vrev obtained by tail currents at pHo 8 (G) and 9 (H) was nearly Nernstian. Both cells in this figure contained pHi 8 solutions. The holding potential was −20 (G), −30 (C), −40 (A, B, E, and H), or −60 mV (D and F). Prepulses were to 60 (E and F), 20 (G), or 10 mV (H).
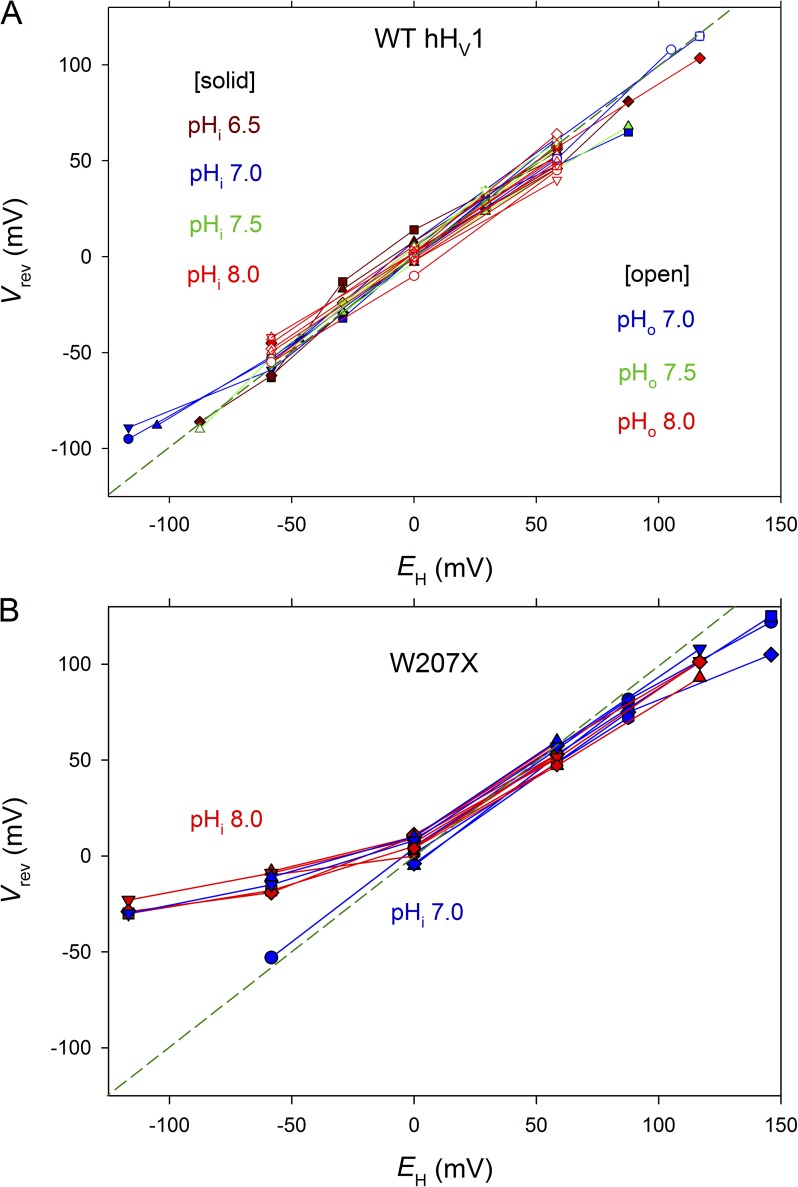
Fig. 8 A confirms the proton specificity of the WT hHV1. The reversal potential, Vrev, measured over a wide range of pHo (5.0–9.0) and pHi (5.5–9.0) was close to the Nernst potential, EH, shown as a dashed line. Surprisingly, Trp207 mutants were imperfectly proton selective. Fig. 8 B shows that although they were highly selective at neutral and acidic pH, at high pHo (8–10), the measured reversal potential, Vrev, deviated consistently and substantially from EH.
Figure 8.
Proton selectivity is perfect in WT hHV1 (A) but compromised in Trp207 mutants (B). Proton selectivity is indicated by the proximity of measured values of Vrev and the Nernst potential for H+, EH. Data were obtained in whole-cell (closed symbols) and inside-out patch configuration (open symbols). In whole-cell measurements, the color-coded pHi solution was in the pipette, and pHo was varied, with lines connecting measurements in each cell. In inside-out patch measurements, pHo was the pipette solution and pHi was varied. Whole-cell measurements in B include eight W207F, 12 W207A, and one W207S cell. For W207X mutants, currents in patches were too small to allow reliable estimation of Vrev.
The mean shift in Vrev for a 1-U change in pH is plotted in Fig. 4 (blue circles). The Vrev of WT hHV1 (closed blue circles) was reasonably near EH at all pH studied, whereas Vrev of the W207X mutants (open blue circles) was distinctly sub-Nernstian at pHo 8–10, being significantly lower than WT at pHo 7–8 and 8–9. Trp207 mutation produces loss of proton selectivity, but only at high pHo.
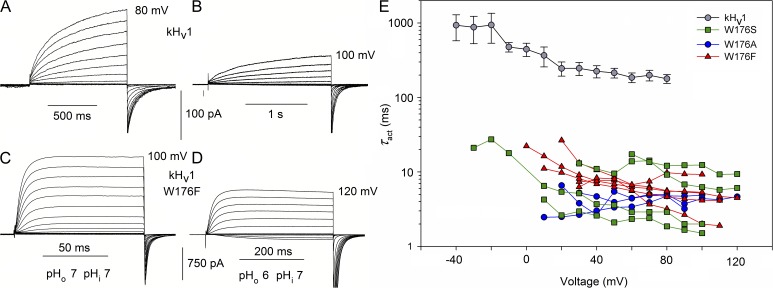
Replacement of Trp in a dinoflagellate HV1 (kHV1) speeds activation
Given that replacing Trp greatly speeds activation in hHV1, we were curious as to whether the same would be true in other species. To make this test rigorous, we selected an evolutionarily distant species in which the amino acid identity with hHV1 is only 15%, namely K. veneficum (Smith et al., 2011). We made the same three substitutions to the corresponding Trp176 in kHV1 (Fig. S1): W176A, W176F, and W176S. These mutants generated proton-selective currents in the pH range explored that exhibited qualitatively similar changes when pHo was changed (Fig. 9, A vs. B, and C vs. D). Like their human counterparts, the Trp mutants activated extremely rapidly. Activation time constants were nearly two orders of magnitude faster than in the WT channel (Fig. 9 E). WT closing kinetics was faster in kHV1 than in hHV1, and in the Trp176 mutants, tail current decay was often too fast to distinguish reliably from capacity transients. Mirroring the pattern seen in the human Trp mutants, τact was roughly the same whether Trp176 was replaced by Ser, Ala, or Phe (Fig. 9 E). The same remarkable result obtained in both species is that Trp in the signature sequence (RxWRxxR) profoundly slows channel opening by a mechanism that other amino acids tested are unable to replicate. This result suggests a quite specific type of interaction.
Figure 9.
The W176F mutation in the dinoflagellate kHV1 accelerates channel opening. Families of currents in WT kHv1 (A and B) and in the W176F mutant (C and D), studied at pHo 7 (A and C) or 6 (B and D), as indicated, all at pHi 7. Note the different time scales. Voltage steps were applied in 10-mV increments to the final voltage indicated. The holding potential was −60 (A and C) or −40 mV (B and D). (E) Replacement of Trp176 in the dinoflagellate kHV1 with Ala, Ser, or Phe hastens channel opening to the same extent. The activation time constant, τact, was determined by fitting the turn-on of current with a single rising exponential. The WT channel kinetics (mean ± SEM; n = 5–14) was extracted from data for a previous study, all at symmetrical pH 7.0 (Smith et al., 2011). Single mutants, as indicated, were expressed in HEK-293 or COS-7 cells. Measurements were performed in whole-cell configuration at symmetrical pH 7. Data from each cell are connected by lines.
The kHV1 channel does exhibit ΔpH-dependent gating, although its absolute voltage range of opening is 60 mV more negative than in other species (Smith et al., 2011). Therefore, it was of interest to determine whether saturation of this effect occurs. In WT kHV1, saturation was observed above pHo 8.0 or pHi 8.0 (Fig. S2), similar to the pH at which saturation occurs in WT hHV1. Also like hHV1, Trp mutation compromised ΔpH dependence, with saturation occurring at lower pHo in the W176F mutant (Fig. S2 C).
Replacement of Trp in a coccolithophore HV1 (EhHV1) speeds activation and shifts the gH–V relationship negatively
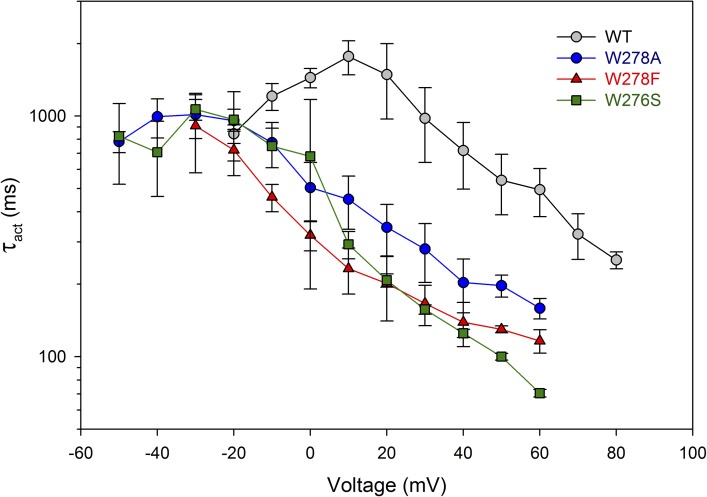
To further test the generality of the roles of Trp, we produced analogous mutations in the coccolithophore, E. huxleyi HV1 (EhHV1), namely W278A, W278S, and W278F (Fig. S1). The EhHV1 sequence differs drastically from hHV1, with only 18% identity, as well as from kHV1, with 29% identity. Fig. 10 shows that activation kinetics was also faster in the EhHV1 when Trp278 was replaced, although the effect was smaller than in the other species examined. Opening of these mutants was accelerated four- to sixfold in the positive voltage range.
Figure 10.
Replacement of Trp278 in the coccolithophore EhHV1 with Ala, Ser, or Phe hastens channel opening to the same extent. The activation time constant, τact, was determined by fitting the turn-on of current with a single rising exponential. The mean ratio of WT/W278X within the range of 10–60 mV was 3.5 (W278A), 5.7 (W278F), and 6.3 (W278S). Numbers of cells are: WT, three to six; W278A, three to four; W278F; two to four; W278S, two to three. Single mutants were expressed in HEK-293 or COS-7 cells, and measurements were performed in whole-cell configuration at symmetrical pH 7. Error bars represent mean ± SEM.
Another pronounced change in EhHV1 mutants was a negative shift of the gH–V relationship, with the voltage at which the gH was 10% of gH,max averaging −5.2 ± 2.9 (12) in WT EhHV1 and −33.4 ± 2.3 (14) in the mutants, a −28-mV shift. Some of the slowing of τact may be ascribable to this shift; the peak value of τact occurs in a more negative voltage range in W278X than in WT.
Intriguingly, the ΔpH dependence of EhHV1 appeared consistently steeper (∼50 mV/U pH) both for changes in pHo and pHi than in HV1 in other species (Fig. S3). Saturation of ΔpH dependence was evident only above pHo 8.0 in WT, essentially identical to both hHV1 and kHV1. No significant change in this property was detected in the EhHV1 mutants, although the mean shift from pHo 7 to pHo 8 decreased from −53 ± 2 mV (SEM; n = 4) in WT to −43 ± 5 mV (n = 4) in mutants. The ΔpH dependence of EhHV1 would thus be maintained reasonably well over the normal pH range experienced by coccolithophores; the pH of seawater is 7.5–8.4 (Chester and Jickells, 2012). That saturation of ΔpH-dependent gating occurred at the same pHo and pHi in all three species suggests that the same or similar group(s) may be involved in pH sensing.
DISCUSSION
Activation kinetics
Cation–π interaction between Trp207 and Arg211 in hHV1 latches the channel closed.
The most obvious effect of replacing Trp207 with smaller amino acids was acceleration of channel opening by two orders of magnitude. The precise physical mechanism by which Trp slows WT HV1 opening can only be speculated, but several possibilities exist. In the closed mHV1 structure (78% sequence identity to hHV1), Trp is oriented toward the lipid (Takeshita et al., 2014), suggesting that hydrophobic interactions might stabilize it in this position. Hydrophobic interactions with membrane lipids have been considered for closed-state stabilization by Val363 in the Shaker K+ channel VSD (Lacroix et al., 2013). However, the aromatic Phe, which engages in purely hydrophobic interactions (Killian and von Heijne, 2000), does not share this behavior in HV1. In all three species studied here, the Trp→Phe mutant did not exhibit intermediate behavior but rather activated as rapidly as Trp→Ser or Trp→Ala mutants. This result suggests that the ambivalence of Trp in having a polar amide group plays a key role. As discussed in the Introduction, the mHV1 structure (Takeshita et al., 2014) appears to indicate that Trp engages in cation–π interaction with R3, stabilizing the closed structure. In fact, Trp207 and Arg211 are an example of the second most common cation–π interaction that occurs within α helices between residues i and (i + 4) (Gallivan and Dougherty, 1999).
The portion of mHV1 containing Trp and R3 is compared with the corresponding region in the Ciona intestinalis voltage-sensing phosphatase (CiVSP) in Fig. 11, with both proteins considered to be in their closed or “down” position. It seems surprising that in both structures, R3 appears to lack direct aqueous contact. Burying acidic or basic amino acids can shift their pKa by as much as 5 pH units, but Arg is the most likely to remain ionized (Kim et al., 2005). In fact, Arg appears uniquely able to remain protonated inside proteins (Harms et al., 2011). In the down structure of CiVSP, R3 is found in a pocket surrounded by hydrophobic residues that faces away from the center of the VSD; the guanidinium group of R3 is oriented somewhat “sideways” to the plane of the lipid and it salt-bridges with Asp186 (Fig. 11 A). In the mHV1 structure (Fig. 11 B), Trp203 appears to provide part of a similar pocket in which R3 interacts both with Asp170 (equivalent to Asp174 in hHv1 and Asp186 in CiVSP) and with Asn210, although in this structure the guanidinium of R3 appears to be pointing nearly directly away from the center of the channel (Fig. 11 B). We speculate that Phe may be unable to stabilize R3 in this position, possibly because of Phe’s weaker cation–π interaction capability. The electrostatic R3–Asp interaction in closed HV1 may also contribute a stabilizing function like that of the corresponding Lys374–Asp316 in the closed Shaker K+ channel VSD (Papazian et al., 1995). In all open HV1 models (Ramsey et al., 2010; Wood et al., 2012; Kulleperuma et al., 2013; Chamberlin et al., 2014), R3 has rotated to face the pore, whereas Trp still appears to face the lipid. For this kind of conformational change to occur during opening, the interactions between the Trp–Arg pair would have to be disrupted. The much more rapid opening in the W207X mutants may reflect the absence of these stabilizing interactions.
Figure 11.
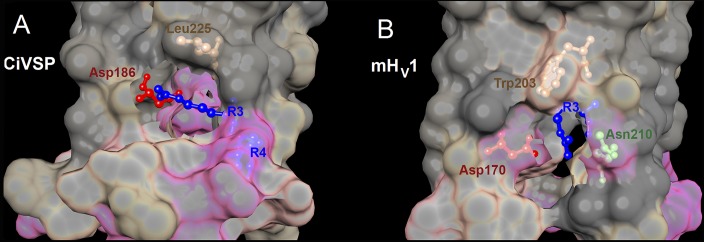
Crystal structures of CiVSP in the “down” state and mHV1 chimera in the closed state reveal pockets that enclose electrostatic interactions involving R3. The crystal structures of the down state of CiVSP (A) (Li et al., 2014) and the closed mHV1 chimera (B) (Takeshita et al., 2014) were superimposed with the same orientation. The top is toward the extracellular surface, and the view is from the side. The interfacial surface between protein and lipid is cut away to show the pocket containing R3 and its interacting partners. Local hydrophobicity is indicated by gray (hydrophobic), tan (intermediate), and pink (hydrophilic). (A) In the down structure of CiVSP (Protein Data Bank [PDB] accession no. 4G80), which is more closely related to HV1 phylogenetically than is the VSD of other ion channels (Smith et al., 2011), R3 faces away from the center of the VSD. The guanidinium group of R3 forms a salt-bridge with Asp186. (B) In the closed structure of mHV1 (PDB accession no. 3WKV), Trp203 and R3 both face away from the center of the channel; the guanidinium of R3 appears to point nearly directly away from the center of the channel. In the mHV1 structure, Trp203 forms the roof of the pocket, in which R3 interacts both with Asp170 (equivalent to Asp174 in hHv1 and Asp186 in CiVSP) and with Asn210. Molecular graphics and analyses were performed with the University of California, San Francisco (UCSF), Chimera package (resource for Biocomputing, Visualization, and Informatics, UCSF, San Francisco; supported by NIGMS P41-GM103311) (Pettersen et al., 2004).
The faster activation kinetics after Trp mutation in hHV1 qualitatively resembles that seen in forced monomerization. Monomeric constructs open four to seven times faster than their dimeric counterparts (Koch et al., 2008; Tombola et al., 2008; Musset et al., 2010; Fujiwara et al., 2012). Might Trp mutation eliminate interaction at the dimer interface between Trps from each protomer that normally contribute to closed-state stabilization? This notion is contradicted by a cysteine cross-linking study indicating that the two S4 helices appear not to interact (Lee et al., 2008). On the other hand, the S4 segments are close to each other in a proposed dimer model based on the closed structure of mHV1 (Takeshita et al., 2014).
Is it possible that the primary reason for the conservation of Trp207 is to slow gating?
It is not immediately obvious why slow HV1 activation would be evolutionarily advantageous. On the other hand, rapid opening would not confer any advantage for most of the functions proposed for HV1 in mammalian cells. For example, HV1 is activated in phagocytes to compensate for the electrogenic activity NADPH oxidase (Henderson et al., 1987; DeCoursey et al., 2003); the latter enzyme is turned on by most stimuli only after a delay and on a time scale of seconds (DeCoursey and Ligeti, 2005). In cells in which acid extrusion via HV1 occurs for the purpose of pH homeostasis or signaling, such as airway epithelia (Fischer, 2012), basophils (Musset et al., 2008b), B lymphocytes (Capasso et al., 2010), neutrophils (Morgan et al., 2009), or sperm (Lishko et al., 2010), rapid opening is less important than simply remaining open as long as necessary. The absence of inactivation is thus arguably more critical than rapid activation would be. Another possibility is that because a single HVCN1 gene codes for proton channels in a multiplicity of cell types in which HV1 serves diverse purposes, the properties of the protein must be compatible with the physiology of all cells. An excitable cell might be ill-served by a rapidly activating proton channel that could interfere with the action potential.
Trp207 in hHV1 anchors the S4 segment and stabilizes the closed channel.
By retarding channel opening by 100-fold, while slowing closing only 29-fold, Trp207 might tend to produce a net stabilization of the closed state. In fact, Trp207 mutants did activate at voltages 18 mV more negative than WT hHV1; therefore Trp207 does contribute to stabilizing a closed state. Native proton channels open almost exclusively positive to EH and thus never produce significant inward current (DeCoursey, 2003). The negative shift of the gH–V relationship in Trp207 mutants resulted in distinct inward currents just above Vthreshold, especially with inward H+ gradients (e.g., Figs. 2, C–E, and 7, A and C). Substitution of Trp207 thus subverts this principal feature of HV1 and compromises the task of proton extrusion. One could speculate that Trp207 fine tunes the voltage dependence of HV1 to prevent premature opening. Because of the proximity of Vthreshold and Vrev in WT HV1 (Cherny et al., 1995; Musset et al., 2008a), without the stabilization of the closed state by Trp207, channel opening would result in proton influx. Given that mammalian cells are largely concerned with extrusion of metabolically produced acid (Roos and Boron, 1981), such a propensity would be deleterious to cell homeostasis.
In EhHV1, Trp also decidedly stabilized the closed state; the gH–V relationship was 28 mV more negative in W278X mutants than in WT. In contrast, W176X mutants in kHV1 activated 25 mV more positively than WT. The atypical behavior of kHV1 in this regard may reflect distinct teleological considerations. EhHV1 exists to extrude acid (Taylor et al., 2011), and like hHV1, must therefore be poised to open just above EH. In contrast, kHV1 activates 60 mV more negatively than HV1 in any other species (Smith et al., 2011), which is ideal for its quite different function in dinoflagellates of mediating H+ influx that triggers the flash in bioluminescent species (Fogel and Hastings, 1972). The molecular mechanism responsible for the unique kHV1 voltage dependence is unknown.
Temperature dependence
The gating kinetics of HV1 in several mammalian cells is extraordinarily temperature dependent, with Q10 values of 6–9 (Ea for the delay, τact, and τtail were identically 30–38 kcal/mol) (DeCoursey and Cherny, 1998). The Arrhenius activation energy, Ea, of channel opening (τact) of W207S was 20–25 kcal/mol (Q10 of 3.5–4.0), distinctly smaller than in WT, indicating that the factors that establish the kinetics in W-free HV1 have more modest Ea. Evidently, the perfectly conserved Trp207 is the dominant contributor to the exotic temperature sensitivity of WT hHV1, and replacing it lowers the Q10 of gating into the range of most ordinary ion channels; two dozen examples are given in Table II of DeCoursey and Cherny (1998). That Trp is involved in the rate-limiting step in HV1 opening is consistent with the idea that opening a closed HV1 requires disrupting the cation–π interactions between Trp and Arg, and perhaps also inter-protomer Trp–Trp interactions in the dimer that stabilize closed channels.
The gating kinetics of hHV1 is much slower than that of many voltage-gated channels; removing Trp eliminates this distinctive property as well. In WT hHV1, the activation time constant, τact, is in the range of seconds at room temperature, but it plummets into the low millisecond range in Trp207 mutants. Thus, in terms of both channel-opening kinetics and temperature dependence, the effect of removing Trp207 is like Kryptonite, turning a Super-channel into an ordinary mortal channel.
Proton selectivity
Over a wide range of pH, the WT hHV1 appears to be perfectly selective. In a previous study, WT HV1 appeared to lose selectivity between pH 9 and 10 (DeCoursey and Cherny, 1997); the present study extended only up to pH 9. The Trp207 mutants, however, lost selectivity at less extreme pH, beginning at pHo 8 (Figs. 4, 7, E and F, and 8 B). It is unlikely that this loss of proton selectivity reflects a direct participation of Trp in the selectivity mechanism. It is well established that an Asp in the S1 helix is crucial to proton selectivity (Musset et al., 2011; Smith et al., 2011). This Asp can be relocated from position 112 to 116 in hHV1 without loss of proton selectivity, but charge compensation by interaction with one or more S4 Arg appears essential (Morgan et al., 2013). Recently, quantum mechanical calculations demonstrated an explicit mechanism by which Asp–Arg interaction is sufficient to produce proton-selective conduction without requiring contribution from the rest of the protein beyond providing a scaffold and focusing aqueous access to the selectivity filter (Dudev et al., 2015). If Trp helps anchor the S4 helix in the membrane, its removal may allow sufficient intramolecular movement to disrupt the Asp–Arg interaction that is critical to proton-selective conduction. That the loss of selectivity manifests only at high pHo may reflect deprotonation of a cationic group that stabilizes the open channel.
Trp207 is essential for normal ΔpH-dependent gating
Perhaps the most striking consequence of Trp mutation is the weakening of ΔpH-dependent gating, a quintessential feature that provides the basis for HV1 function in all cells. In all species, and even among all known HV1 mutations described to date (Ramsey et al., 2010; Musset et al., 2011; DeCoursey, 2013), the gH–V relationship shifts a roughly 40-mV/U change in ΔpH over a wide range of pHo and pHi. With the single exception of the dinoflagellate K. veneficum (Smith et al., 2011), this behavior results in HV1 opening only when the electrochemical gradient is outwards, so that HV1 extrudes acid. The mechanism of ΔpH-dependent gating remains one of the most elusive unsolved mysteries regarding HV1. The first and only explicit model of ΔpH-dependent gating (but see Villalba-Galea, 2014) postulated titratable sites that were alternatively accessible to external or internal solutions (Cherny et al., 1995). A systematic attempt to identify which site(s) was (were) involved revealed no single ionizable residue whose mutation to a non-ionizable residue abolished this phenomenon (Ramsey et al., 2010). Rather than protonation of a site, interaction of protonated water with the Arg residues in the S4 helix was suggested to effect ΔpH-dependent gating (Ramsey et al., 2010). The possibility remained that multiple titratable sites are involved, an appealing idea because of the wide pH range over which the gH–V relationship shifts according to the 40-mV rule. If one or more titratable sites are involved, the effect of pH might be expected to saturate. We demonstrate here that saturation of ΔpH-dependent gating does occur above pH 8.0 in WT hHV1 (Fig. 3), consistent with previous observations in native rat proton currents (Cherny et al., 1995). Correspondingly, the regulatory sites proposed in our model were assigned a pKa of 8.5, which was assumed to be same for access from internal or external solutions (Cherny et al., 1995).
Although distinct ΔpH-dependent gating persists in hHV1 Trp207 mutants, the pH at which saturation occurred dropped to pHo 7 but did not change for pHi. Evidently, mutation of Trp207 lowers the apparent pKa of the putative external site(s) without changing internal pH sensing. Despite not being titratable itself, Trp is evidently a key component in this mechanism, and may regulate the accessibility of another pH-sensing site, evidently increasing its effective pKa. Because the same kind of pKa shift occurs in both kHV1 and hHV1, the putative titratable group(s) may be conserved in these species. In the closed mHV1 crystal structure (Takeshita et al., 2014) Arg207 (R3) is twisted away from the pore into a hydrophobic pocket of the protein. If R3 is directed away from the aqueous pore, its pKa may be decreased substantially (Kim et al., 2005) and could thus be a candidate pH regulatory site. However, mutation of R3 did not eliminate ΔpH-dependent gating (Ramsey et al., 2010). Furthermore, experimental evidence indicates that Arg remains charged even inside proteins (Harms et al., 2011). Rather than viewing these effects as a change in effective pKa, it is equally possible that mutation might alter the accessibility to protons of a site buried within the membrane electric field, by changing the effective dielectric constant in the pathway or the voltage drop to reach the site.
The results of this study indicate that both external and internal pH sensing may be accomplished by titratable groups with similar effective pKa in WT hHV1 and in two unicellular marine species: kHV1 and EhHV1. Furthermore, because the Trp207 mutation in hHV1 (or the Trp176 mutation in kHV1) selectively lowered the apparent pKa for external but not internal pH, there appear to be distinct internal and external pH-sensing sites. This result speaks against the idea that a single site might alternatively sense pHo and pHi (Cherny et al., 1995), and indicates that distinct internal and external sensors (that must nevertheless interact with each other) are involved.
The kinetics of ion channel gating is often critical to their specialized function. The activation kinetics of voltage-gated Na+ and K+ channels is tunable by specific residues in S2 and S4 transmembrane segments (Lacroix et al., 2013). Here, we show that the highly conserved tryptophan residue in the S4 signature sequence of HV1 (RxWRxxR) is responsible for the characteristically slow kinetics of hHV1. By stabilizing the closed state and optimizing ΔpH sensing, Trp fine-tunes the threshold for channel opening, which in most species is just positive to EH. Trp thus acts as a mechanism for adjusting the ΔpH-dependent gating that is prerequisite to the functions of HV1 in all species.
Supplementary Material
Acknowledgments
We appreciate the generous gift of the EhHV1 construct by Alison Taylor (University of North Carolina, Chapel Hill, NC) and Colin Brownlee and Glen Wheeler (Marine Biological Association of the UK, The Laboratory, Plymouth, UK). The authors appreciate helpful discussions with Artem Ayuyan (Rush University, Chicago, IL) and Valerij Sokolov (Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia).
This work is supported by US National Science Foundation award MCB-0943362 and US National Institutes of Health (NIH) grant GM102336 (to T.E. DeCoursey and S.M.E. Smith). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors declare no competing financial interests.
Merritt C. Maduke served as editor.
Note added in proof. A recent EPR spectroscopy study of hHV1 (Li et al. 2015. The resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl. Acad. Sci. USA. In press) showed that the dimer interface includes the top of S1 and the lower part of S4.
Footnotes
Abbreviations used in this paper:
- CiVSP
- Ciona intestinalis voltage-sensing phosphatase
- hHV1
- human voltage-gated proton channel
- HV1
- voltage-gated proton channel
- VSD
- voltage-sensing domain
References
- Burley S.K., and Petsko G.A.. 1986. Amino-aromatic interactions in proteins. FEBS Lett. 203:139–143. 10.1016/0014-5793(86)80730-X [DOI] [PubMed] [Google Scholar]
- Capasso M., Bhamrah M.K., Henley T., Boyd R.S., Langlais C., Cain K., Dinsdale D., Pulford K., Khan M., Musset B., et al. 2010. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 11:265–272. 10.1038/ni.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin A., Qiu F., Rebolledo S., Wang Y., Noskov S.Y., and Larsson H.P.. 2014. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 111:E273–E282. 10.1073/pnas.1318018111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny V.V., Markin V.S., and DeCoursey T.E.. 1995. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105:861–896. 10.1085/jgp.105.6.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester R., and Jickells T.D.. 2012. Marine Geochemistry. Vol. Third edition Wiley-Blackwell, Hoboken, NJ: 420 pp 10.1002/9781118349083 [DOI] [Google Scholar]
- DeCoursey T.E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83:475–579. 10.1152/physrev.00028.2002 [DOI] [PubMed] [Google Scholar]
- DeCoursey T.E. 2010. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda). 25:27–40. 10.1152/physiol.00039.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E. 2013. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the HV family. Physiol. Rev. 93:599–652. 10.1152/physrev.00011.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., and Cherny V.V.. 1997. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J. Gen. Physiol. 109:415–434. 10.1085/jgp.109.4.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., and Cherny V.V.. 1998. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J. Gen. Physiol. 112:503–522. 10.1085/jgp.112.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., and Ligeti E.. 2005. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 62:2173–2193. 10.1007/s00018-005-5177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E., Morgan D., and Cherny V.V.. 2003. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 422:531–534. 10.1038/nature01523 [DOI] [PubMed] [Google Scholar]
- Dudev T., Musset B., Morgan D., Cherny V.V., Smith S.M.E., Mazmanian K., DeCoursey T.E., and Lim C.. 2015. Selectivity mechanism of the voltage-gated proton channel, HV1. Sci Rep. 5:10320 10.1038/srep10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. 2012. Function of proton channels in lung epithelia. Wiley Interdiscip Rev Membr Transp Signal. 1:247–258. 10.1002/wmts.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., and Hastings J.W.. 1972. Bioluminescence: mechanism and mode of control of scintillon activity. Proc. Natl. Acad. Sci. USA. 69:690–693. 10.1073/pnas.69.3.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Kurokawa T., Takeshita K., Kobayashi M., Okochi Y., Nakagawa A., and Okamura Y.. 2012. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat. Commun. 3:816 10.1038/ncomms1823 [DOI] [PubMed] [Google Scholar]
- Gallivan J.P., and Dougherty D.A.. 1999. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA. 96:9459–9464. 10.1073/pnas.96.17.9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M.J., Schlessman J.L., Sue G.R., and García-Moreno B.. 2011. Arginine residues at internal positions in a protein are always charged. Proc. Natl. Acad. Sci. USA. 108:18954–18959. 10.1073/pnas.1104808108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M., Chappell J.B., and Jones O.T.G.. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329. 10.1042/bj2460325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E., Brown M.A., Musset B., Morgan D., Cherny V.V., Taubert C., Bhamrah M.K., Coe D., Marelli-Berg F., Gribben J.G., et al. 2014. Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells. Proc. Natl. Acad. Sci. USA. 111:18078–18083. 10.1073/pnas.1411390111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovannisci D., Illek B., and Fischer H.. 2010. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 136:35–46. 10.1085/jgp.200910379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian J.A., and von Heijne G.. 2000. How proteins adapt to a membrane-water interface. Trends Biochem. Sci. 25:429–434. 10.1016/S0968-0004(00)01626-1 [DOI] [PubMed] [Google Scholar]
- Kim J., Mao J., and Gunner M.R.. 2005. Are acidic and basic groups in buried proteins predicted to be ionized? J. Mol. Biol. 348:1283–1298. 10.1016/j.jmb.2005.03.051 [DOI] [PubMed] [Google Scholar]
- Koch H.P., Kurokawa T., Okochi Y., Sasaki M., Okamura Y., and Larsson H.P.. 2008. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 105:9111–9116. 10.1073/pnas.0801553105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulleperuma K., Smith S.M.E., Morgan D., Musset B., Holyoake J., Chakrabarti N., Cherny V.V., DeCoursey T.E., and Pomès R.. 2013. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 141:445–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Ando H., Morihata H., Sakai H., Mori H., Sawada M., and Oiki S.. 2009. Temperature dependence of proton permeation through a voltage-gated proton channel. J. Gen. Physiol. 134:191–205. 10.1085/jgp.200910213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J.J., Campos F.V., Frezza L., and Bezanilla F.. 2013. Molecular bases for the asynchronous activation of sodium and potassium channels required for nerve impulse generation. Neuron. 79:651–657. 10.1016/j.neuron.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Letts J.A., and Mackinnon R.. 2008. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA. 105:7692–7695. 10.1073/pnas.0803277105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wanderling S., Paduch M., Medovoy D., Singharoy A., McGreevy R., Villalba-Galea C.A., Hulse R.E., Roux B., Schulten K., et al. 2014. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21:244–252. 10.1038/nsmb.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko P.V., Botchkina I.L., Fedorenko A., and Kirichok Y.. 2010. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 140:327–337. 10.1016/j.cell.2009.12.053 [DOI] [PubMed] [Google Scholar]
- MacCallum J.L., Bennett W.F.D., and Tieleman D.P.. 2008. Distribution of amino acids in a lipid bilayer from computer simulations. Biophys. J. 94:3393–3404. 10.1529/biophysj.107.112805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., and DeCoursey T.E.. 2014. Analysis of electrophysiological properties and responses of neutrophils. Methods Mol. Biol. 1124:121–158. 10.1007/978-1-62703-845-4_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Capasso M., Musset B., Cherny V.V., Ríos E., Dyer M.J.S., and DeCoursey T.E.. 2009. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. USA. 106:18022–18027. 10.1073/pnas.0905565106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Musset B., Kulleperuma K., Smith S.M.E., Rajan S., Cherny V.V., Pomès R., and DeCoursey T.E.. 2013. Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 142:625–640. 10.1085/jgp.201311045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Cherny V.V., Morgan D., Okamura Y., Ramsey I.S., Clapham D.E., and DeCoursey T.E.. 2008a. Detailed comparison of expressed and native voltage-gated proton channel currents. J. Physiol. 586:2477–2486. 10.1113/jphysiol.2007.149427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Morgan D., Cherny V.V., MacGlashan D.W. Jr., Thomas L.L., Ríos E., and DeCoursey T.E.. 2008b. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc. Natl. Acad. Sci. USA. 105:11020–11025. 10.1073/pnas.0800886105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Smith S.M.E., Rajan S., Cherny V.V., Morgan D., and DeCoursey T.E.. 2010. Oligomerization of the voltage-gated proton channel. Channels (Austin). 4:260–265. 10.4161/chan.4.4.12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Smith S.M.E., Rajan S., Morgan D., Cherny V.V., and DeCoursey T.E.. 2011. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature. 480:273–277. 10.1038/nature10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B., Clark R.A., DeCoursey T.E., Petheo G.L., Geiszt M., Chen Y., Cornell J.E., Eddy C.A., Brzyski R.G., and El Jamali A.. 2012. NOX5 in human spermatozoa: expression, function, and regulation. J. Biol. Chem. 287:9376–9388. 10.1074/jbc.M111.314955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D.M., Shao X.M., Seoh S.A., Mock A.F., Huang Y., and Wainstock D.H.. 1995. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 14:1293–1301. 10.1016/0896-6273(95)90276-7 [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., and Ferrin T.E.. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Ramsey I.S., Moran M.M., Chong J.A., and Clapham D.E.. 2006. A voltage-gated proton-selective channel lacking the pore domain. Nature. 440:1213–1216. 10.1038/nature04700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey I.S., Mokrab Y., Carvacho I., Sands Z.A., Sansom M.S.P., and Clapham D.E.. 2010. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17:869–875. 10.1038/nsmb.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., and Boron W.F.. 1981. Intracellular pH. Physiol. Rev. 61:296–434. [DOI] [PubMed] [Google Scholar]
- Santiveri C.M., and Jiménez M.A.. 2010. Tryptophan residues: scarce in proteins but strong stabilizers of β-hairpin peptides. Biopolymers. 94:779–790. 10.1002/bip.21436 [DOI] [PubMed] [Google Scholar]
- Smith S.M.E., Morgan D., Musset B., Cherny V.V., Place A.R., Hastings J.W., and DeCoursey T.E.. 2011. Voltage-gated proton channel in a dinoflagellate. Proc. Natl. Acad. Sci. USA. 108:18162–18167. 10.1073/pnas.1115405108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Sakata S., Yamashita E., Fujiwara Y., Kawanabe A., Kurokawa T., Okochi Y., Matsuda M., Narita H., Okamura Y., and Nakagawa A.. 2014. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21:352–357. 10.1038/nsmb.2783 [DOI] [PubMed] [Google Scholar]
- Tatko C.D., and Waters M.L.. 2003. The geometry and efficacy of cation-π interactions in a diagonal position of a designed β-hairpin. Protein Sci. 12:2443–2452. 10.1110/ps.03284003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.R., Chrachri A., Wheeler G., Goddard H., and Brownlee C.. 2011. A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol. 9:e1001085 10.1371/journal.pbio.1001085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F., Ulbrich M.H., and Isacoff E.Y.. 2008. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 58:546–556. 10.1016/j.neuron.2008.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A. 2014. Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 107:1564–1572. 10.1016/j.bpj.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li S.J., Wu X., Che Y., and Li Q.. 2012. Clinicopathological and biological significance of human voltage-gated proton channel Hv1 protein overexpression in breast cancer. J. Biol. Chem. 287:13877–13888. 10.1074/jbc.M112.345280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M.L., Schow E.V., Freites J.A., White S.H., Tombola F., and Tobias D.J.. 2012. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta. 1818:286–293. 10.1016/j.bbamem.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J., Wu G., Akhavan Sharif M.R., Baker A., Jia Y., Fahey F.H., Luo H.R., Feener E.P., and Clapham D.E.. 2012. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 15:565–573. 10.1038/nn.3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.