Abstract
CRABP-II, a retinoid acid binding protein, shuffles retinoid acid from cytoplasm into nucleus and forms a complex with nuclear retinoid acid receptor to facilitate transcriptional activities of retinoid acid. In this study, we studied the expression patterns of CRABP-II in pancreatic ductal adenocarcinoma (PDAC) compared to those in normal pancreas, chronic pancreatitis and pre-cancerous lesions. We showed no detectable expressions of CRABP-II in normal pancreatic parenchyma, normal ductal epithelium and chronic pancreatitis. In contrast, the expression of CRABP-II was readily detected in all PDACs including metastatic PDACs. CRABP-II staining was also observed and progressively increased from PanIN 1 to 3. In addition, when FNA specimens were evaluated from patients with PDAC, CRABP-II was positive in 55.6% cases if cytology diagnosis was “atypia”, and in 87.5% cases if was “malignancy”. Our study suggests that CRABP-II is highly and specifically expressed in PDAC, and is more commonly expressed in high-grade precursor cancerous lesions than in low-grade lesions. Therefore, over-expression of CRABP-II is a late event of pancreatic carcinogenesis, and it could be used as a diagnostic marker to distinguish PDAC from other benign pancreatic conditions in both resection and cytology specimens.
Keywords: CRABP-II, pancreatic duct adenocarcinoma, pancreatic intraepithelial neoplasm, retinoid acid, cytology, immunohistochemistry, pathology
Introduction
Pancreatic cancer is the fourth leading cause of cancer death (1). Pancreatic ductal adenocarcinomas (PDAC) constitute 80% to 90% of all pancreatic cancers. Most patients with PDAC have a locally advanced or metastatic disease at the time of diagnosis. Once clinically evident, PDAC progresses rapidly and is largely resistant to conventional chemotherapy and radiotherapy (2). Therefore, early detection of PDAC is crucial for a possible curative resection and a better prognosis. In this context, pancreatic intraepithelial neoplasia (PanINs) have recently been proposed as noninvasive precursor lesions of PDAC (3). PanINs are believed to progress from flat to papillary lesions without atypia, to papillary lesions with atypia, to lesions with severe architectural and cytologic atypia (PanIN-1A to PanIN-1B to PanIN-2 to PanIN-3). The progression from PanINs to PDAC is accompanied by accumulation of multiple genetic alterations (2, 4, 5). During early genetic events such as activating point mutations in K-ras oncogene and overexpression of HER-2/neu gene product, pancreatic duct lesions show minimal cytological and architectural atypia (6–9). Inactivation of the p16 tumor suppressor gene appears to occur at a later stage followed by the loss of p53, SMAD4, and BRCA2 tumor suppressor genes (10, 11). According to this model and various studies since then, the initial genetic changes serve as a trigger for subsequent molecular and genetic events to occur, and this sequential acquisition of mutations results in progression of the disease. Therefore, identification of the early genetic alterations may provide potential targets for future therapy and also markers for early diagnosis.
The natural metabolite of vitamin A, Retinoic acid (RA) has been shown to inhibit cancer cell growth in various types of carcinomas including pancreatic cancer (12). Two lipid binding proteins, CRABP-I and CRABP-II bind RA in high binding affinity and selectivity (13). Both of them localize in cytoplasm, bind and protect RA from the cytosol aqueous microenvironment. CRABP-II has been reported to be important for RA signaling. CRABP-II shuttles RA from the cytosol into the nucleus through its ligand-activated nuclear localization signal (NLS) (14). The RA associated CRABP-II also binds RA receptors (RAR) to form a CRABP-II/RAR complex that channels RA to the nuclear receptors, thereby facilitating its ligation and enhancing its transcriptional activities. Thus, CRABP-II functions as a co-activator for RA dependent transcription.
While CRABP-II expression up-regulation has been reported in primary ovarian tumors (15), uterine leiomyoma (16) and promyelocytic leukemia (17, 18), the down-regulation of CRABP-II was observed in prostate cancer (19) and RA resistant medulloblastoma cells (20). In head and neck square cell carcinoma, the absence of CRABP-II was associated with decreased disease-free survival rates (21). It has been reported that CRABP-II is expressed in certain types of PDAC cell lines (22), but the role of CRABP-II in patient sample with pancreatic ductal adenocarcinoma has not been investigated. In this study, we evaluated the expression of CRABP-II in PDACs in comparison to normal pancreatic parenchyma and chronic pancreatitis, and explored the possibility of using CRABP-II as a diagnostic marker to facilitate the differential diagnosis of PDAC on morphologically challenging cases.
Materials and methods
Sample collection
Hematoxylin- and eosin-stained sections retrieved from the files of the Department of Pathology; University Hospitals Case Medical Center, were reviewed independently by two pathologists. We selected 50 cases of primary pancreatic ductal adenocarcinoma, 48 of normal pancreatic tissue, and 49 of chronic pancreatitis. The normal pancreatic tissue was from patients with non-pancreatic disease, most of which were Whipple resection of duodenal adenocarcinoma or neoendocrine tumor. Foci of PanIN were included as well: 36 PanIN 1, 47 PanIN 2 and 25 PanIN 3. All PanIN 1 and 2 cases are not associated with PDACs, while all PanIN3 cases are associated with PDACs. All these patients had undergone surgical resection between 2001 and 2005. All specimens analyzed were formalin-fixed and paraffin-embedded tissue sections. Tissue microarray (TMA) was made. The diagnosis was confirmed by H&E staining. Cellblocks from pancreatic fine needle aspiration (FNA) specimen were also included. Based on the cytologic diagnosis, the cases were divided into three groups—benign (n=14), atypia (n=9) and malignancy (n=16). The diagnosis of all selected FNA specimens was confirmed by following surgical resection. On resection, the “benign” group had 8 PDACs and 6 non malignant. All the “atypia” and “malignancy” cases were confirmed as PDACs. Institutional Research Board (IRB) of the University Hospitals Case Medical Center approved the research protocol.
Immunohistochemical (IHC) staining
Expression levels of CRABP-II were examined by immunohistochemistry (IHC) on all cases included in this study. IHC was performed on paraffin sections of tissue microarrays. Immunohistochemistry was performed by the clinical diagnostic lab of Immunohistochemistry of University Hospitals Case Medical Center. Briefly, unstained 4 μm-sections were prepared from paraffin blocks and baked for 30 minutes at 60° C in a Boekel Lab oven. The slides were then processed using a BondMax Automated Immunostainer (Leica). The slides were deparaffinized, antigen retrieved, incubated in primary antibody, polyclonal anti-CRABPII (1:1000 dilution; Abcam, Cambridge, MA) and subsequently counterstained onboard the automated instrument. Antigen retrieval was performed with Bond Epitope Retrieval Solution 2 (Leica), a EDTA based pH 9.0 solution for 20 minutes at 100 ° C. Histological images were obtained with the use of a ScanScope® XT digital scanning system (Aperio Technologies, Vista, CA, USA). Nuclear immunoreactivity was considered as a positive expression. Immunoreactivity was scored by two investigators based on the percentage and intensity of positive epithelium cells (percentage: 0: <1%, 1+: <25%, 2+: 25–50%, 3+: 50%–75%, 4+: 75%–100%; intensity: undetectable, weak, moderate and strong). Score 0 was considered as negative, score 1 or above as positive. IHC results of TMA slides were further randomly confirmed with whole tissue section slides.
Statistical analysis
Comparison of the CRABP-II expression rates among different groups was done using the Fisher’s exact test (two-tailed) and student t-test (two-tailed).
Results
CRABP-II expression in normal pancreas and chronic pancreatitis
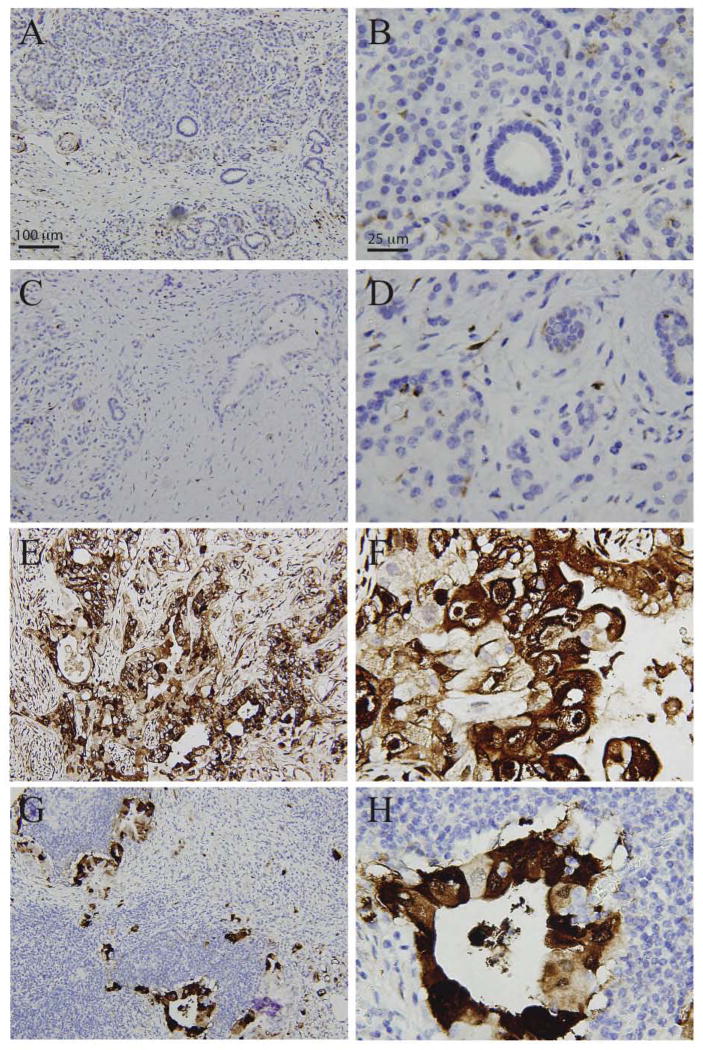
We assessed CRABP-II protein expression levels by immunohistochemical analysis in normal pancreatic tissue and chronic pancreatitis. We observed no CRABP-II staining in normal parenchyma and normal ductal epithelium, including acinar cells, islets, intercalated ducts, intralobular ducts, interlobular ducts and main pancreatic ducts (Figure 1A–B, Table 1). Expression pattern of CRABP-II in chronic pancreatitis was similar to that in normal tissue (Figure 1C–D); demonstrating that CRABP-II is largely negative in benign pancreatic conditions.
Figure 1.
CRABP-II expression in pancreatic tissue by immunohistochemical staining. CRABP-II is completely negative in parenchym and ducts in normal control (A, B) and chronic pancreatitis (C, D), but it is strongly positive in PDACs (E, F) and metastasis in lymph nodes (G,H). Objective magnification x4 (A,C,E,G) and x20 (B,D,F,H).
Table 1.
CRABP-II expression in pancreatic tissue
| Normal parenchyma | Normal ducts | Chronic pancreatitis# | PanIN 1 | PanIN 2 | PanIN 3 | PDAC | ||
|---|---|---|---|---|---|---|---|---|
| Positive (n) | 0 | 0 | 0 | 5 | 30 | 22 | 50 | |
| Negative (n) | 48 | 23 | 49 | 31 | 17 | 3 | 0 | |
|
| ||||||||
| Positive rate (%) | 0 | 0 | 0 | 13.9* | 63.8**,† | 88**,† | 100**,† | |
including parenchyma and ducts.
p <0.05,
p<0.001 as compared to normal parenchyma;
p<0.001 as compared to PanIN1.
CRABP-II expression in PDACs
We then evaluated CRABP-II expression in pancreatic ductal adenocarcinoma; CRABP-II staining was strongly diffusely positive in all 50 cases (100%, 50/50) with pancreatic ductal adenocarcinoma (Figure 1E–F), regardless of the tumor stage and grade. In cancer cells, CRABP-II staining was uniformly localized in cytoplasm and to some extent nuclei. In contrast to pancreatic duct adenocarcinoma, normal pancreatic tissue did not show any specific staining of CRABP-II. We further examined CRABP-II expression in metastatic PDACs in lymph nodes. As expected, CRABP-II was strongly positive in 100% (12/12) of metastatic PDACs (Figure G–H).
CRABP-II expression in PanINs
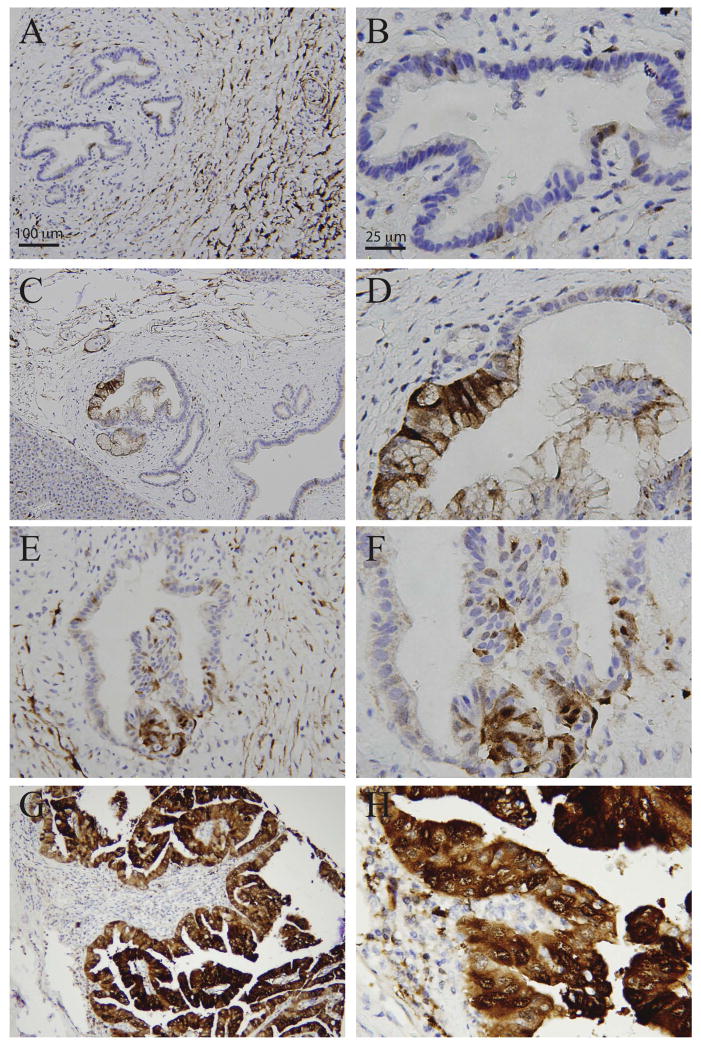
PanIN has been considered as precursor lesions for pancreatic duct adenocarcinoma (3). We therefore examined CRABP-II expression in PanIN. CRABP-II staining was observed from PanIN 1 to 3 in a pattern similar to pancreatic duct adenocarcinoma (Figure 2A–H); although the intensity and positive rate were lower than that in cancer (Table 1 and 2). Interestingly, CRABP-II expression progressively increased from PanIN 1 to 3 (Table 2). Fewer than 15% of PanIN-1 lesions expressed CRABP-II, whereas more than 80% of PanIN-3 lesions and 100% of PDACs expressed it (Table 1), showing that expression of CRABP-II is associated with higher-grade lesions.
Figure 2.
CRABP-II expression in PanIN by immunohistochemical staining. CRABP-II is focally/weakly positive in PanIN1 (A, B), weakly positive in PanIN2 (C–F) and diffusely/strongly positive in PanIN3 (G,H). Objective magnification x4 (A,C,E,G) and x20 (B,D,F,H).
Table 2.
Intensity of CRABP-II expression in pancreatic neoplasm
| PanIN 1 (n=5) | PanIN 2 (n=30) | PanIN 3 (n=22) | PDAC (n=50) | |
|---|---|---|---|---|
| Scores# (mean±SD) | 1.6±0.5 | 2.1±1.0 | 3.3±0.8**,† | 4±0.1**,† |
Including only CRABP-II positive cases;
p<0.001 as compared to PanIN1;
p<0.001 as compared to PanIN2.
CRABP-II expression in FNA specimens
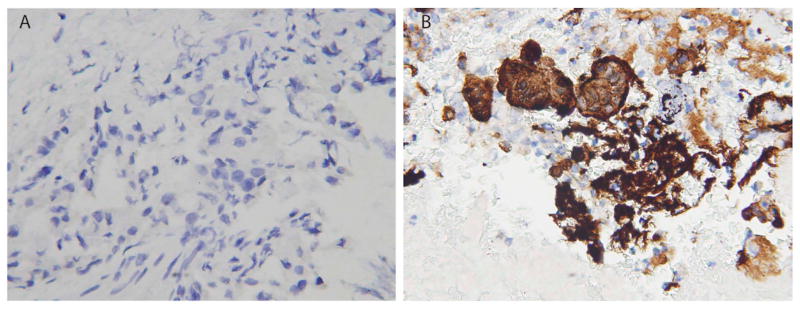
FNA has been widely used to help establish the diagnosis of pancreatic tumor before resection (23). Although the specificity of a PDAC diagnosis on cytology specimen is high, the sensitivity is relatively low (24). As CRABP-II is uniformly expressed in every PDAC cases examined, we hypothesized that CRABP-II be a helpful diagnostic marker in morphologically challenging cytology specimen. To this end, we included 39 cases that had FNA performed with evaluable cellblocks before surgical resection. We classified the cases based on final resection diagnosis. In all 8 cases with no malignancy found in the resection specimen, cytologic diagnosis was all “benign” and CRABP-II was negative in all 8 cases. Next, 31 cases with PDAC revealed by resection specimen were classified into 3 groups based on cytologic diagnosis, “benign”, “atypia”, and “malignancy” (Table 3). In “benign” group, all 6 cases were CRABP-II negative (Figure 3A). Nine cases were diagnosed as “atypia”, 5 of which had positive CRABP-II expression. In “malignancy” group, 14 out of 16 cases were positive for CRABP-II expression (Figure 3B). In summary, the sensitivity of CRABP-II for PDAC was 61.3% (19/31), slightly higher than that of cytology (51.2%, 16/31). By combination of CRABP-II and morphology, the sensitivity of detecting PDAC increased to 67.7% (21/31).
Table 3.
CRABP-II expression in FNA specimens
| Resection diagnosis | FNA diagnosis | CRABP-II expression
|
||
|---|---|---|---|---|
| Positive (n) | Negative (n) | Sensitivity | ||
| Benign | Benign (n=8) | 0 | 8 | |
|
| ||||
| PDAC | Benign (n=6) | 0 | 6 | 0 |
| Atypia (n=9) | 5 | 4 | 5/9 (55.6%) | |
| Malignant (n=16) | 14 | 2 | 14/16 (87.5%) | |
Figure 3.
CRABP-II expression in FNA cytology specimens from patients diagnosed as PDAC on resection specimen. A. Cytology diagnosis was “Benign”. B. Cytology diagnosis was “Malignant”. Objective magnification x40 (A,B).
Discussion
Morphologically, distinguishing pancreatic ductal adenocarcinoma (PDAC) from benign pancreatic conditions, such as extensive chronic pancreatitis, can be challenging. Molecular biomarkers that have high sensitivity and specificity for PDAC have been rigorously searched. Many biomarkers have been studied in this context including survivin (25), MUC4 (26), mapsin (27), and CD44 (28, 29), etc. However, none of these markers is comparable to CRABP-II in terms of high sensitivity and specificity for PDAC in this context.
FNA has been widely used to help establish the diagnosis of pancreatic tumor before resection (23). It is also frequently a daunting task to diagnose PDAC on cytology specimen. The sensitivity of diagnosing PDAC base on morphology can be as low as 50% (24). Molecular markers such as MUC4, S100P and XIAP have been shown to increase the detection sensitivity on FNA specimens (30, 31). Our results implicate that CRABP-II might also be potentially helpful in this regard especially when the cytology diagnosis is “atypia”. CRABP-II was positive in more than 50% of these cases. It is of interest to notice that if the cytology diagnosis is “benign”, CRABP-II was negative in all the cases even though the resection turned out to be PDAC, suggesting that the false negativity might be due to sampling errors.
Our study shows that CRABP-II expression is upregulated in PDACs and the up-regulation is progressive from PanIN 1 to 3, suggesting that CRABP-II might play an important role in the tumorigenesis of PDACs. Oncogenes such as K-ras and tumor suppressor genes such as DPC4 and p53 have been extensively studied and reported to affect the behavior of primary carcinomas and the progression of pancreatic neoplasia (6, 32–35). Herein we report the overexpression of CRABP-II as an additional alteration with a role in the progression from normal pancreatic ductal epithelium to PDACs. The significance of CRABP-II overexpression in PDACs is unclear. CRABP-II shuffles RA from cytoplasm into nucleus and thus is critical for RA-induced cell differentiation. Down-regulation of CRABP-II has been associated with poor survival in head and neck squamous cell carcinoma and breast cancer (21). On the other hand, CRABP-II overexpression has been reported in various types of malignancy including ovarian cancer (15) and leukemia (17, 18). The overexpression of CRABP-II in PDACs might suggest two possibilities: (1) CRABP-II is an oncoprotein, or (2) CRABP-II is tumor suppressor and has loss-of-function alterations that lead to feedback overexpression in PDACs. Our preliminary results didn’t show any mutations in CRABP-II coding region (data not shown). We are currently investigating the possibility of mutations in other regions. Regardless of the mechanism, our results implicate that CRABP-II could be a diagnostic molecular marker and also be a potential therapeutic target in future.
In conclusion, we observed a gradual increase in CRABP-II expression that went along with the progression of PanINs to PDACs. Expression of CRABP-II was associated with higher-grade lesions. Fewer than 15% of PanIN-1 lesions expressed CRABP-II, whereas it was expressed by more than 80% of PanIN-3 lesions and 100% of PDACs. Our finding adds further support to the proposed progression model for PDACs, suggesting that CRABP-II might provide a reasonable target for the chemoprevention of invasive pancreatic adenocarcinoma at early stages.
Acknowledgments
The study was supported by small research grant from Department of Pathology, University Hospital Case Medical Center and faculty startup funds (L.Z. and W.X.) from School of Medicine at Western Reserve University
Footnotes
Disclosure
None
Conflict of Interest
The authors have no relevant financial interest in the products or companies described in this article. The study was approved by University Hospital Case Medical Center Institutional Review Board.
References
- 1.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 4.Singh P, Srinivasan R, Wig JD. Major molecular markers in pancreatic ductal adenocarcinoma and their roles in screening, diagnosis, prognosis, and treatment. Pancreas. 2011;40:644–652. doi: 10.1097/MPA.0b013e31821ff741. [DOI] [PubMed] [Google Scholar]
- 5.Hong SM, Park JY, Hruban RH, Goggins M. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med. 2011;135:716–727. doi: 10.5858/2010-0566-ra.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Kunz J, Beger HG, Korc M. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24:1127–1134. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]
- 8.Battifora H. HER2/neu immunostaining in pancreatic carcinoma. Hum Pathol. 1994;25:549–550. doi: 10.1016/0046-8177(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 9.Lei S, Appert HE, Nakata B, Domenico DR, Kim K, Howard JM. Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. Int J Pancreatol. 1995;17:15–21. doi: 10.1007/BF02788354. [DOI] [PubMed] [Google Scholar]
- 10.Jones J, Bentas W, Blaheta RA, Makarevic J, Hudak L, Wedel S, Probst M, Jonas D, Juengel E. Modulation of adhesion and growth of colon and pancreatic cancer cells by the histone deacetylase inhibitor valproic acid. Int J Mol Med. 2008;22:293–299. [PubMed] [Google Scholar]
- 11.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, Kern SE. Tumor-suppressive pathways in pancreatic carcinoma. Cancer research. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 12.Riecken EO, Rosewicz S. Retinoids in pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):197–200. [PubMed] [Google Scholar]
- 13.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 14.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol Cell. 2005;18:343–353. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Hibbs K, Skubitz KM, Pambuccian SE, Casey RC, Burleson KM, Oegema TR, Jr, Thiele JJ, Grindle SM, Bliss RL, Skubitz AP. Differential gene expression in ovarian carcinoma: identification of potential biomarkers. Am J Pathol. 2004;165:397–414. doi: 10.1016/S0002-9440(10)63306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O’Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78:114–121. doi: 10.1016/s0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delva L, Cornic M, Balitrand N, Guidez F, Miclea JM, Delmer A, Teillet F, Fenaux P, Castaigne S, Degos L, et al. Resistance to all-trans retinoic acid (ATRA) therapy in relapsing acute promyelocytic leukemia: study of in vitro ATRA sensitivity and cellular retinoic acid binding protein levels in leukemic cells. Blood. 1993;82:2175–2181. [PubMed] [Google Scholar]
- 18.Zhou DC, Hallam SJ, Lee SJ, Klein RS, Wiernik PH, Tallman MS, Gallagher RE. Constitutive expression of cellular retinoic acid binding protein II and lack of correlation with sensitivity to all-trans retinoic acid in acute promyelocytic leukemia cells. Cancer Res. 1998;58:5770–5776. [PubMed] [Google Scholar]
- 19.Okuducu AF, Janzen V, Ko Y, Hahne JC, Lu H, Ma ZL, Albers P, Sahin A, Wellmann A, Scheinert P, Wernert N. Cellular retinoic acid-binding protein 2 is down-regulated in prostate cancer. Int J Oncol. 2005;27:1273–1282. [PubMed] [Google Scholar]
- 20.Fu YS, Wang Q, Ma JX, Yang XH, Wu ML, Zhang KL, Kong QY, Chen XY, Sun Y, Chen NN, Shu XH, Li H, Liu J. CRABP-II methylation: a critical determinant of retinoic acid resistance of medulloblastoma cells. Mol Oncol. 2012;6:48–61. doi: 10.1016/j.molonc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calmon MF, Rodrigues RV, Kaneto CM, Moura RP, Silva SD, Mota LD, Pinheiro DG, Torres C, de Carvalho AF, Cury PM, Nunes FD, Nishimoto IN, Soares FA, da Silva AM, Kowalski LP, Brentani H, Zanelli CF, Silva WA, Jr, Rahal P, Tajara EH, Carraro DM, Camargo AA, Valentini SR. Epigenetic silencing of CRABP2 and MX1 in head and neck tumors. Neoplasia. 2009;11:1329–1339. doi: 10.1593/neo.91110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Pramanik D, Mukherjee R, Campbell NR, Elumalai S, de Wilde RF, Hong SM, Goggins MG, De Jesus-Acosta A, Laheru D, Maitra A. Molecular determinants of retinoic acid sensitivity in pancreatic cancer. Clin Cancer Res. 2012;18:280–289. doi: 10.1158/1078-0432.CCR-11-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David O, Green L, Reddy V, Kluskens L, Bitterman P, Attal H, Prinz R, Gattuso P. Pancreatic masses: a multi-institutional study of 364 fine-needle aspiration biopsies with histopathologic correlation. Diagn Cytopathol. 1998;19:423–427. doi: 10.1002/(sici)1097-0339(199812)19:6<423::aid-dc4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Jing X, Wamsteker EJ, Li H, Pu RT. Combining fine needle aspiration with brushing cytology has improved yields in diagnosing pancreatic ductal adenocarcinoma. Diagn Cytopathol. 2009;37:574–578. doi: 10.1002/dc.21062. [DOI] [PubMed] [Google Scholar]
- 25.Bhanot U, Heydrich R, Moller P, Hasel C. Survivin expression in pancreatic intraepithelial neoplasia (PanIN): steady increase along the developmental stages of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2006;30:754–759. doi: 10.1097/00000478-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 27.Cao D, Zhang Q, Wu LS, Salaria SN, Winter JW, Hruban RH, Goggins MS, Abbruzzese JL, Maitra A, Ho L. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Mod Pathol. 2007;20:570–578. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- 28.Takada M, Yamamoto M, Saitoh Y. The significance of CD44 in human pancreatic cancer: II. The role of CD44 in human pancreatic adenocarcinoma invasion. Pancreas. 1994;9:753–757. doi: 10.1097/00006676-199411000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Takada M, Yamamoto M, Saitoh Y. The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas. 1994;9:748–752. doi: 10.1097/00006676-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, Eloubeidi M, Jones JJ, Grizzle WE. Biomarkers in Diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 31.Kosarac O, Takei H, Zhai QJ, Schwartz MR, Mody DR. S100P and XIAP expression in pancreatic ductal adenocarcinoma: potential novel biomarkers as a diagnostic adjunct to fine needle aspiration cytology. Acta Cytol. 2011;55:142–148. doi: 10.1159/000320913. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, O’Reilly EM. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34:321–325. doi: 10.1097/COC.0b013e3181e84b1f. [DOI] [PubMed] [Google Scholar]
- 33.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P, Herman JM, Cameron JL, Yeo CJ, Halushka MK, Eshleman JR, Raben M, Klein AP, Hruban RH, Hidalgo M, Laheru D. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 35.Blackford A, Parmigiani G, Kensler TW, Wolfgang C, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Eshleman JR, Goggins M, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Klein A, Cameron JL, Olino K, Schulick R, Winter J, Vogelstein B, Velculescu VE, Kinzler KW, Hruban RH. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer research. 2009;69:3681–3688. doi: 10.1158/0008-5472.CAN-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]