Abstract
Background
The effect of red blood cell (RBC) storage on oxygenation in critically ill patients is still unknown. The objective of this study was to determine the association of RBC storage with oxygenation, long-term neurological recovery, and death after traumatic brain injury.
Methods
We used data from a 2×2 factorial randomized controlled trial of administration of Erythropoietin or placebo and of assignment to transfusion threshold <7g/dl or <10 g/dl in neurosurgical intensive care units in 2 US level 1 trauma centers. Patients had severe traumatic brain injury with closed head injury, were unable to follow commands, and were enrolled within 6 hours of injury. Blood oxygenation one hour after the transfusion as measured by jugular venous oxygen saturation (SjvO2, n=59) was the primary outcome. Secondary outcomes were brain tissue oxygenation (PbtO2, n=77), 6-month Glasgow Outcome Scale (GOS, n=122) collected using a structured interview and dichotomized into favorable (good recovery or moderate disability) or unfavorable outcome (severe disability, vegetative state, or dead), and mortality (n=125). RBC age was defined as the maximum age of RBCs over all units in one transfusion per patient. For long-term outcomes RBC age was defined as the mean age over all units given.
Results
We failed to detect an association of RBC age with SjvO2 (linear regression β=1.59, 95% confidence interval [CI] = -2.99-6.18, P=0.49), PbtO2 (linear regression β=0.20, 95% CI =-0.23-0.63, P=0.36), GOS (odds ratio = 1.37, 95% CI = 0.53-3.57, P=0.52), and with mortality (hazard ratio=1.35, 95% CI=0.61-2.98, P=0.46).
Discussion
Limitations of this study include that although this was a prospective study, the RBC ages were not randomized. We conclude that older blood does not appear to have adverse effects in severe TBI.
Level Of Evidence
Prospective study, level III.
Background
Approximately 50% of all traumatic brain injury (TBI) patients receive a transfusion (1). Longer duration of the red blood cell (RBC) storage has been hypothesized to alter RBC function, leading to more complications. There are multiple alterations that result from the ex vivo storage of the RBCs which result in modification of the RBC properties and the supernatant, referred to as the “storage lesion”. A variety of metabolic and structural changes occur in the RBC during the ex vivo storage. There is progressive decrease in the 2,3-diphosphoglycerate (DPG) levels and decrease in adenosine triphosphate levels which may impair oxygen delivery and reduce Na+-K+ ATPase activity, respectively. There are also irreversible membrane changes resulting in deformed sphero-echinocytes which result in increased adherence to the endothelium and increased susceptibility to phagocytosis (2). Additionally, there are changes that occur in the supernatant including decrease in pH and release of proinflammatory cytokines which may exacerbate the underlying condition (2). In trauma studies, age of RBCs has been associated with increased mortality (3-6), increased risk of infections (3,6), and increased risk of deep vein thrombosis (DVT) (3). However, whether or not transfusions with fresh RBCs are associated with improved outcomes has remained inconclusive (2).
Determining the effect of age of RBCs on TBI patient outcomes, both short-term and long-term, has been challenging. A patient may have multiple transfusions and each transfusion could have multiple units, each unit with their own age of RBCs. The definition of patient-level and transfusion-level age of RBCs can be confounded with the patient's injury severity; e.g., if the patient-level age of RBCs is defined as the maximum RBC age of all units given, a patient given more transfusions is more likely to have a larger value of their age of RBCs as well as more likely to have a more severe injury, resulting in a bias in favor of finding an association between age of RBCs and worse outcomes. Alternatively, taking the mean of all units given for that patient is unbiased but can mask any benefit of young RBCs or harm of older RBCs in patients with a lot of variability in their RBC age among units given.
Whereas there have been many studies assessing the possible effect of age of RBCs, there are few prospective studies in trauma. These few studies have been limited by very small sample sizes and in the types of outcomes assessed. Furthermore, none are in severe TBI patients. Using data from a recently completed 2×2 factorial randomized trial (7), we studied the associations between older age of RBCs and post-transfusion blood oxygenation measures assessed by jugular venous oxygen saturation (SjvO2) and brain tissue PO2 (PbtO2). Additionally, we examined the association between older age of RBCs and long-term neurological outcome at six months after injury and mortality.
Methods
Primary Hypothesis and Outcomes
Although this was a prospective study, the age of RBCs was not randomized. The blood bank was blinded to the patient's characteristics and the oldest blood was used first regardless of the severity of the patient's injury. Therefore the age of the RBCs is not likely to be confounded with predictors of the outcomes. Because the oldest blood was used first as part of standard management, consecutive units of blood (e.g., for a transfusion with multiple units) are likely to have more similar ages of RBCs than overall (e.g., between transfusions). Therefore, within a single transfusion there is lower likelihood of there being a mix of younger and older RBCs than among all transfusions administered to a patient within 6 months of their injury. Therefore, the effect of the age of RBCs on short-term outcomes after the transfusion is less likely to be confounded than on long-term outcomes. Hence, our primary hypothesis was that an older age of RBCs was associated with less improvement in blood oxygenation one hour after transfusion. Our secondary hypotheses were that older ages of RBCs among all units of blood given were associated with worse neurological outcome at six months after injury and increased mortality.
The transfusion-specific oxygenation was measured by the post-transfusion SjvO2 (primary outcome) and PbtO2 (secondary outcome) among transfusions given within the first five days after injury. The patient-specific neurological outcome was assessed by the dichotomized Glasgow Outcome Scale (GOS), collected using a structured interview at six months after injury and dichotomized per protocol into favorable outcome (good recovery or moderate disability) or unfavorable outcome (severe disability, vegetative state, or dead).
Age of RBCs
Age of the RBCs was defined at the transfusion level and at the patient level for short-term and long-term outcomes, respectively. The first transfusion administered within 5 days of injury with the most complete outcome data recorded was considered for the analysis of the primary outcome. The rationale was to limit any confounding effect on the outcomes from the randomization groups, limit bias if we had omitted patients that had some missing data in their first transfusion and were possibly not missing at random, and to maintain as large of a sample size as possible. The transfusion-level age of blood was the maximum age of RBCs over all units administered in this transfusion. All transfusions per patient were used for long-term outcomes (GOS and mortality) because of possible multiple transfusion additive effects and the difficulty associating a single transfusion (where there could be multiple transfusions per patient) with a long-term outcome. The patient-level age of blood was the mean age of RBCs over all units given to that patient.
Study Population
A total of 200 subjects were enrolled into the Erythropoietin TBI trial between 2006 and 2012. The details of the design of the study and the results of the primary analysis have been previously reported (7). Briefly, severe TBI participants were randomly assigned to administration of erythropoietin (Epo) or placebo and to hemoglobin transfusion thresholds of 7 or 10 g/dl in a 2×2 factorial design. Patients received Epo (Epogen, Amgen, Inc., Thousand Oaks, CA) 500 IU/kg or an equal volume of saline intravenous bolus infusion for each dose of the study drug. Patients received an initial dosage regimen of the assigned study drug followed by two additional doses, one per week for the next two weeks provided that the patient remained in ICU and their hemoglobin concentration levels remained below 12 g/dl. The initial dosage regimen was one dose given within six hours of injury followed by two additional doses given every 24 hours (Epo 1 dose regimen) or one dose given within six hours of injury (Epo 2 dose regimen). The assigned transfusion threshold was maintained during the acute ICU management with transfusion of leukoreduced packed RBCs. Neither the administration of Epo nor maintaining hemoglobin concentration of greater than 10 g/dL resulted in improved neurological outcome at 6 months after injury. A higher incidence of deep venous thrombosis events were observed with the transfusion threshold of 10 g/dL.
Study Procedures
To study changes in brain oxygenation with transfusion, we attempted to collect cerebral hemodynamic measurements before and after all transfusion events during the first 5 days after injury when SjvO2 monitoring was most likely to be available. Before the transfusion, and one hour after transfusion of sufficient units of red blood cells to raise the hemoglobin concentration to the assigned transfusion threshold, we collected SjvO2 as well as other hemodynamic variables. Additional details of the hemodynamic measurements have been reported (8).
Data Analysis
All statistical analyses were performed using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) or SAS version 9.3 (SAS Institute Inc) with 2-sided statistical tests at a .05 significance level.
SjvO2 and PbtO2 outcomes analysis
Covariates considered in the analysis of SjvO2 included pre-transfusion SjvO2, Epo treatment group, transfusion threshold (<7 g/dl versus <10 g/dl), baseline motor GCS (1–3 versus 4–5), number of units transfused (1 vs. >1), day after injury that the transfusion occurred (≤2 vs. >2 days), age of patient (≤40 vs. >40), and CT category. In the PbtO2 analysis, the covariates considered were pre-transfusion PbtO2 (instead of pre-transfusion SjvO2), location of catheter (normal vs. contused brain), and all the other covariates included in the SjvO2 analysis. The stratifying variables used in the randomization of the trial (treatment group, transfusion threshold) were included per standard statistical procedures when analyzing data from a randomized trial. Baseline motor GCS and CT category were included as indicators of severity of injury; days from injury to transfusion was included because injury evolves over time and response might be different on day 1 than on day 5; age of patient was included as a prognostic factor related to oxygenation; and location of catheter was included due to its known association with PbtO2 measurement. A Wilcoxon signed rank test was used to compare the difference in pre-transfusion and post-transfusion hemodynamic variables and a Wilcoxon rank sum test was used to compare these differences among the older and younger RBC age groups. We conducted transformations of continuous variables when needed to satisfy normality assumptions. Multiple linear regression was used to assess the effect of age of RBCs (dichotomized at 21 days) on post-transfusion SjvO2 and PbtO2, adjusting for covariates. We also included interaction terms pre-transfusion mean arterial pressure (MAP) × age of RBCs, pre-transfusion intracranial pressure (ICP) × age of RBCs, and PbtO2 catheter location × age of RBCs. Variable selection was conducted using lasso penalized regression with the shrinkage parameter selected using 5-fold cross-validation. Age of RBCs, pre-transfusion SjvO2 or PbtO2, and the Epo and hemoglobin transfusion threshold randomization groups were forced in the lasso model. The final model fit was assessed using quantile-quantile plots and examining the residuals.
GOS secondary outcome analysis
Fisher's exact test was used to compare categorical covariates to 6-month dichotomized GOS. Logistic regression was used to model the effect of age of RBCs on GOS, adjusting for covariates transfusion threshold group, Epo treatment group, injury severity score, and IMPACT predicted probability of poor GOS outcome (9). The Hosmer-Lemeshow goodness-of-fit test and the area under the receiver operating characteristic curve were used to assess the goodness of fit.
Mortality secondary outcome analysis
Cox proportional-hazards model was used to assess the relationship between age of RBCs and mortality, adjusting for covariates transfusion threshold group, Epo treatment group, injury severity score, and IMPACT predicted probability of death (9). Schoenfeld residual plots and a global test for interactions of all predictors with log(time) simultaneously were used to assess the assumptions of proportional hazards. Because RBC age may have differential effects over time, we conducted a stratified Cox analysis in separate time intervals (days 0–3, 4–13, ≥14 days after injury), omitting patients that had died in a previous interval and calculating the age of RBC using transfusions that had occurred up to that time point (10).
Sensitivity analyses of age of RBCs definition
As sensitivity analyses we also treated age of RBCs as quartiles, dividing our data into four groups based on the sample range (<16, 16–23, 23–32, and >32 days) and based on ranges used by Leal-Noval (11) in their data (<10, 10–14, 15–19, and >19 days), treated the RBC age as a continuous variable, and dichotomized age of RBCs at <14 days versus older.
Results
Of the 200 patients enrolled in the study, 125 were given at least one transfusion with a median of 5 total units per transfused patient. The mean of the average age of RBCs given was 22.6 days (range 5–42 days, standard deviation=8.1). There were 68 (54%) patients that had a mean RBC age >21 days and 57 (46%) patients ≤21 days.
A subset of 116 patients required transfusion during the first 5 days after injury of which 59 had complete Pre- and Post- transfusion SjvO2. The reasons for not having complete SjvO2 data included (1) the transfusions were too urgent or were given in the operating room (n=21), (2) the patient was unstable or unable to insonate vessels for transcranial Doppler measurements of flow velocity (n=3), (3) the transfusion was given from post-op, in the EC, or for over 24 hours (n=3), (4) the patients were recruited at a hospital where this part of the study was not being performed (n=5), and (4) the patient was given a transfusion before enrolled (n=1). Reasons for incomplete data were not recorded or ambiguous for 24 of the transfused patients. The demographic and injury characteristics of the subset of patients with complete pre- and post-transfusion cerebral hemodynamic data are shown in Supplemental Digital Content - Table A.1 and are not different from the total population except for expected injury severity differences for transfused versus not transfused cohorts. We did not detect any differences in baseline variables between patients with younger and older age of RBCs (data not shown).
Table 1 describes the univariate pre- and post-transfusion changes in cerebral hemodynamics. There was an overall increase in SjvO2, MAP, and hemoglobin, and a decrease in middle cerebral artery flow volume (Fvol) and partial pressure of oxygen in arterial blood (PaO2) during the transfusions. We did not observe a statistically significant difference between the older and younger age of RBCs for any hemodynamic outcome.
Table 1. Pre- and Post-Transfusion Values for Cerebral Hemodynamics.
| Variable | Total Median (IQR) n |
P-value (Posttfx-Pretfx) |
RBC (maximum) ≤ 21 days old Median (IQR) n |
P-value (Posttfx-Pretfx) |
RBC (maximum) > 21 days old Median (IQR) n |
P-value (Posttfx-Pretfx) |
P-value for difference in RBC≤21 vs. difference in RBC>21 groups |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Pretfx | Posttfx | Pretfx | Posttfx | Pretfx | Posttfx | |||||
| Primary outcome: SjvO2 (n=59) | 73.0 (15.0) 63 | 76.0 (15.0) 63 | 0.02 | 74.0 (15.0) 27 | 76.0 (17.0) 26 | 0.07 | 71.5 (15.5) 36 | 76.0 (13.0) 37 | 0.11 | 0.54 |
| Secondary outcome: PbtO2 (n=77) | 21.0 (16.7) 82 | 24.0 (18.1) 84 | 0.32 | 18.2 (16.6) 38 | 22.3 (18.8) 39 | 0.46 | 21.9 (15.7) 44 | 25.0 (19.4) 45 | 0.5 | 0.81 |
| ICP (n=90) | 16.0 (8.0) 94 | 17.0 (9.0) 96 | 0.67 | 16.0 (9.0) 43 | 17.0 (9.0) 42 | 0.53 | 16.0 (9.0) 51 | 17.0 (9.0) 54 | 0.26 | 0.19 |
| MAP (n=103) | 86.0 (21.0) 105 | 89.0 (16.0) 106 | 0.03 | 86.0 (17.0) 47 | 89.0 (15.0) 47 | 0.37 | 84.0 (23.0) 58 | 89.0 (17.0) 59 | 0.04 | 0.42 |
| CPP (n=90) | 70.0 (21.0) 94 | 73.0 (19.0) 96 | 0.2 | 70.0 (21.5) 44 | 73.0 (21.0) 43 | 0.38 | 70.0 (20.0) 50 | 73.0 (16.0) 53 | 0.5 | 0.85 |
| FV (n=71) | 90.0 (28.0) 71 | 88.0 (36.0) 71 | 0.22 | 90.0 (25.0) 37 | 88.0 (35.0) 37 | 0.76 | 90.5 (36.0) 34 | 88.0 (34.0) 34 | 0.14 | 0.46 |
| FVOL (n=63) | 541.3 (380.3) 63 | 451.7 (316.7) 65 | <.01 | 545.0 (414.0) 33 | 462.2 (309.2) 34 | 0.15 | 529.9 (373.1) 30 | 380.0 (349.3) 31 | <.01 | 0.37 |
| Hgb (n=110) | 9.0 (1.7) 110 | 10.2 (1.5) 110 | <.01 | 9.0 (1.5) 49 | 10.0 (1.4) 49 | <.01 | 8.7 (2.8) 61 | 10.2 (1.6) 61 | <.01 | 0.12 |
| PaO2 (n=59) | 165.0 (84.0) 63 | 157.0 (71.0) 62 | 0.01 | 160.5 (81.5) 32 | 129.0 (82.0) 31 | 0.05 | 165.0 (104.0) 31 | 165.0 (63.0) 31 | 0.09 | 0.82 |
| PaCO2 (n=60) | 36.5 (10.5) 64 | 38.0 (8.0) 63 | 0.62 | 35.0 (11.0) 33 | 36.0 (7.5) 32 | 0.95 | 38.0 (5.0) 31 | 38.0 (8.0) 31 | 0.34 | 0.51 |
Abbreviations: posttfx, post transfusion; pretfx, pre transfusion; RBC, red blood cell age
SjvO2 Outcome
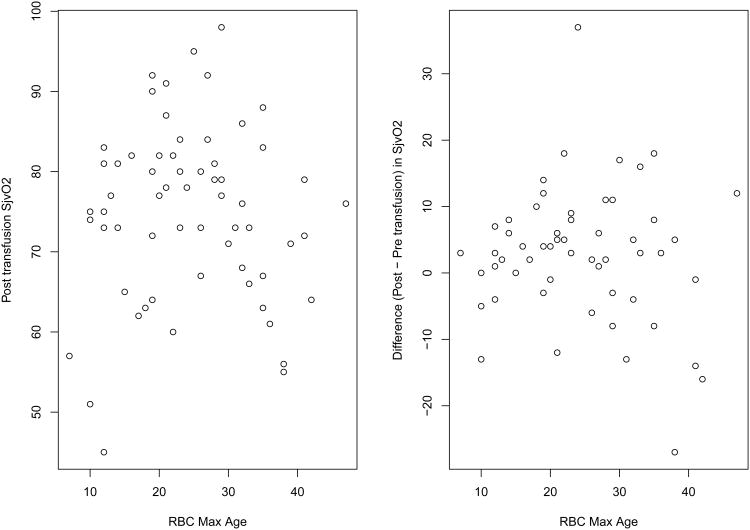
There were 59 complete cases for the SjvO2 outcome. SjvO2 increased significantly after the transfusion (pre-transfusion median = 73.0%, post-transfusion median =76.0%; p=0.02, Table 1). We observed a greater increase in the older age of RBCs group (medians 71.5 versus 76.0%, p=0.11) compared to the younger age of RBCs group (medians 74.0% versus 76.0%, p=0.07), but this was not statistically significant (p=0.54). After variable selection, the multiple linear regression adjusted for variables pre-transfusion SjvO2, Epo regimen group, transfusion threshold group, and number of units transfused. In both univariate and adjusted analyses, we did not detect an effect of the age of RBC on post-transfusion SjvO2 (Table 1, 2). Figure 1 illustrates the overall relationship between age of RBCs and SjvO2. The sensitivity analyses treating RBC age as a continuous variable and creating quartiles were consistent in not revealing any trends towards improvement in oxygen delivery with younger age of the transfused RBCs. When dichotomizing using a different threshold of <14 days, a significant p-value was detected (p=0.045). However, the direction was opposite from our hypothesis (the younger RBC age had lower oxygenation compared to the older RBC age group) and there were only 10 observations in the younger RBC age group. Because all other definitions of RBC age consistently did not suggest an association, and due to the multiple testing and thus increased probability of type I error, we believe this result is likely to be spurious.
Table 2. Multiple Linear Regression for Outcomes Post-Transfusion Jugular Venous Oxygen Saturation (Sjvo2) and Brain Tissue Oxygenation (Pbto2), Adjusted for Lasso-Selected Covariates.
| Coefficient | 95% CI | p-value | ||
|---|---|---|---|---|
|
|
||||
| Post-transfusion SjvO2 Outcome | Intercept | 34.06 | 16.90-51.22 | <.001 |
| RBC (maximum) > 21 days old | 1.59 | -2.99-6.18 | 0.49 | |
| Pre-Transfusion SjvO2 | 0.54 | 0.34-0.74 | <.001 | |
| Epo 1 regimen | 2.49 | -3.69-8.68 | 0.42 | |
| Epo 2 regimen | 0.85 | -4.57-6.27 | 0.75 | |
| Transfusion Threshold <10 g/dl | -2.63 | -8.60-3.34 | 0.38 | |
| Number of units transfused (>1) | 3.16 | -1.96-8.29 | 0.22 | |
|
| ||||
| Post-transfusion PbtO2 Outcome* | Intercept | 0.84 | -0.02-1.69 | 0.06 |
| RBC (maximum) > 21 days old | 0.20 | -0.23-0.63 | 0.36 | |
| Pre-Transfusion PbtO2* | 0.68 | 0.56-0.80 | <0.001 | |
| Epo 1 regimen | -0.16 | -0.74-0.42 | 0.58 | |
| Epo 2 regimen | 0.29 | -0.20-0.78 | 0.24 | |
| Transfusion Threshold <10 g/dl | 0.61 | 0.12-1.09 | 0.02 | |
| Number of units transfused (>1) | 0.27 | -0.25-.79 | 0.30 | |
Square-root transformed
Figure 1.
PbtO2 Outcome
There were 77 complete cases for the PbtO2 outcome. PbtO2 increased after the transfusion overall and within each of the age of RBCs groups, but the change was not statistically significant neither within nor between groups (Table 1). We used a square root transformation for pre- and post- transfusion PbtO2 to normalize the outcome in the multiple regression analysis. After adjusting for covariates, there was no statistically significant effect of maximum age of RBCs more than 21 days old on Post-transfusion PbtO2 (Table 2). Pre-transfusion PbtO2 was statistically associated with Post-transfusion PbtO2 (p<0.001). None of the interaction terms were statistically significant.
GOS Outcome
The 6 month GOS was available in 122 of the 125 transfused patients (23 in the Epo1 dose regimen group, 37 in the Epo2 dose regimen group, and 62 in the placebo group; 73 in the 10 g/dl threshold group and 49 in the 7 g/dl threshold group). Twenty-five (20%) transfused patients had a favorable outcome, and 97 (77.6%) had an unfavorable outcome at 6 months after injury. There were no detectable differences found in GOS outcome when comparing average age of RBCs that was greater than 21 (21.5% favorable) versus less than or equal to 21 days old (19.3% favorable) in transfused patients (p=0.82). After adjusting for covariates, we failed to detect an association between age of RBCs and GOS outcome (Table 3). Similar results were obtained in the subset of patients having either all units of transfused RBCs ≤21 versus >21 days old (n=73, 9/37=24.3% favorable GOS in <21 days group and 7/36=19.4% in ≥21 days group; p=0.78; adjusted odds ratio=0.84, 95% CI 0.26–2.77, p=0.78).
Table 3. Logistic Regression Results for Analysis of Glasgow Outcome Scale Adjusted for Pre-Specified Covariates.
| GOS Outcome | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
|
| |||
| Intercept | 0.92 | 0.09-9.70 | 0.77 |
| RBC age (average) > 21 days old | 1.37 | 0.53-3.57 | 0.52 |
| Epo1 regimen (compared to Placebo) | 1.58 | 0.48-5.20 | 0.46 |
| Epo2 regimen (compared to Placebo) | 0.75 | 0.24-2.31 | 0.61 |
| Transfusion Threshold <10 g/dL (compared to TT7) | 1.46 | 0.55-3.92 | 0.45 |
| Injury Severity Score | 1.00 | 0.95-1.05 | 0.90 |
| IMPACT probability of unfavorable GOS | 0.96 | 0.94-0.99 | 0.002 |
Mortality Outcome
Twenty-seven (21.6%) transfused patients died and 98 (78.4%) survived at 6 months after injury. Of patients who received an average age of RBCs greater than 21 and less than or equal to 21, 16 (23.5%) and 11 (19.3%) died, respectively (p=0.66). There was no detectable association between age of RBCs on the risk of mortality after adjustment for other covariates (Table 4). Similar results were obtained when we included a time-dependent variable for first episode of hypoxia or hypotension, in the subset of patients having either all units of transfused RBCs ≤21 versus >21 days old (n=73, 6/37=16.2% died in <21 days group and 6/36=16.7% in ≥21 days group; p>0.99; adjusted hazard ratio=0.90, 95% CI 0.27–3.00, p=0.86), and in the stratified-by-time Cox models (Table 5).
Table 4. Cox Regression Analysis-average age of RBCs Adjusted for Pre-specified Covariates.
| Mortality | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
|
| |||
| RBC (average) >21 days | 1.35 | 0.61 -2.98 | 0.46 |
| Epo1 regimen (compared to Placebo) | 1.17 | 0.40-3.45 | 0.77 |
| Epo2 regimen (compared to Placebo) | 1.13 | 0.43-2.97 | 0.78 |
| Transfusion Threshold <10 g/dL (compared to TT7) | 1.03 | 0.47-2.30 | 0.94 |
| Injury Severity Score | 1.01 | 0.97-1.05 | 0.79 |
| IMPACT probability of Death | 1.05 | 1.02-1.07 | <.0001 |
Table 5. Cox Regression Analysis-average age of RBCs Adjusted for Pre-specified Covariates stratified by time.
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Time interval (day after injury) | Number dead in interval | Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value |
|
|
|
|
|||||
| 0-3 | 8 | 2.68 | 0.54-13.29 | 0.23 | 1.88* | 0.29-12.32 | 0.51 |
| 4-13 | 7 | 0.70 | 0.16-3.11 | 0.63 | 0.73† | 0.16-3.35 | 0.69 |
| ≥14 | 12 | 1.28 | 0.41-4.05 | 0.67 | 1.70* | 0.50-5.78 | 0.40 |
Adjusted for baseline variables EPO treatment group, the randomization transfusion threshold, injury severity score, and the IMPACT predicted probability of death.
Adjusted for baseline variables EPO treatment group, injury severity score, and the IMPACT predicted probability of death (the randomization transfusion threshold was omitted from the adjustment of the model in time interval 4-13 days because all 7 dead were in the transfusion threshold <10g/dL group).
Model Diagnostics
Model diagnostics for all adjusted models are displayed in the Supplemental Digital Content.
Discussion
This study is a secondary analysis of a recently completed randomized trial of erythropoietin in TBI, in which we investigated the association between age of the RBCs transfused and blood oxygenation as measured by SjvO2 and intracerebral PbtO2, as well as its possible impact on the neurologic outcome at 6 months after injury and mortality. In this study, the effect of transfusion on SjvO2 did not appear to be dependent on the age of the RBCs. In addition, we were not able to detect associations of the age of RBCs transfused with PbtO2, the neurologic outcomes at 6 months after injury, or with mortality.
There has been significant interest in the effect of age of blood transfused on outcomes in the critically ill patients. Many publications over the past 5 years have attempted to answer this question with focus on a variety of outcomes, from mortality to infection to length of hospital stay and organ failure. The results of these studies have been conflicting. In a recent structured literature review by Lelubre, they identified 55 studies reporting on this potential association and found that 47% of the studies found detrimental effect of blood storage whereas the remainder did not find any detrimental effect (2). What is clear is that the critically ill population represents a diverse population with different baseline risk, and the effect of the age of blood transfused may not translate across diverse populations. Therefore it is important to assess this potential relationship in individual cohorts of patients to determine its effect.
In our study, we chose to examine this question in patients with severe TBI, defined as patients with a baseline GCS motor score ≤5. One of the main reasons for transfusion of blood in the care of patients with brain injury is the belief that an injured brain requires at least normal oxygen delivery and may even require supranormal oxygen delivery (1). Our analysis does not seem to support the premise that younger blood, as it has had less time to develop the changes associated with “storage lesion”, would have better oxygen delivery. This is conflicting to the previous study published by Leal-Noval, et al (11).
In addition, we did not find that there was a positive effect on the long term favorable neurologic outcome or mortality in patients who received younger RBCs, defined as the mean age of RBCs across all transfusions received by a patient to be <21 days. These findings are consistent with the Age of Transfused Blood in Critically Ill Adults (ABLE) trial, a multicenter, randomized, blinded trial that did not detect an association between RBC storage groups and 90-day mortality (12). In contrast, in this study we measured long-term (6-month) neurological recovery which may be more important for TBI patients than mortality. Additionally, we were able to assess oxygenation differences before and after the transfusion. Finally, in our sensitivity analyses using various definitions of younger and older RBCs, we examined both questions of whether old RBCs had deleterious effects and whether young RBCs improved outcomes, whereas the ABLE trial assessed the latter.
The deleterious effects of older donor leukocytes, including decreased red cell viability and oxygen delivery, may be reduced by leukoreduced RBCs. Older leukoreduced blood has been associated with a decreased risk of mortality (13). In our study, all transfusions used leukoreduced blood. This may have reduced any adverse effects of blood storage.
There are various limitations to our study. This is a secondary analysis of a prospective trial designed to answer a separate question. As a result, the results can only be considered in terms of hypothesis generation. The analysis of 6-month GOS and mortality may be difficult to assess because patients did not have a randomized RBCs age resulting in varying RBCs ages per patient. It is therefore difficult to determine whether the potential beneficial effects of the younger RBCs were offset by the potential deleterious effects of the older RBCs, resulting in a net no effect on these outcomes. However, the analysis of the subset of patients receiving all younger or all older RBCs suggests no effect. Similar results were also found in a stratified sensitivity analysis where Cox models were fit in separate time intervals. We had many missing values for the post-transfusion oxygenation. We have attempted to mitigate these limitations by accounting for many of the typical confounders. The age of RBC was not randomized and therefore patients received variable ages of RBC within a transfusion. However, only 8% (5/59) and 14% (11/77) of patients had a mix of young and old RBCs in the transfusion used for the analysis of SjvO2 and PbtO2, respectively, and these patients were all classified in the old group. Finally, we did not record the age of plasma and platelets or details about other fluids that were given as part of standard management.
Despite these limitations, our study is the first prospective study in severe TBI with a relatively large sample size. The only other prospective study assessing this question was in trauma patients with a small sample size of 32 and did not assess any long term outcomes (14).
Conclusions
Based on the results of our secondary post hoc analysis of a randomized trial of patients presenting with severe traumatic brain injury, we were not able to detect an association between the non-randomized age of RBCs transfused and tissue oxygenation capacity of the blood, long term neurologic outcome, and mortality. Although our study represents the largest dataset examining the effect of age of RBCs on the outcomes of patients with severe traumatic brain injury with standardized data collection including follow-up time, to truly answer this question a prospective randomized trial is required.
Supplementary Material
Acknowledgments
We thank the National Institute of Neurological Disorders and Stroke, National Institutes of Health for funding to conduct this study.
Source of Funding: This work was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (grant No. P01NS38660).
Footnotes
Author Contribution: Jose-Miguel Yamal, Julia Benoit, Pratik Doshi, and Claudia Robertson wrote the manuscript draft. Jose-Miguel Yamal, Julia Benoit, M. Laura Rubin, Barbara C. Tilley, and Claudia Robertson conducted the data analysis. Julia Hannay and Claudia Robertson collected the data. All authors critically revised the manuscript and interpreted the data.
Conflicts of Interest: No conflicts of interest declared.
Clinical Trial Registration: www.clinicaltrials.gov, NCT00313716
Protection of Human Subjects: The protocol was approved by the US Food and Drug Administration and by institutional review boards at each clinical site. Subjects were enrolled either using prospective written consent from their family members or through exception from informed consent.
Contributor Information
Jose-Miguel Yamal, Department of Biostatistics, University of Texas School of Public Health, Houston, TX, USA.
Julia S. Benoit, Email: Julia.Benoit@times.uh.edu, Department of Basic Vision Sciences, University of Houston, Houston, TX, USA.
Pratik Doshi, Department of Emergency Medicine and Internal Medicine, University of Texas Health Science Center at Houston, Houston, TX, USA.
Maria Laura Rubin, Email: Maria.L.Rubin@uth.tmc.edu, Department of Biostatistics, University of Texas School of Public Health, Houston, TX, USA.
Barbara C. Tilley, Email: Barbara.C.Tilley@uth.tmc.edu, Department of Biostatistics, University of Texas School of Public Health, Houston, TX, USA.
H. Julia Hannay, Email: Julia.Hannay@times.uh.edu, Department of Psychology, University of Houston, Houston, TX, USA.
Claudia S. Robertson, Email: claudiar@bcm.edu, Department of Neurosurgery, Baylor College of Medicine, Houston, TX, USA.
References
- 1.Utter GH, Shahlaie K, Zwienenberg-Lee M, Muizelaar JP. Anemia in the setting of traumatic brain injury: the arguments for and against liberal transfusion. J Neurotrauma. 2011;28(1):155–165. doi: 10.1089/neu.2010.1451. [DOI] [PubMed] [Google Scholar]
- 2.Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care. 2013;17(2):R66. doi: 10.1186/cc12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg JA, McGwin G, Jr, Griffin RL, Huynh VQ, Cherry SA, 3rd, Marques MB, Reiff DA, Kerby JD, Rue LW., 3rd Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65(2):279–82. doi: 10.1097/TA.0b013e31817c9687. discussion 282-4. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., 3rd Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69(6):1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg JA, McGwin G, Jr, Marques MB, Cherry SA, 3rd, Reiff DA, Kerby JD, Rue LW., 3rd Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65(4):794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 7.Robertson CS, Hannay HJ, Yamal J, Gopinath S, Goodman JC, Tilley BC the EPO Study Group. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamal J, Rubin ML, Benoit JS, Tilley BC, Gopinath SP, Hannay HJ, Doshi PB, Aisiku I, Robertson CS. Effect of Hemoglobin Transfusion Threshold on Cerebral Hemodynamics and Oxygenation. J Neurotrauma. doi: 10.1089/neu.2014.3752. Epub 2015 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JDF. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. Public Library of Science Medicine. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Junco DJ, Fox EE, Camp EA, Rahbar MH, Holcomb JB. Seven deadly sins in trauma outcomes research: An epidemiologic post-mortem for major causes of bias. J Trauma Acute Care Surg. 2013;75:S97–S103. doi: 10.1097/TA.0b013e318298b0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, Marin-Caballos A, Amaya-Villar R, Marin A, Puppo-Moreno A, Ferrandiz-Millon C, Flores-Cordero JM, Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36(4):1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, et al. for the ABLE Investigators and the Canadian Critical Care Trials Group. Age of Transfused Blood in Critically Ill Adults. N Engl J Med. 2015;372(15):1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 13.Phelan HA, Gonzalez RP, Patel HD, Caudill JB, Traylor RK, Yancey LR, Sperry JL, Friese RS, Nakonezny PA. Prestorage leukoreduction ameliorates the effects of aging on banked blood. J Trauma. 2010;69(2):330–337. doi: 10.1097/TA.0b013e3181e0b253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67(1):29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.