To the Editor
We recently published an article in Ear and Hearing on “Auditory Function in Normal-Hearing, Noise-Exposed Human Ears” (Stamper & Johnson, 2015). In this article, we presented electrophysiological and otoacoustic emission (OAE) data from 30 normal-hearing human subjects with varying amounts of noise exposure background (NEB), as quantified by a detailed self-report questionnaire, the noise exposure questionnaire (NEQ). The results revealed that high-level click-evoked wave I amplitudes of the auditory brainstem response (ABR) decreased as a function of NEB. In contrast, distortion-product OAE (DPOAE) level was not found to vary with NEB. These findings are consistent with previous reports from noise-exposed guinea pigs and mice (Kujawa & Liberman 2009; Lin et al 2011; Furman et al. 2013) that suggest noise exposures that do not result in permanent threshold change may result in permanent auditory damage. Furthermore, evidence of this damage is only present when examining ABR wave I amplitudes in response to high-level stimuli.
Since the publication of this manuscript, we have been contacted by several individuals regarding a possible confounding influence of subject sex on the results reported in Stamper and Johnson (2015). Based on these discussions, and consultations with the editorial staff at Ear and Hearing, we are providing additional information regarding the sex of subjects who participated in the study. Specifically, we present the sex distribution of all 30 subjects and briefly revisit data published in Stamper and Johnson (2015).
Table 1 displays subject NEQ values (rounded to the nearest integer) along with subject number, age, sex, and ABR wave I and V amplitude and latency. These data were collected in response to click stimuli presented at 90 dB nHL and were recorded using a mastoid electrode (see Stamper & Johnson, 2015, for a full description of testing methods). On average, female subjects exhibited larger wave I amplitudes when compared to males subjects (479 nV versus 359 nV, respectively). There was essentially no difference in wave V amplitudes between the two sexes (662 nV versus 660 nV, respectively). NEQ values for female subjects (n=20) ranged from 67 to 83 LAeq8760h and were distributed across this range. NEQ values for male subjects (n=10) were somewhat more restricted, ranging from 70 to 82 LAeq8760h, with the majority reporting NEQ values > 75 LAeq8760h (80% of male participants) and no male subjects with NEQ values < 70 LAeq8760h. These data indicate male and female subjects were not evenly distributed across the NEQ range.
Table 1.
Study participant characteristics.
| ABR Amplitude (nV) | ABR Latency (msec) | ||||||
|---|---|---|---|---|---|---|---|
| NEQ | Age | Sex | Wave I | Wave V | Wave I | Wave V | |
| 1 | 67 | 23 | F | 518.36 | 789.59 | 1.46 | 5.68 |
| 2 | 67 | 22 | F | 572.48 | 989.03 | 1.60 | 5.64 |
| 3 | 67 | 26 | F | 561.14 | 595.92 | 1.64 | 5.56 |
| 4 | 67 | 23 | F | 586.01 | 733.96 | 1.56 | 5.46 |
| 5 | 68 | 24 | F | 401.20 | 909.18 | 1.79 | 5.39 |
| 6 | 69 | 21 | F | 611.00 | 641.02 | 1.62 | 5.27 |
| 7 | 69 | 23 | F | 554.06 | 512.33 | 1.56 | 5.70 |
| 8 | 69 | 23 | F | 529.70 | 500.27 | 1.50 | 5.62 |
| 9 | 69 | 23 | F | 781.23 | 535.76 | 1.60 | 5.58 |
| 10 | 70 | 25 | M | 344.90 | 723.62 | 1.50 | 5.58 |
| 11 | 70 | 22 | M | 224.68 | 771.96 | 1.68 | 5.70 |
| 12 | 71 | 22 | F | 336.70 | 453.97 | 1.68 | 5.70 |
| 13 | 72 | 19 | F | 671.68 | 519.09 | 1.62 | 5.80 |
| 14 | 73 | 21 | F | 411.65 | 709.63 | 1.54 | 5.33 |
| 15 | 74 | 21 | F | 299.87 | 1110.99 | 1.59 | 5.07 |
| 16 | 74 | 26 | F | 555.25 | 788.44 | 1.56 | 5.33 |
| 17 | 75 | 23 | M | 488.12 | 568.29 | 1.60 | 5.15 |
| 18 | 76 | 21 | F | 416.18 | 654.78 | 1.54 | 5.56 |
| 19 | 77 | 23 | M | 167.47 | 457.04 | 1.62 | 5.48 |
| 20 | 77 | 19 | F | 372.08 | 673.21 | 1.52 | 5.46 |
| 21 | 77 | 24 | F | 308.55 | 626.42 | 1.66 | 5.58 |
| 22 | 78 | 21 | M | 249.22 | 765.50 | 1.56 | 5.62 |
| 23 | 78 | 21 | M | 372.90 | 755.49 | 1.56 | 5.46 |
| 24 | 79 | 20 | M | 485.81 | 287.33 | 1.58 | 5.52 |
| 25 | 81 | 28 | M | 387.80 | 868.44 | 1.64 | 5.53 |
| 26 | 82 | 23 | M | 308.14 | 638.80 | 1.60 | 5.87 |
| 27 | 82 | 21 | F | 335.14 | 723.79 | 1.44 | 5.44 |
| 28 | 82 | 20 | M | 564.29 | 760.78 | 1.54 | 5.41 |
| 29 | 82 | 27 | F | 313.61 | 219.43 | 1.56 | 5.64 |
| 30 | 83 | 28 | F | 447.64 | 553.45 | 1.68 | 5.46 |
Subjects are ordered by NEQ value (rounded to the nearest integer).
ABR, auditory brainstem response; NEQ, noise exposure questionnaire.
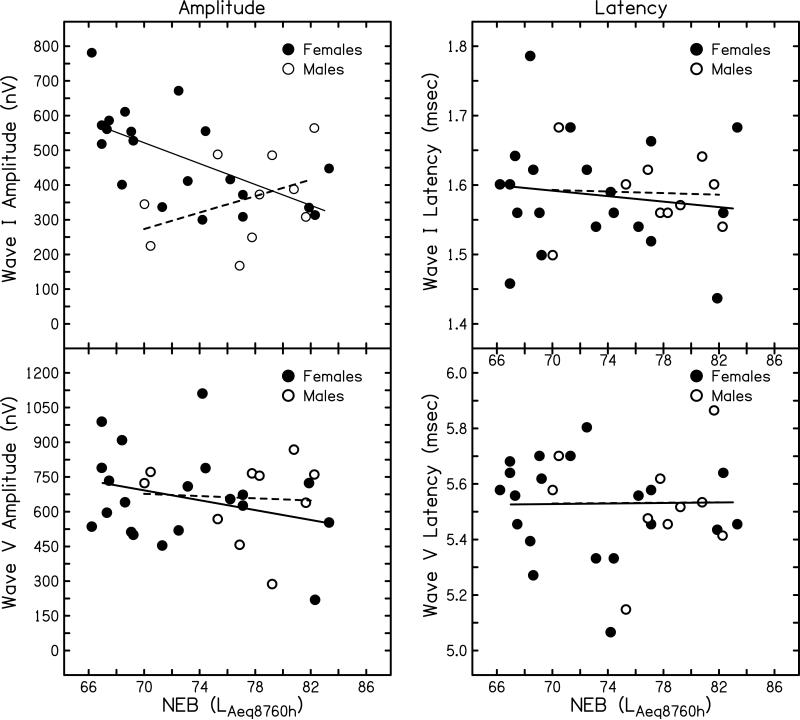
ABR amplitudes (left column) and latencies (right column) are plotted as a function of NEB in Fig. 1. Data for wave I are plotted in the top row and data for wave V are plotted in the bottom row. These data were collected with a mastoid recording electrode in response to click stimuli presented at 90 dB nHL and correspond to the numbers reported in Table 1. The amplitude data plotted in Fig. 1 also correspond to the data shown the upper left panels of Fig. 4 (wave I) and Fig. 6 (wave V) published in Stamper and Johnson (2015). In contrast to the previously published versions of these data, the data plotted in Fig. 1 include different symbols to indicate subject sex. Specifically, female subjects are plotted as filled circles and male subjects are plotted as open circles. Linear regression analysis was completed on each sex individually and the resulting regression lines are shown (solid line = females; dashed line = males). For females, ABR wave I amplitude decreased as a function of NEB (p=0.005, r2=0.360). For males, although the trend was for wave I amplitude to increase with NEB, ABR wave I amplitude was not significantly correlated with NEB (p=0.302, r2=0.132). When compared to the results with males and females combined, as described in Stamper and Johnson (2015), female participants revealed the same overall findings as initially described while males did not. While the reasons for this difference between males and females are not clear, several possible explanations will be discussed below. In contrast to the findings for wave I, wave V amplitude was not significantly correlated with NEB in either sex (females: p=0.231, r2=0.079; males: p=0.888, r2=0.003), consistent with the findings reported in Stamper and Johnson when data for the sexes were pooled.
Figure 1.
Suprathreshold ABR wave I and wave V amplitude and latency recorded with a mastoid electrode in response to click stimuli at 90 dB nHL. ABR amplitude (in nV) is shown as a function of NEB for wave I (top left) and wave V (bottom left). ABR latency (in msec) is shown as a function of NEB for wave I (top right) and wave V (bottom right). Symbols represent individual responses (females: filled circles; males: open circles). Linear regression lines are shown separately for each sex (females: solid line; males: dashed line).
The latency data plotted in Fig. 1 (and reported in Table 1) were not included in Stamper and Johnson (2015). However, because latency has been reported to vary between males and females, particularly for wave V (e.g., Picton et al., 1981), latency data are included here for a complete description of the variability in ABR features between the sexes. There were essentially no differences between the two sexes for wave I latency or wave V latency. On average, females had wave I and wave V latencies of 1.59 and 5.51 msec, respectively, while males had wave I and wave V latencies of 1.59 and 5.53 msec, respectively. Linear regression analyses did not reveal any significant relationship between NEB and wave I latency (females: p=0.535, r2=0.022; males: p=0.898, r2=0.002) or wave V latency (females: p=0.522, r2=0.023; males: p=0.908, r2=0.002) for either sex.
Sex differences in ABR amplitudes and latencies have been reported previously in the literature (e.g., Jerger and Hall, 1980; Picton et al, 1981; Mitchell et al, 1989). In general, normal-hearing males exhibit smaller amplitudes for ABR waves I, III, and V. Likewise, latency for ABR waves III and V is shorter in females than males. A less consistent difference between the sexes is reported for ABR wave I latency, with some studies reporting shorter latencies in females (e.g., Trune et al., 1988; Mitchell et al., 1989) and others not reporting a difference (e.g., Picton et al., 1981; Watson et al., 1996). One proposed reason for these sex differences is that males tend to have larger head and brain sizes when compared to females (Allison et al., 1983; Dempsey et al., 1986). However, even when head size is controlled, a sex effect appears to remain (Trune et al. 1988). Dehan and Jerger (1990) suggested that a combination of head size and hormonal factors are responsible for the sex differences. Finally, Don et al. (1993) have argued that sex differences in latency and amplitude are due to faster cochlear response times and increased synchrony in females that arise as a consequence of the shorter cochlear length in females (Sato et al., 1991).
Although sex differences are reported throughout the literature for both wave I and V amplitude, only wave I amplitude appears to differ between the sexes in our sample. The lack of a difference in male and female latency is consistent with the generally equivocal findings in the literature for wave I latency, but is not consistent with previous reports of sex differences for wave V latency. It is possible that our male-female amplitude difference for wave I is driven by the factors that have been suggested by others (e.g., physical size differences between the sexes, especially cochlear length, or hormonal influences). It is not clear why these factors would influence wave I amplitude but not wave V amplitude. In addition to having smaller average wave I amplitude, our male subjects also had higher average NEQ values than our female subjects. It is possible that the higher average NEQ levels in our male subjects resulted in these subjects having smaller wave I amplitudes but not wave V amplitudes. A disassociation between wave I and V amplitudes was described by Schaette and McAlpine (2011), where wave I amplitude was reduced in normal-hearing subjects with tinnitus relative to controls, while wave V amplitude did not vary between the groups. If noise exposure is a factor contributing to a difference in wave I amplitude between males and females, it is not clear why wave I amplitude did not vary statistically with NEB and showed a trend opposite of what would be expected in male subjects (i.e., larger wave I amplitude in males with greater NEB). We do note that the NEB range for our male subjects is more restricted than for our female subjects. No male subject had NEQ values < 70 LAeq8760h. Considerable variability in wave I amplitude for a given NEQ value was observed for both male and female subjects; however, the largest amplitude values for females tended to be for those subjects with NEQ values < 70 LAeq8760h. Without similar data for low NEQ values in males, it is impossible to know if males would show a similar relationship between wave I amplitude and NEB. Although we feel that exploring the interaction of sex, NEB, and ABR variables is an important question, our sample size is too limited to draw any definitive conclusions.
To summarize, the results of Stamper and Johnson (2015) showed a statistically significant relationship between suprathreshold ABR wave I amplitude and NEB. When analyzed for individual sex differences, female participants show the same pattern of a decrease in wave I amplitude with increases in NEB. In male participants, it is our belief that not enough data exist to clearly understand the relationship and the reader is cautioned regarding drawing firm conclusions. Future studies investigating the influence of noise exposure history and electrophysiological markers in normal-hearing, noise-exposed human ears should consider sex as a potentially confounding variable. Without further empirical evidence, it is unknown what sex influence, if any, is present in this population.
ACKNOWLEDGEMENTS
The work described here was supported by a Student Investigator Research Grant from the American Academy of Audiology/American Academy of Audiology Foundation and by a grant from the NIH NIDCD (R03 DC011367).
REFERENCES
- Allison T, Wood CC, Goff WR. Brain stem auditory, pattern-reversal visual, and short-latency somatosensory evoked potentials: Latencies in relation to age, sex, and brain and body size. Electroencephalogr Clin Neurophysiol. 1983;55:619–636. doi: 10.1016/0013-4694(83)90272-9. [DOI] [PubMed] [Google Scholar]
- Dehan CP, Jerger JJ. Analysis of gender differences in the auditory brainstem response. Laryngoscope. 1990;100:18–24. doi: 10.1288/00005537-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Dempsey J, Censoprano E, Mazor M. Relationship between head size and latency of the auditory brainstem response. Int J Audiol. 1986;25:258–262. [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Masuda A. Gender differences in cochlear response time: An explanation for gender amplitude differences in the unmasked auditory brainstem response. J Acoust Soc Am. 1993;94:2135–2148. doi: 10.1121/1.407485. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger G, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerson SC. Published Dissertation, University of Kansas. ProQuest; Ann Arbor: 2010. Development of a screening tool for identifying young people at risk for noise-induced hearing loss. [Google Scholar]
- Mitchell C, Phillips DS, Trune DR. Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear Res. 1989;40:75–86. doi: 10.1016/0378-5955(89)90101-9. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stapells BA, Campbell KB. Auditory evoked potentials from the human cochlea and brainstem. J Otolaryngol Suppl. 1981;10:1–41. [PubMed] [Google Scholar]
- Sato H, Sando I, Takahashi H. Sexual dimorphism and development of the human cochlea. Computer 3-D measurement. Acta Otolaryngol. 1991;111:1037–1040. doi: 10.3109/00016489109100753. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015;36:172–184. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS. The relative importance of head size, gender and age on the auditory brainstem response. Hear Res. 1988;32:165–174. doi: 10.1016/0378-5955(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Watson DR. The effects of cochlear hearing loss, age and sex on the auditory brainstem response. Audiology. 1996;35:246–258. doi: 10.3109/00206099609071945. [DOI] [PubMed] [Google Scholar]