Synopsis
Contrast enhanced breast MRI is increasingly being used to diagnose breast cancer and to perform biopsy procedures. The American Cancer Society has advised women at high risk for breast cancer to have breast MRI screening as an adjunct to screening mammography. This article places special emphasis on biopsy and operative planning involving MRI and reviews utility of breast MRI in monitoring response to neoadjuvant chemotherapy. We describe peer-reviewed data on currently accepted MR-guided therapeutic methods for addressing benign and malignant breast diseases, including intraoperative imaging.
Keywords: Breast cancer, needle biopsy, MR-guidance, lumpectomy, extent of disease, tumor ablation, neoadjuvant chemotherapy
Problem/clinical presentations
Use of contrast enhanced breast MRI: Contrast enhanced breast MRI is an important adjunctive modality for screening and diagnosis of breast cancer. MRI has been demonstrated as beneficial and used increasingly as an adjunct to mammography [1] in screening in a subset of women at high risk for developing breast cancer because of its high sensitivity and negative predictive value. MRI is being used to assess response for neoadjuvant chemotherapy treatment (NACT), detect otherwise occult breast cancer presenting as metastatic axillary or systemic disease, evaluate extent of disease in patients with newly diagnosed breast cancer, and assess contralateral breast. Additional clinical trials are needed to determine the significance of MRI-detected, otherwise occult disease [2].
MR-guided tissue sampling: In cases in which MRI alone detects a suspicious lesion (i.e., no correlative finding with other methods), MR-guided tissue sampling is needed to determine the underlying histopathology.
Margin status at breast conserving therapy (BCT). The current positive or close margin rate at initial surgery requiring an additional operation with re-excision estimated ranging from 30% to 60% [3, 4]. There is no ideal method for margin evaluation during surgery. However, there are trials in progress on the use of MRI guidance and MR evaluation of the margins intra-operatively with the goal of reducing the need for additional operations [5].
Need for MR-guided procedures
Recommendations for performance of breast MRI are conditioned on a standard level of quality of MRI studies with high spatial resolution images. The American College of Radiology (ACR) accreditation process includes the requirement for facilities to have the ability to provide MRI-guided biopsy when offering breast MRI [6].
When a suspicious lesion has been detected by breast MRI, and biopsy for histologic diagnosis is suggested, the first step should be to evaluate the area by mammography and targeted ultrasound for a possible correlate [7]. Ultrasound guidance is preferred over MRI for biopsy if a sonographic correlate can be identified [7]. Ultrasound is readily available and ultrasound-guided biopsies are quicker, more comfortable for the patient, do not require intravenous contrast and are less expensive. An ultrasound correlate can be identified in approximately half of the cases [7, 8]. If the findings of this approach are unrevealing or uncertain, an MRI-guided biopsy should be performed [9–15].
Breast MR Imaging and techniques
There are widespread variations in breast MR imaging techniques, with different approaches to balance morphology, kinetic information, and use of fat saturation versus subtraction techniques. Obtaining a good quality breast MRI is conditioned on many factors: use of a high-field-strength magnet and a dedicated breast coil, appropriate breast positioning, injection of gadolinium contrast material, high-spatial-resolution imaging without artifacts, and specified adequate timing of the dynamic sequences.
The following MRI equipment specifications and performance must meet all state and federal requirements and should apply and the ACR practice parameters and technical standards guidelines including routine quality control should apply [6]. Field strength: A 1.5Tesla (T) or 3T magnet has typically been utilized for breast MR; Positioning: All routine clinical breast MR examinations are performed with the patient in prone position with simultaneous bilateral imaging utilizing a dedicated (bilateral) breast MRI coil containing 2 individual depressions for the left end right breast. Prone positioning helps to move the breasts away from the chest wall and minimizes respiratory and cardiac motion effects [16]; Resolution, contrast and artifacts: The slice thickness should be 3 mm or less; in-plane pixel resolution should be 1 mm or less so as to reduce the problem of volume averaging and to detect and characterize small abnormalities. Chemical fat suppression is helpful as a method for reducing the fat signal. Misregistration due to patient motion can occur, and both subtraction imaging for assessment of enhancement and fat suppression are recommended. Motion correction may aid in reducing artifacts encountered with image subtraction; Contrast: Gadolinium intravenous contrast is needed in the evaluation of breast cancer. Dynamic kinetic information based on enhancement data at appropriate time intervals is extremely important for lesion classification.
Challenges in MRI-guided breast biopsy targeting
Many of the challenges experienced with MRI-guided biopsy are similar to those encountered using stereotactic biopsy with patients prone on a dedicated table and are related to targeting (i.e. difficulty with posterior targets or those that are superficial), positioning and compression (e.g., an accordion effect at clip deployment or problems with very breasts). Furthermore, the patient needs to be removed from the magnet in order to be repositioned for the biopsy to be performed, as there is somewhat limited access to the medial and posterior breast. Additional difficulties may arise, including contrast washout, lesion location related problems, and/or limitations in confirming lesion sampling. [9, 17]
Cancellation of the procedure is frequent (reported as between 8% to13%) [18]. Non-visualization of the suspicious finding may be due to change in tissue enhancement as the patient is in different phase of her period and/or may be related to compression of breast tissue with decreased inflow of contrast material. Signal-void artifact from needles, obturators and wires used in the MRI setting and hemorrhage (hyperintense on T1 sequences) may obscure the target. Air entered or generated from needle placement frequently interferes with target visualization.
MRI-guided breast core needle biopsy
A dedicated breast MR-coil and prone positioning on a moveable exam table is typically required with MR-conditional biopsy equipment. Usually a larger needle (11–14 gauge) and vacuum assistance are used for sampling, although smaller, spring-activated 14–18-gauge sizes are also available. Needle susceptibility artifact should be reduced by appropriate imaging protocol without compromising image quality and lesion detection.
The grid technique is widely implemented because of its ease of use. Other localizing methods include pillar and post, and free-hand techniques [13, 14]. Protocols may differ among facilities. Usually, after localizing images, axial and sagittal T1-weighted, fat saturated images are obtained prior to and following injection of the contrast agent in the area of interest. The imaging protocol should minimize image acquisition time while maintaining lesion visualization. There is a short period following the administration of the intravenous contrast during which the area of interest can be visualized. Following identification, targeting the lesion includes identifying the correct opening within the grid and the correct hole in the introducer for needle insertion. Most systems offer an approach from the lateral, or from the medial direction. There is frequently tissue displacement during the needle insertion and repeated adjustments in needle positioning may be required (Figure 1.). Placing a marker clip at the biopsy site, typically followed by two mammographic views to document clip location is recommended. Vacuum assisted core biopsy tissue sampling with MR-guided devices has been shown to be technically successful in 94%-98% of cases and is an accepted alternative for histopathologic assessment to surgical biopsy [9, 10, 17].
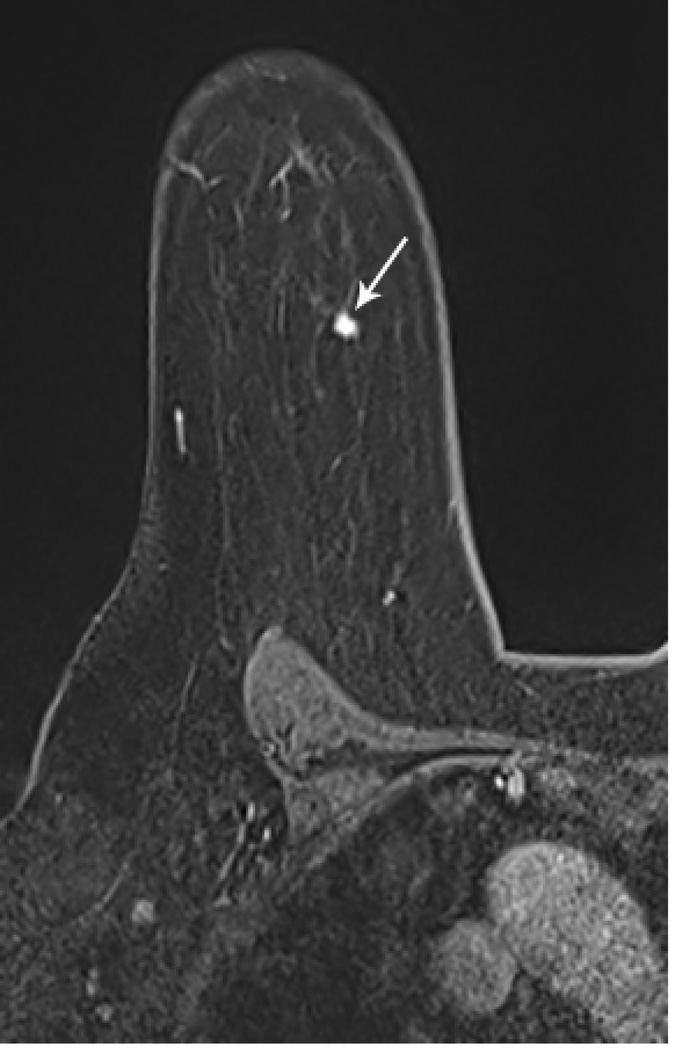
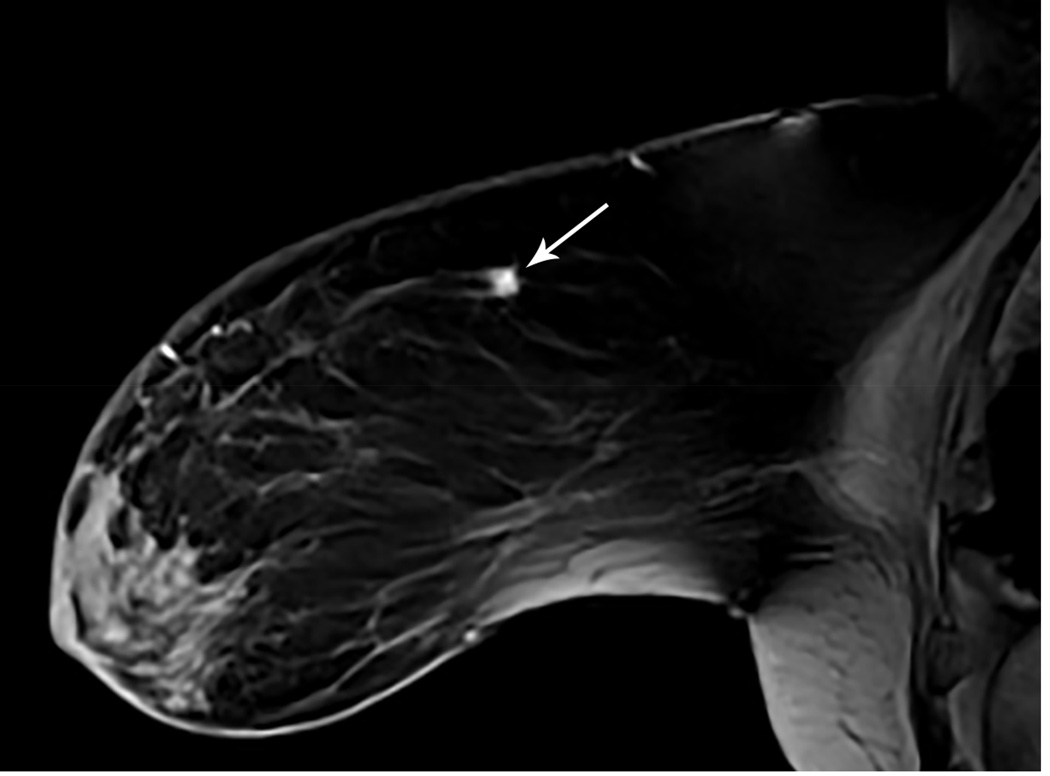
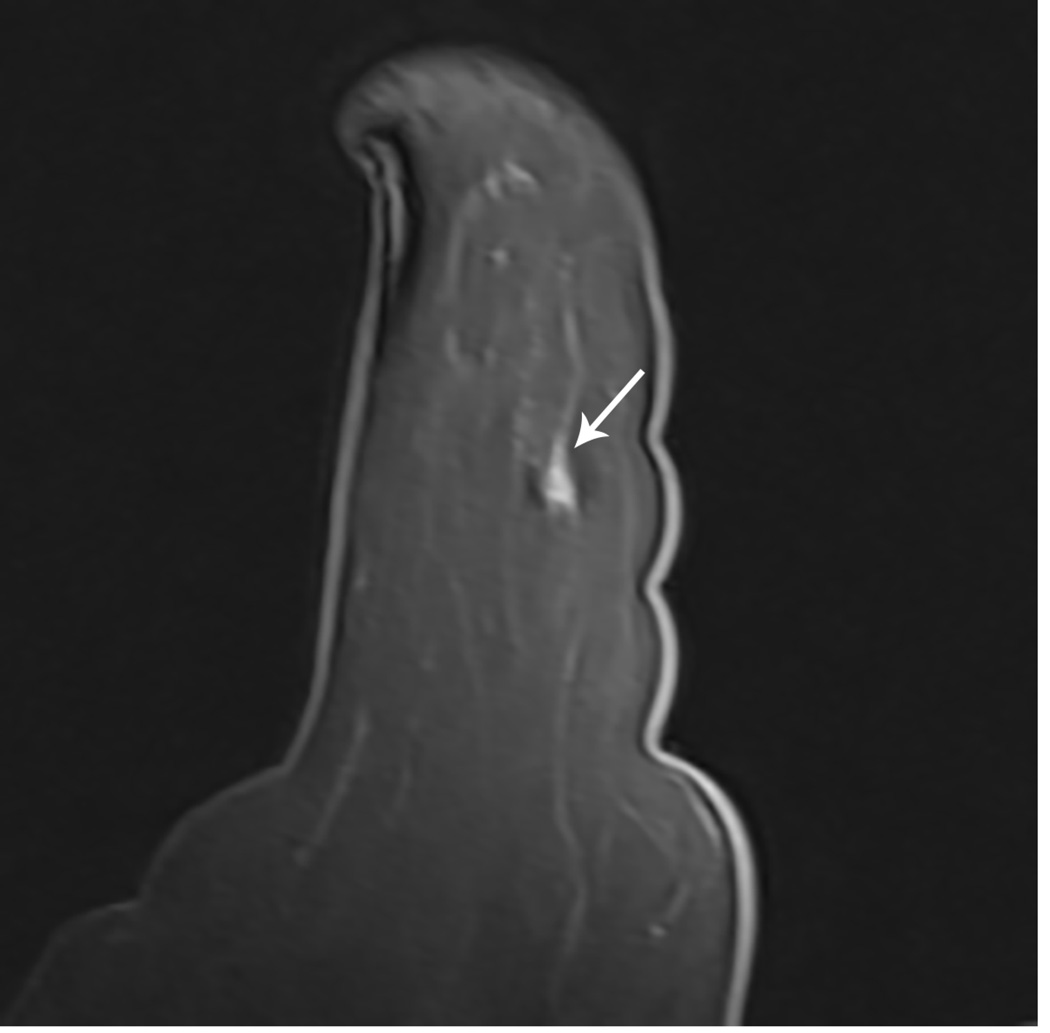
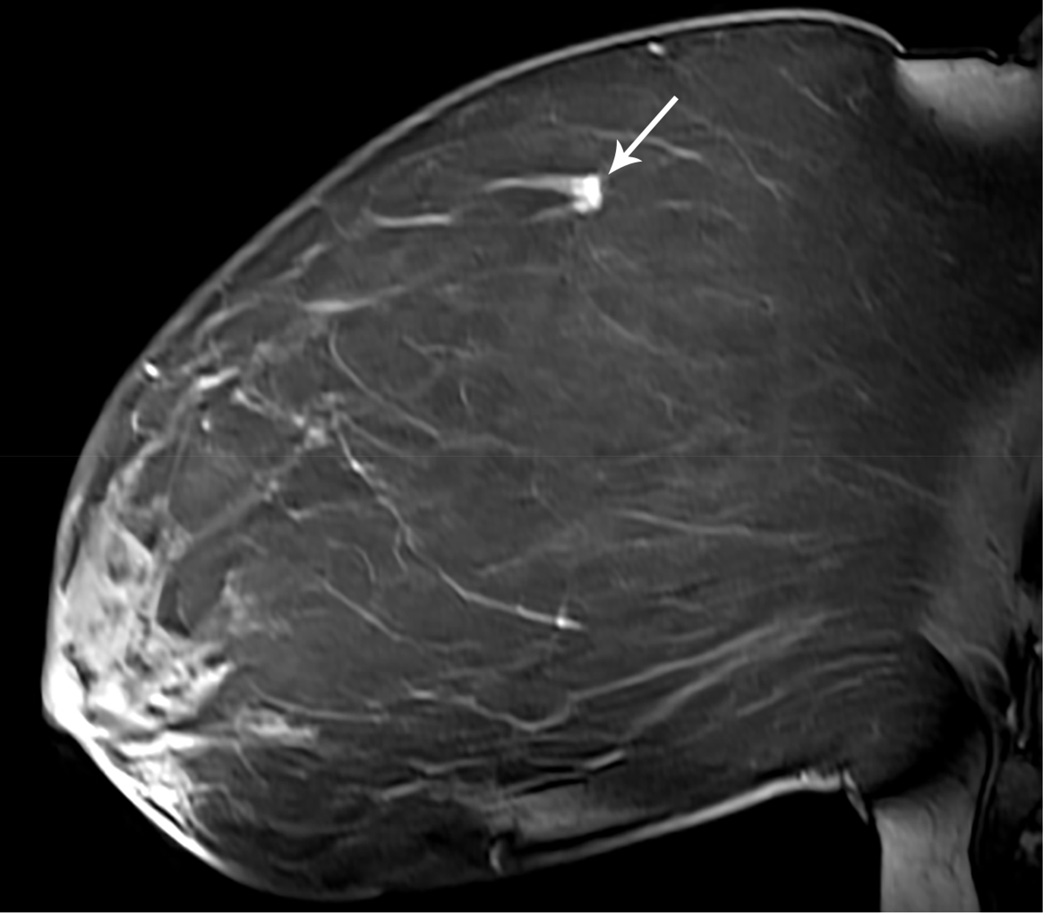
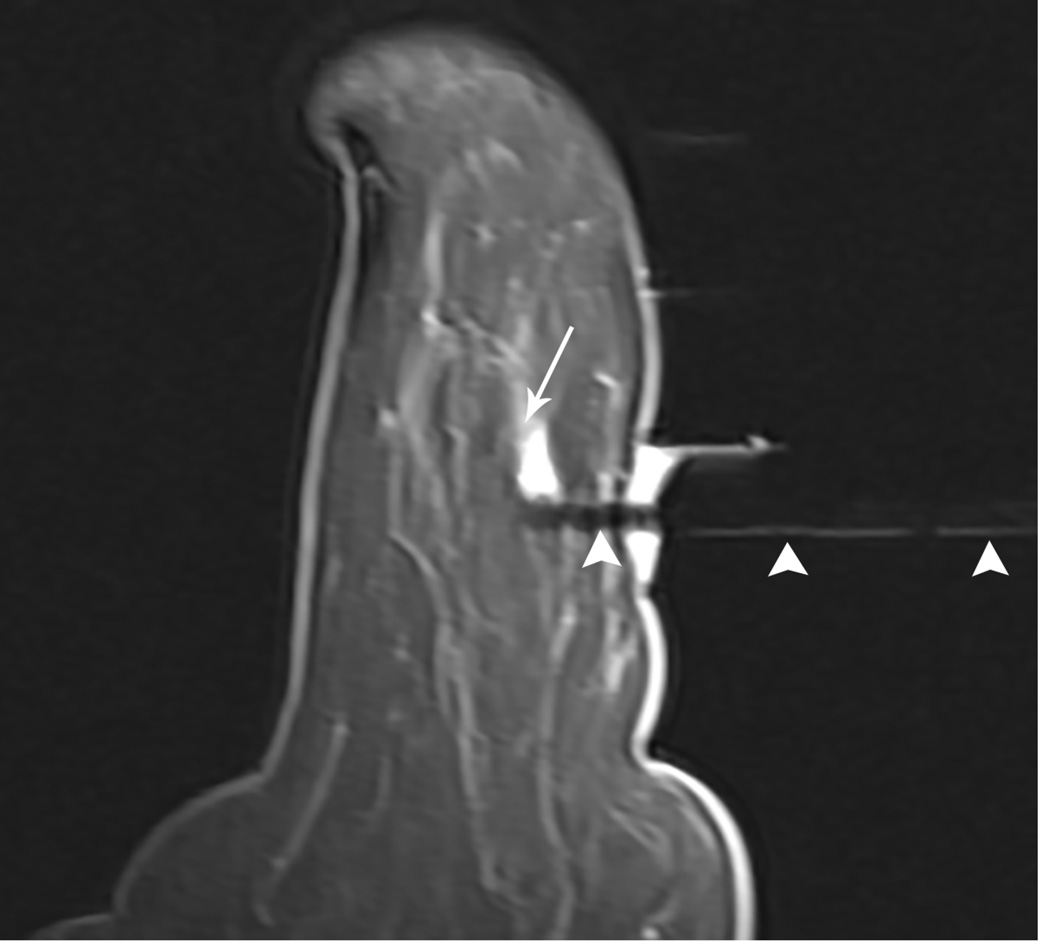
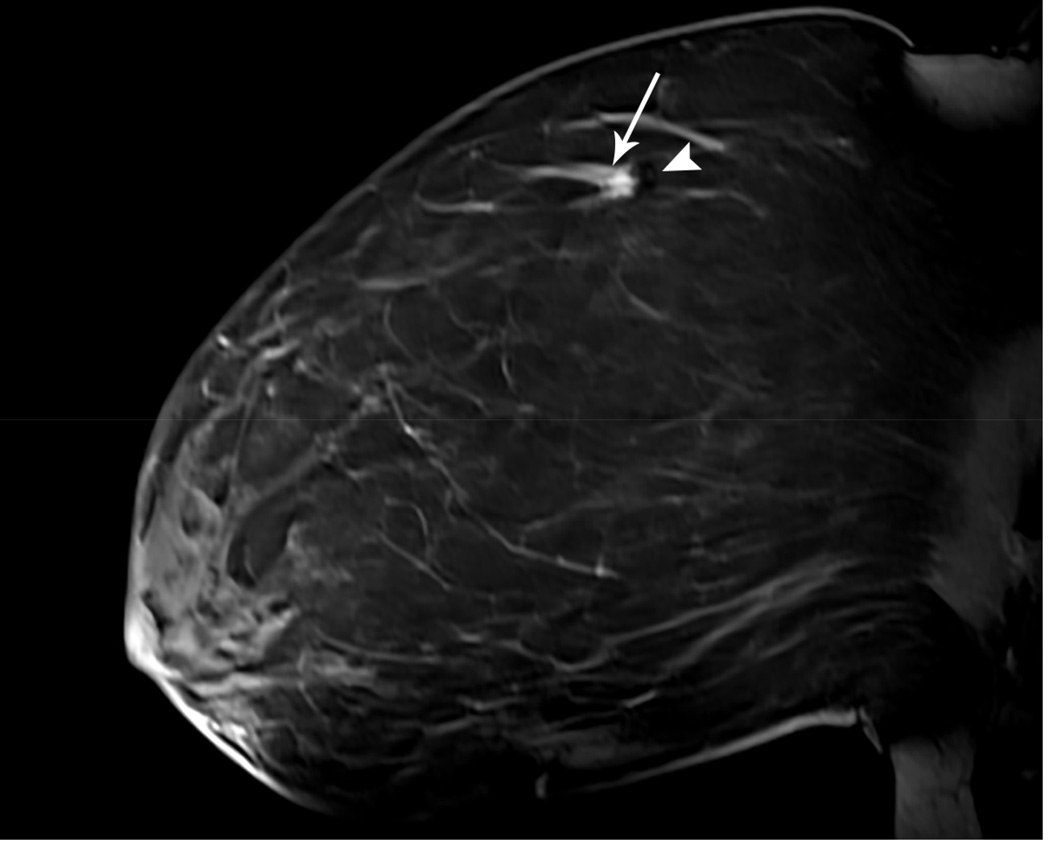
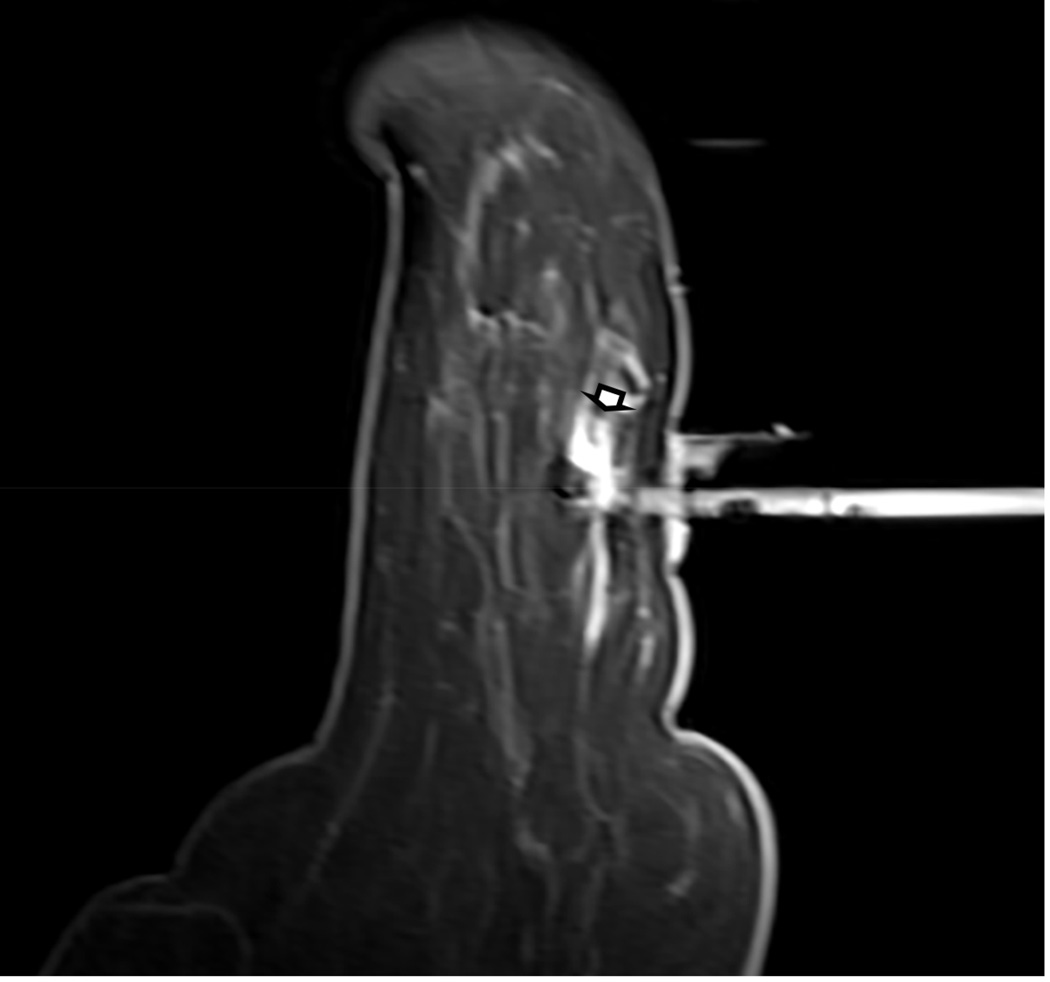
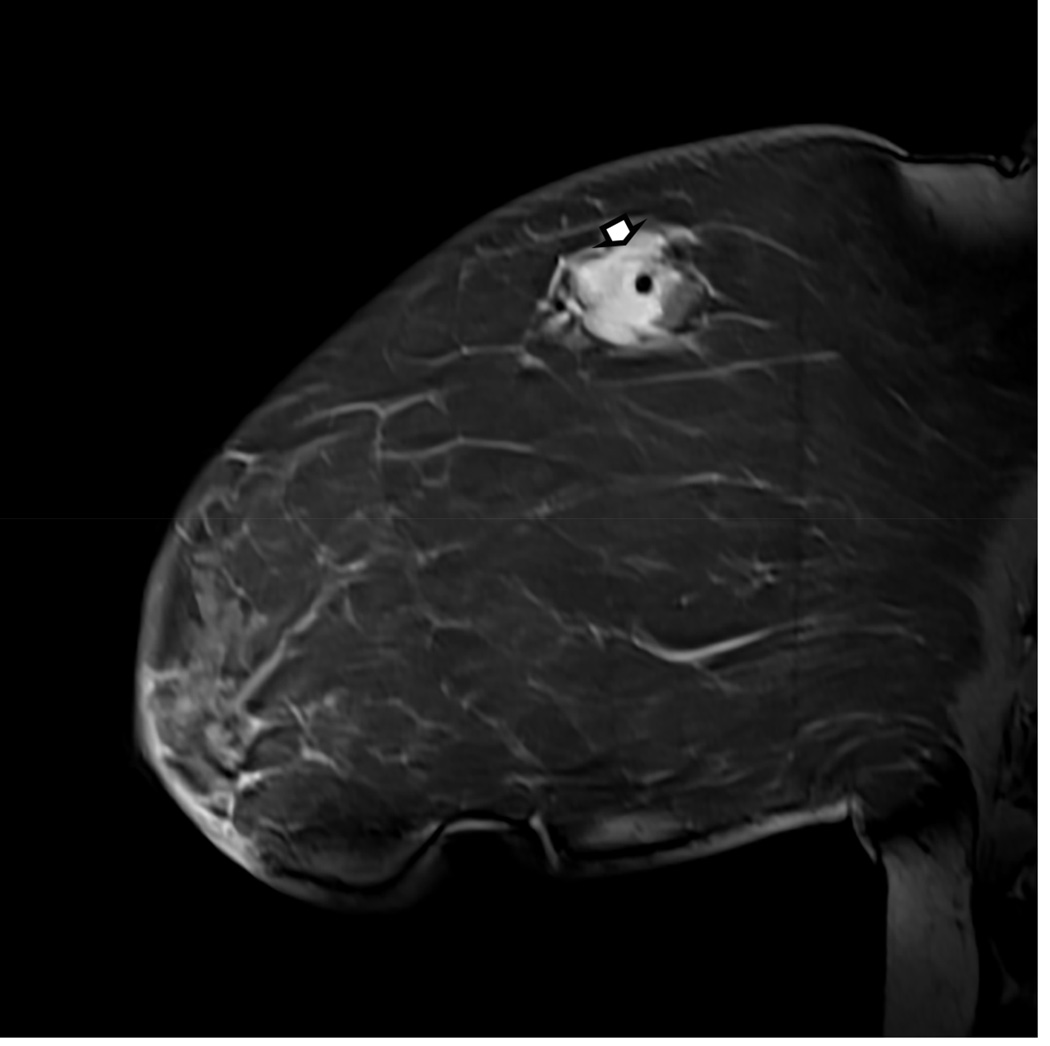
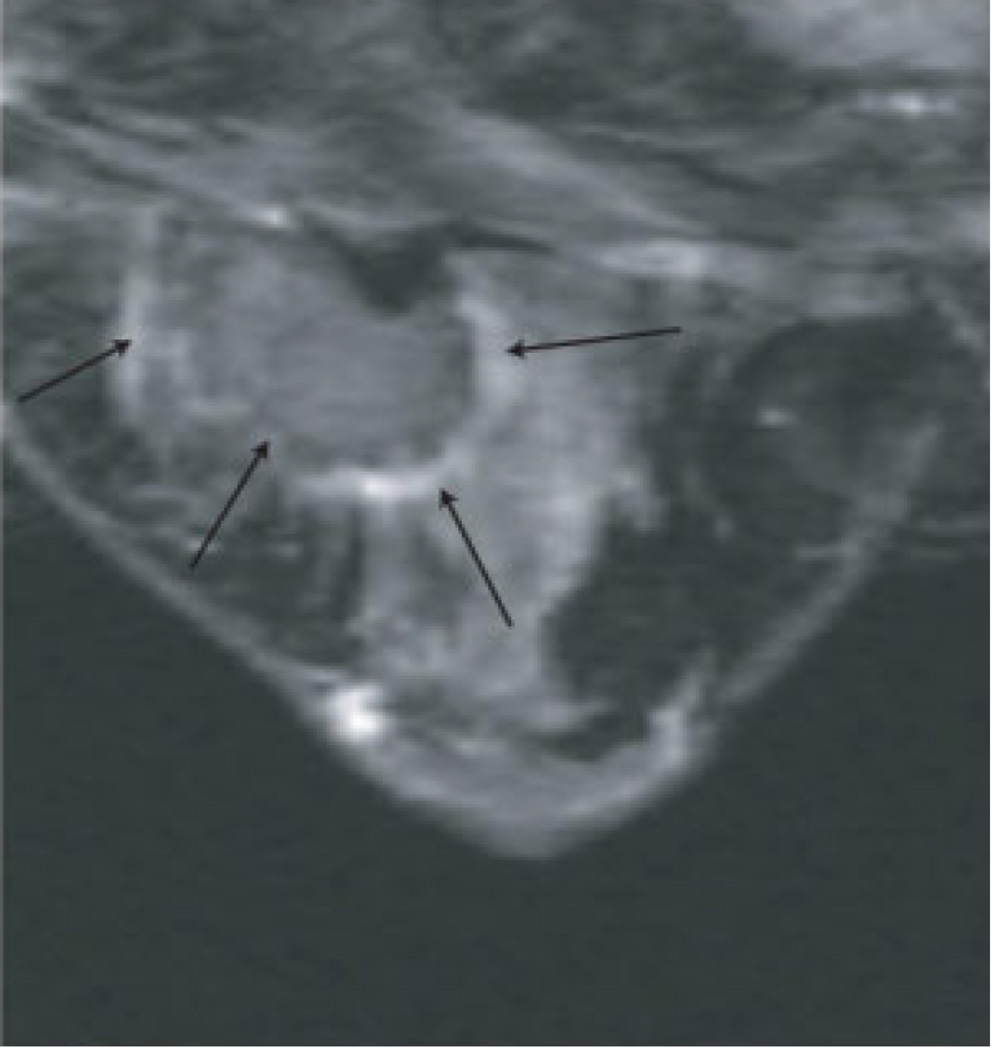
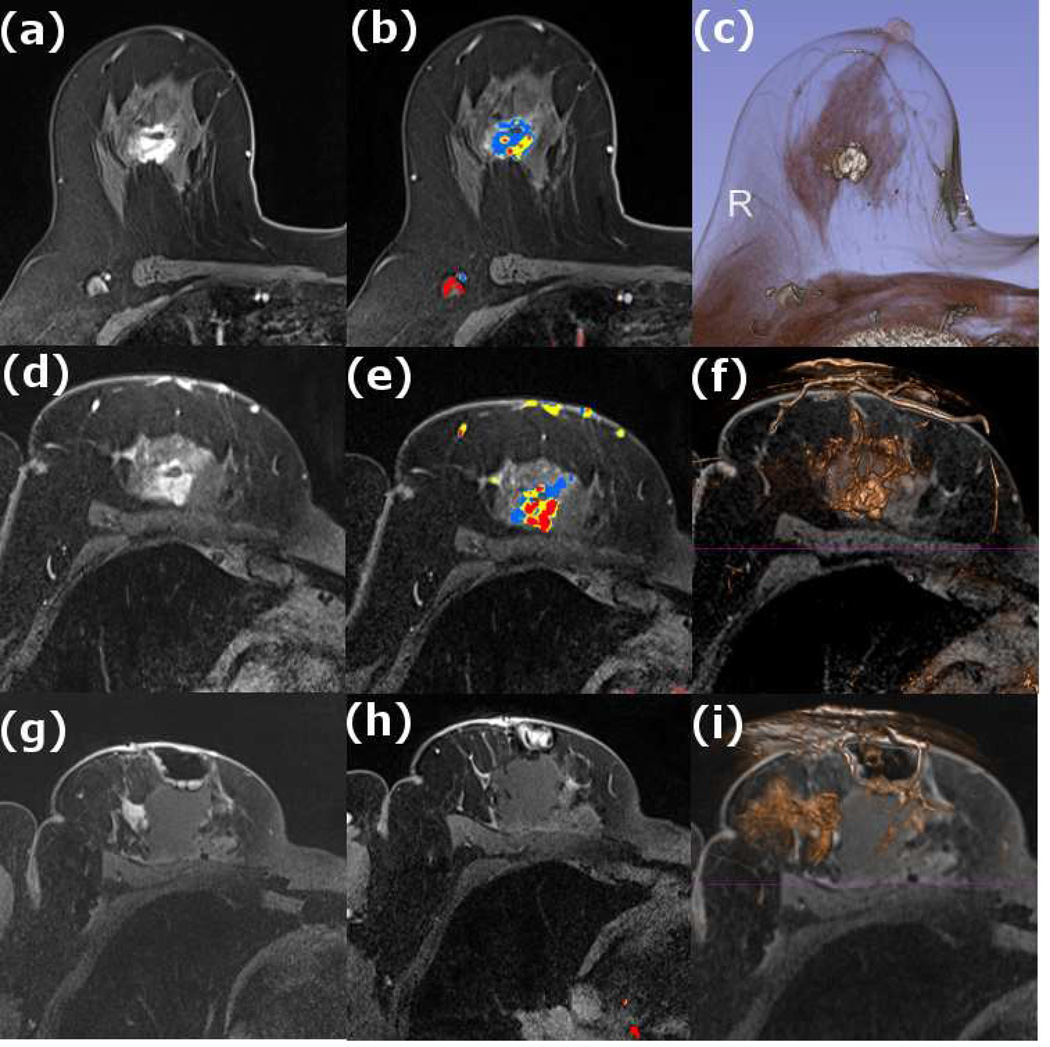
Figure 1. MR guided core needle biopsy planning and procedure.
Baseline MRI identifies a suspicious 7mm mass in the upper inner right breast (axial and sagittal images, A, B), not seen clearly by other imaging modalities; a decision about MR-guided biopsy was made. A high signal intensity fiducial marker is placed on a grid hole. Following localizing images, precontrast images and axial and sagittal postcontrast sequences (C, D) are obtained to re-identify the target. Needle insertion site is determined by measuring the target location relative to the fiducial’s position. After placement of the obturator, axial and sagittal sequences are obtained to confirm proper depth and location prior to the biopsy (E, F). Further sagittal and axial images are performed following the biopsy to show hyper-intense hematoma - developed in this case at the site - and to verify the deployment and location of the marker clip (signal void artifact) (G, H).
Long arrows = targeted mass; short arrow = hematoma; arrow head = obturator.
MR-guided wire localization
The first MR-guided interventional procedure developed was needle localization prior surgery. The procedure is occasionally performed when the extent of disease is not apparent by conventional imaging modalities, and therefore, pre-lumpectomy localization is best done with MRI. Currently, MRI-guided core biopsy has replaced many MRI-needle localizations. Excision is sometimes considered when core biopsy is not possible (e.g., there is a posterior target location or an extremely small breast) or per patient’s preference [12].
The positioning and targeting for needle localization is the same as that for needle biopsy. Following lesion is identification and location determination, a guide needle is introduced to the appropriate depth. After imaging confirms appropriate location and depth, an MR-conditional localization hook wire is deployed through the needle. The guiding needle is similar to the Kopans needle used for mammographic localizations. The MR wire is softer than conventional, non-MR-compatible wires and therefore deployment in hard fibrous tissue may occasionally be difficult and they have a tendency to break during surgery [17]. Following the localization procedure, a mammogram can visualize for the surgeon the site of the wire within breast tissue, and nipple and chest-wall positions.
Pathology correlation of MR-guided biopsies
Evaluating concordance is important in all image-guided biopsies, and especially important for MR-guided biopsies as sampling accuracy is subject to uncertainty. Concordance decisions begin in the planning phase with the radiologist defining the expected pathology result based on original images. As there is no specimen image confirmation of the target (as in a stereotactic core biopsy specimen X-ray showing calcifications), nor direct visualization of sampling (as in ultrasound guided biopsies with real time observation of sampling), accuracy can be difficult to determine from core biopsy images. The procedure radiologist should review images to determine whether procedure images support lesion retrieval. The final decision about concordance vs. discordance is made when the radiologist decides if pathology results agree with the expected outcome.
Based on the radiologist’s degree of certainty regarding satisfactory tissue sampling, benign concordant histologic results may warrant short-term (six-month) follow-up MRI to confirm stability, [11]. For discordant lesions, surgical excision is recommended. Imaging histologic discordance rate has been reported as approximately 7%-9% [9, 18]. Higher rates of imaging–histologic discordance and underestimation of atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS) have been reported with MRI-guided biopsies than with stereotactic mammographic biopsies [19, 20].
For cases that have been assessed as possibly missed or discordant, repeat biopsy or surgical excision is recommended. MRI-guided core biopsy malignancy rates varying between 16–37% have been reported [9, 12, 15, 17, 18].
Pathology examination of excisional biopsy specimen of MR-guided wire localization: MR imaging of a breast specimen with current clinical scanners is not useful for lesion detection as detection is based on visualization by enhancement with the injected contrast agent. Gross examination and specimen radiography do not identify most of the malignancies in MRI-localized procedures. For that reason, optimal pathology processing of MRI-guided excisions requires microscopic examination of the entire specimen tissue [21].
Surgical planning with pre-operative MRI following neoadjuvant chemotherapy
Systemic chemotherapy improves survival for patients with invasive breast cancer. It is the standard of care for node positive patients and is used for many patients with high-risk node-negative disease with invasive breast cancer. During the past approximately 20 years, there has been an option to administer chemotherapy prior to surgery (neoadjuvant chemotherapy) rather than following surgery (adjuvant chemotherapy) for those women requiring systemic therapy. The main advantage of preoperative neoadjuvant chemotherapy (NACT) is the reduction in primary tumor size and conversion from node-positive into node-negative status. NACT is used for treatment of locally advanced breast cancer to allow for surgery in cases in which skin or pectoral muscle is involved (Figure 2.). NACT is also used in early stage breast cancer to enable breast-conserving (BCT) therapy when originally, mastectomy was planned, or to achieve better cosmetic outcomes due to smaller surgical resection volume. Despite less extensive surgery following NACT, several studies showed similar local recurrence rates with preoperative NACT compared to adjuvant chemotherapy, though some studies suggested a trend for higher locoregional recurrence [22, 23]. The National Surgical Adjuvant Breast and Bowel Project B18 and other clinical trials comparing neoadjuvant and adjuvant chemotherapy found that there is no significant difference in overall or disease disease-free survivals between patients receiving adjuvant or neoadjuvant chemotherapy; however, more women undergoing preoperative chemotherapy were eligible and received breast conservation treatment [22, 24, 25].
Figure 2. Tumor response can render previously inoperable tumors operable, leading to increased breast conservation rate and in smaller resection volumes.
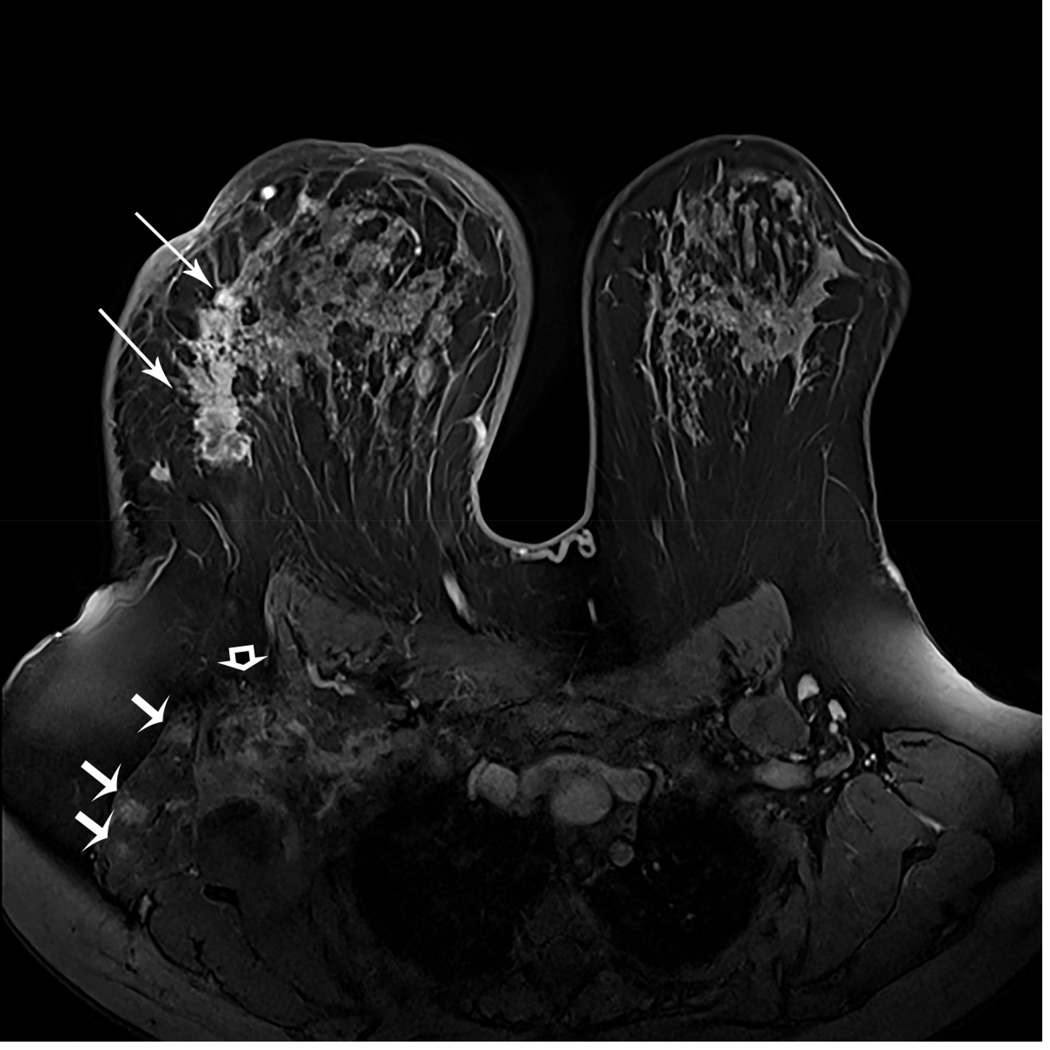
A 43-year-old woman with a new diagnosis of triple negative inflammatory right breast cancer. Baseline MRI shows an extensive, large, irregular mass (long arrows) and multiple ill-defined masses with rim-enhancement in the right lateral chest wall musculature (short arrows). Axillary adenopathy is present (curved arrow) (A). The patient underwent neoadjuvant chemotherapy.
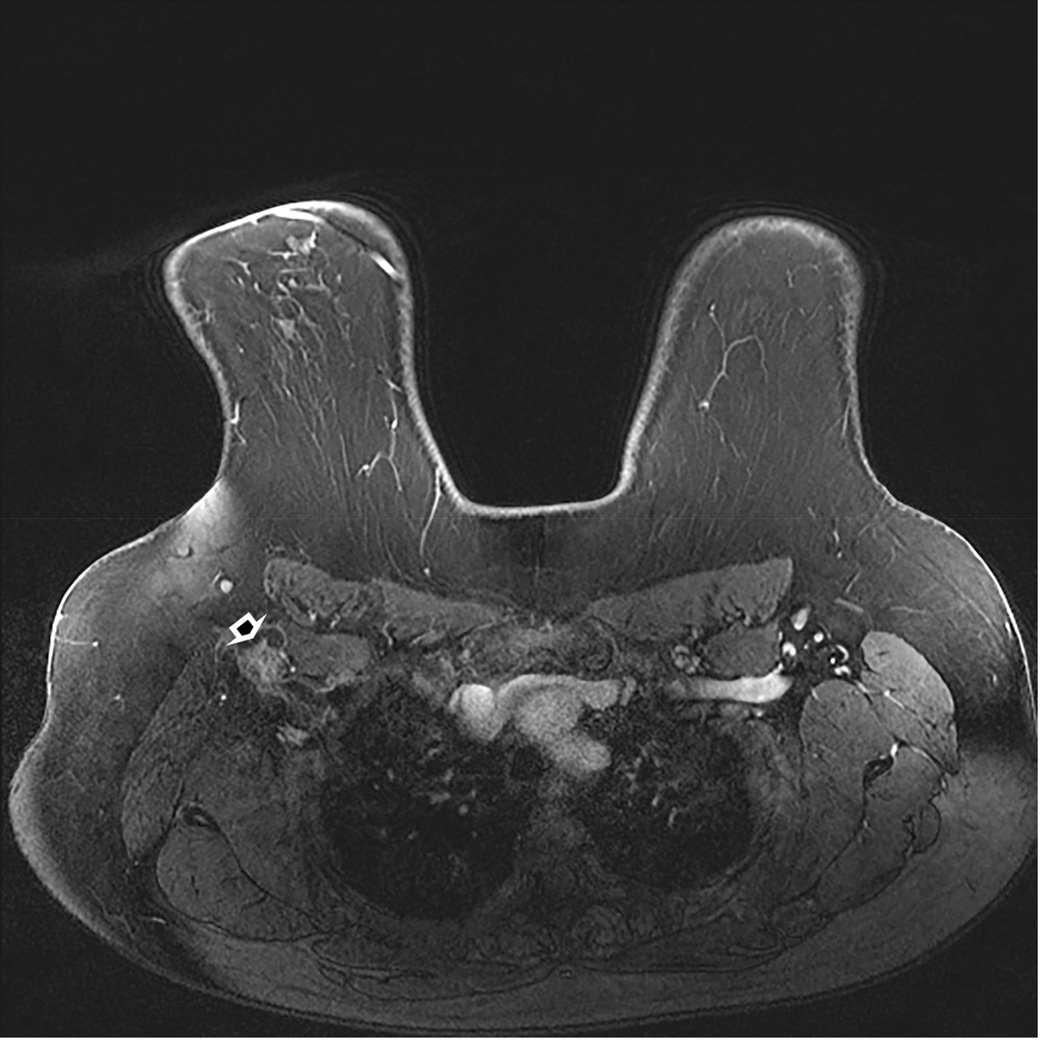
Pre-surgical MRI five months later shows disappearance of the previous large mass and disappearance of the lateral chest wall masses. Remaining ill-defined axillary adenopathy is seen (curved arrow) (B). The patient underwent right mastectomy with axillary dissection. There was no residual carcinoma on histopathology examination in the 8.5 cm fibrous tumor bed. Thirteen removed lymph nodes showed treatment effect but with no carcinoma. Summary: pathologic complete response (Miller-Payne 5 and Residual Cancer Burden 0).
Accurate monitoring of NACT response is essential; imaging may demonstrate stable or progressive disease, or remission, and even complete response (Box 1). Pathological complete response (pCR) is defined as the absence of any residual invasive tumor cells in the original tumor bed; however, residual DCIS may be present (Figure 3.). Attaining pCR following NACT has been shown as a prognostic factor for overall better survival, and for disease-free survival [23].
Box 1. Monitoring of response in neoadjuvant chemotherapy (NACT).
Monitoring of response in neoadjuvant chemotherapy (NACT)
The amount of residual invasive cancer following therapy is an important prognostic predictor.
Pathological complete response (pCR) is consistently associated with a favorable outcome, especially in estrogen receptor (ER) negative [ERBB2 (HER2/NEU) positive and triple negative] tumors.
Close monitoring of tumor response is required.
Best monitoring modality: contrast-enhanced breast MRI (DCE-MRI)
Data have shown that MRI is superior to clinical examination and other breast imaging methods regarding accuracy and PPV in determining post NACT pathologic tumor response.
Accuracy of MRI is highest in ER/PR negative [ERBB2 (HER2/NEU) positive and triple negative] tumors (Figure 4).
Figure 3.
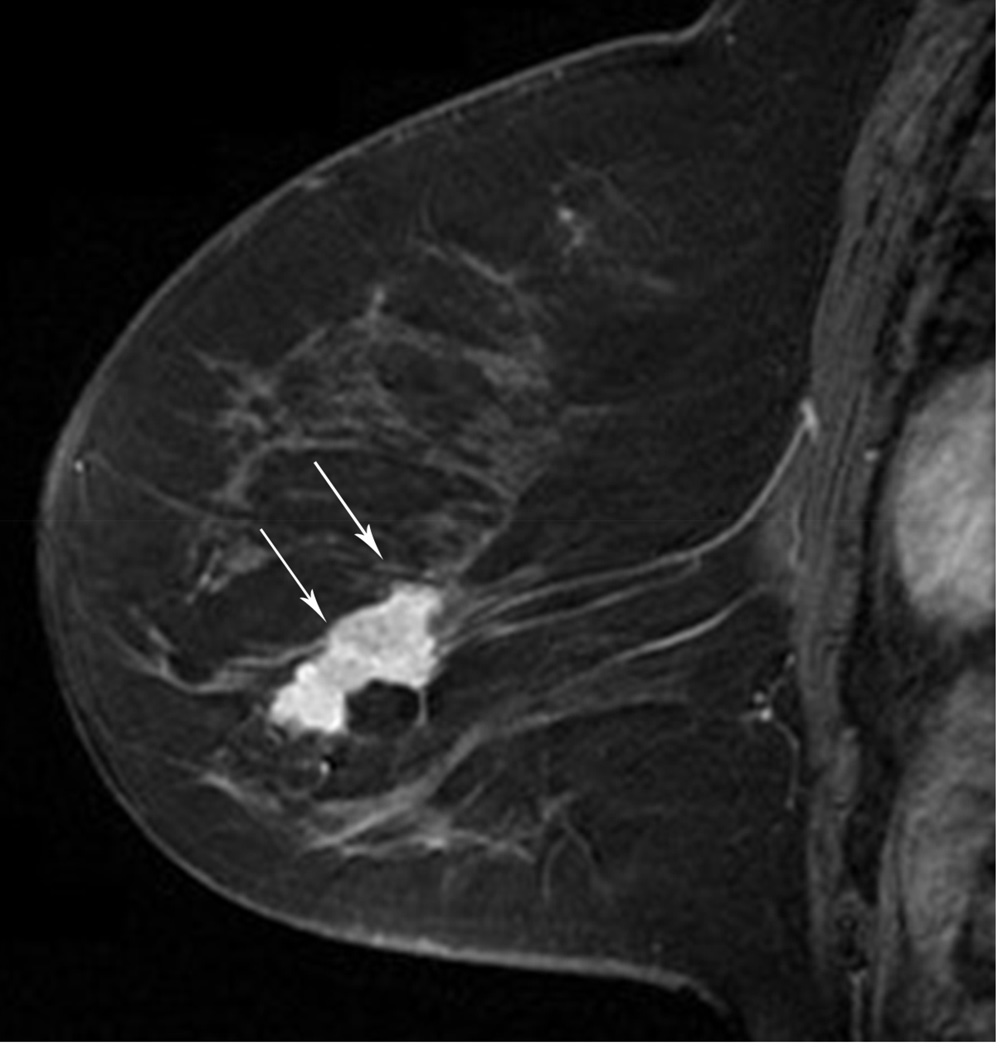
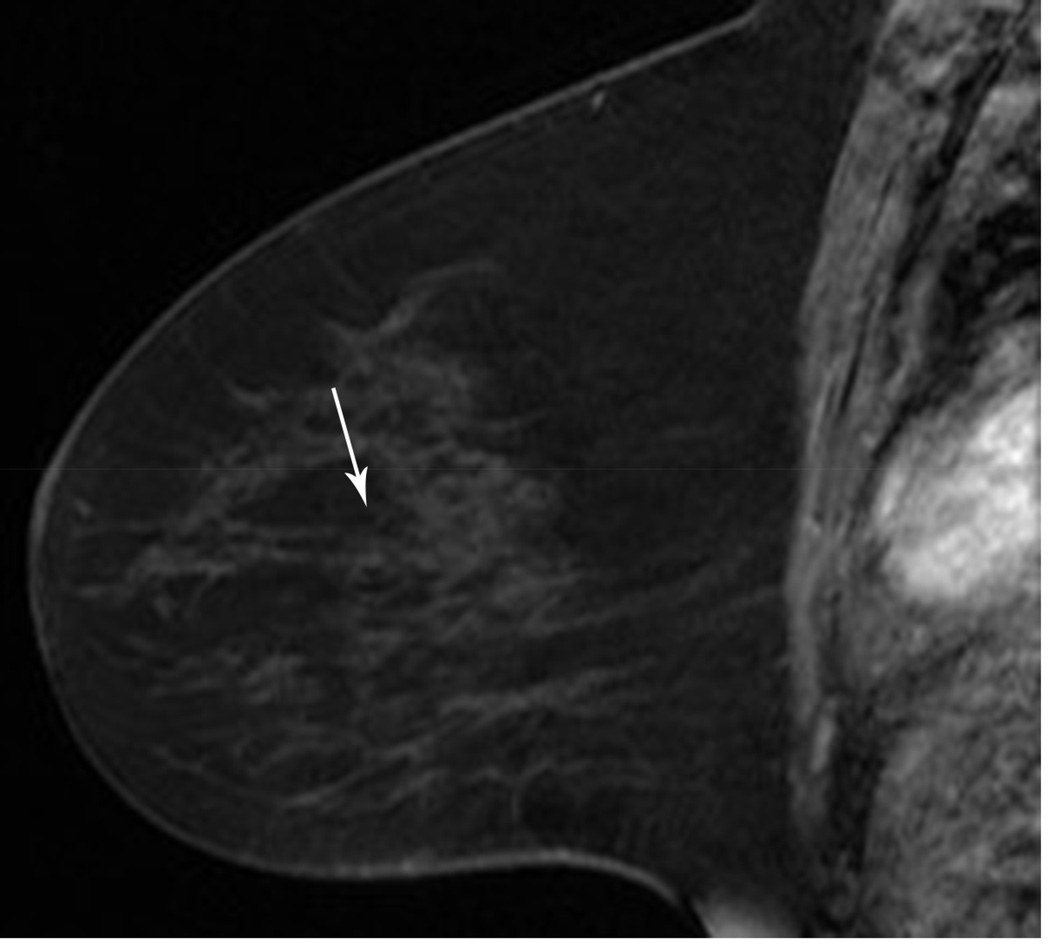
MRI has been shown to be of value in predicting tumor size when there is no response or there is a complete response. A 54 year-old woman with an ER/PR negative, ERBB2(Her-2/neu) positive, high-grade IDC. A. Baseline MR shows the known mass (arrows). B. Post-treatment MR shows no residual mass or enhancement in the area of primary tumor, only artifact from prior treatment (arrow). Lumpectomy pathology showed a 1.6 cm fibrous area consistent with treated tumor bed with scattered small foci of DCIS. Findings remain compatible with a Miller-Payne grade 5 response.
MRI-guided breast ablation
The aim of ablative therapy is to achieve a well-defined area encompassing the tumor, irreversible cell damage, protein denaturation, and coagulation necrosis, while sparing overlying and surrounding tissues. The role of imaging is to aid the clinician in planning the probe placement for optimal coverage, targeting the lesion, and monitoring the deposition of energy. The advantages of MRI-guidance in these tasks are 3D visualization via multiplanar, multi slice acquisition, high sensitivity, and delineation of breast lesions, and tissue thermal sensitivity. A therapeutic probe is percutaneously placed in the lesion to deliver cooling (cryoablation) or heating energy (radio frequency ablation, laser interstitial thermal therapy) so as to cause cell death. High intensity focused ultrasound (HIFU) can achieve these goals without use of an invasive probe. Ablative techniques may be useful in patients with benign lesions [26], those who refuse surgery [27], or patients with Stage 4 breast cancer who need palliative care [36], or patients with recurrent disease [28].
There are uncertainties that may prevent image-guided minimally invasive tumor ablation, in patients with early stage breast cancer, from becoming a viable alternative treatment for lumpectomy. It remains to conclusively show that clinical outcomes (clear margins, recurrence rate, morbitity, and mortality) are comparable to the standard of care, surgery followed by whole breast radiation. Careful inclusion criteria and control measures are critical elements.
MR-guided cryoablation
Percutaneous cryoablation using freezing temperatures is delivered by gas cooled probes [29]. Although most breast ablation has been guided by US [30], MRI is particularly well suited for monitoring the growth of the iceball. The iceball appears as a signal void due to the short T2* of the crystalized water and, unlike with US, the tissue beyond the iceball is not subject to shadowing [31].
In a feasibility study, Morin et al. [32] reported on the MRI-guided cryoablation in 25 patients with breast carcinoma without complications. Four weeks after treatment, surgical excision was performed for histopathologic correlation. Total ablation was achieved in 13 of the 25 tumors treated. Pusztaszeri et al. [33] reported on that in all ten of the evaluated patients undergoing MRI-guided cryotherapy followed by surgical excision, the iceball engulfed the tumor, but only two patients had a complete response. The authors suggested that components of undetected DCIS in the larger tumors were far from the two probes used. Five patients suffered from skin necrosis, a complication that can be avoided by selection criteria of minimum distance between the lesion and the skin or managed with the use of warm saline on the skin or saline injection [34]. In these studies, the patient was supine. More recently, Tozaki et al. [35] treated a single patient with core needle biopsy proven invasive ductal carcinoma without an intraductal component using a non-MRI compatible cryotherapy system. MR imaging of a prone patient with a breast coil was used to define the target tissue. An US system safely integrated into the MRI room was used to place the probes. At the nine-week MRI evaluation, the lesion was not enhancing and was shown to be inside the cryo-zone. No viable cancer cells were noted on histology following a lumpectomy at 14 weeks.
MRI temperature mapping in the breast
A tool common to the ablative techniques that use elevated temperature is non-invasive MRI temperature mapping based on temperature-sensitive MR parameters such as the proton resonance frequency, the diffusion coefficient, T1 and T2 relaxation times, magnetization transfer, the proton density, and temperature-sensitive contrast agents [36]. Through empirical experimentation, cell death can be correlated with thermal dose, which is derived from time-temperature curves [37]. Although Proton Resonance Frequency Shift is useful for measuring temperature in aqueous tissue, the chemical shift in fat is almost constant with the temperatures used in thermal ablation. However, the T1 temperature dependence can be exploited in fat [38, 39].
MR-guided Radio Frequency Ablation (RFA)
Radiofrequency ablation (RFA) refers to the destruction of tissue via the application of electromagnetic fields created by interstitial electrode delivery of energy (0.4 – 8 MHz). A dispersive electrode on the thigh or back is used to complete the electrical circuit. Current density is induced in the tissue, causing resistive heating. RF energy deposition is a function of tissue conductivity and is difficult to predict and control. The formation of the thermal lesion may be inhomogeneous, especially in regions of the tissue boundaries. Susceptibility artifacts around the probe during MR imaging may prevent accurate temperature monitoring. No monopolar commercial solution is currently available to remedy the problem of electromagnetic interference emitting from the RF generator manifesting as noise in the MR images. Several research sites have implemented gating [40] or filtering solutions.
Van den Bosch et al. performed MRI-guided RFA on three patients followed immediately by surgical excision for histopathologic correlation (Figure 5) [41]. US-guided large-core needle biopsy confirmed invasive ductal carcinoma in all three patients, with DCIS adjacent to the invasive lesion in the second and third patients. Patients were positioned prone in a 0.5T vertically open MRI scanner. Measurements from a fiber optic temperature probe were used for comparisons to MRI temperature mapping. Histopathology confirmed successful (100%) tumor ablation in one patient, and partial tumor destruction (33% and 50%, respectively) in two patients. The lesion size was probably underestimated on the MR in the latter two cases. It was noted that susceptibility artifact caused by the 6 mm diameter probe would create a challenge for temperature mapping in lesions <10 mm. A high success rate for the technique in other organs [42] may encourage industry to provide complete solutions for breast MRI-guided RFA.
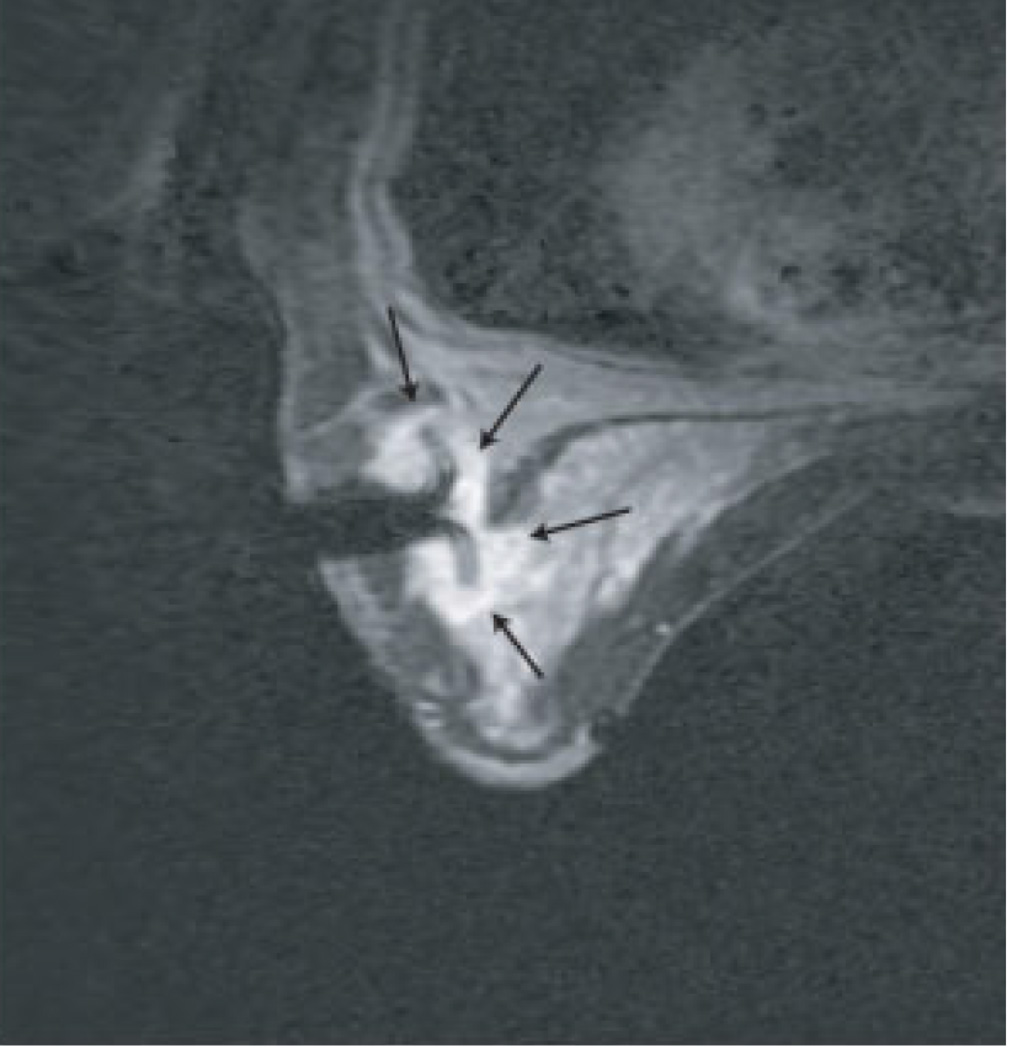
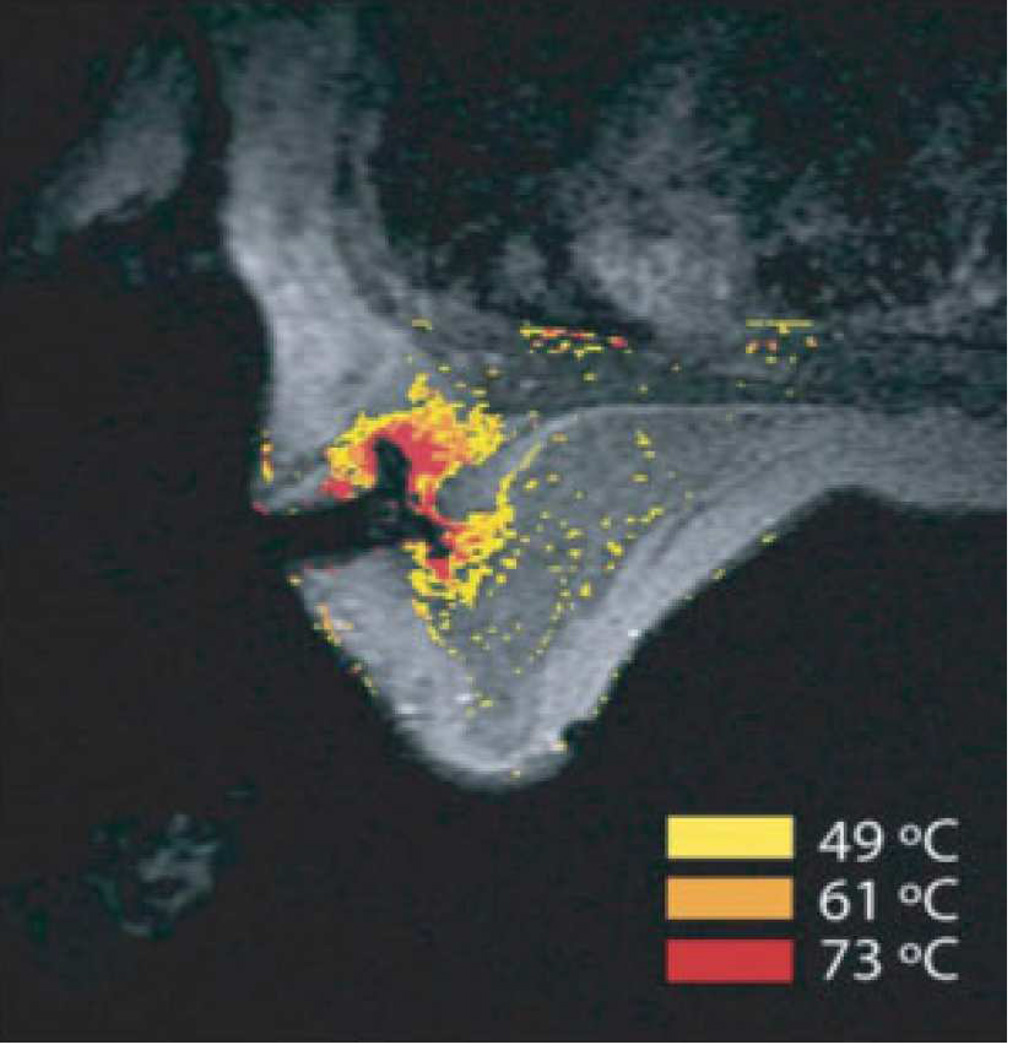
Figure 5. MRI-guided RF ablation.
Contrast-enhanced three-point Dixon gradient-echo images with patient in prone position showing the fully deployed LeVeen needle electrode (signal void) centrally in the enhancing tumor mass (arrows) in the right breast (A). Same axial positioning showing the magnetic resonance PRF shift thermomap (yellow zone 49 °C, orange 61 °C, red 73 °C) around the deployed RFA electrode centrally in the mass (B). Post-procedure contrast-enhanced water-selective, spectral-spatial [AU11] FSE image of the right breast demonstrates a small enhancing rim representing the border of the ablation zone corresponding to fresh scar tissue (C) (arrows).
(From van den Bosch et al. MRI-guided radiofrequency ablation of breast cancer: preliminary clinical experience. J Magn Reson Imaging 2008;27(1):204–208, with permission.)
MR-Guided Laser Interstitial Thermal Therapies (LITT)
During laser interstitial thermal therapy (LITT), light energy is delivered directly to tissue via percutaneous optical fiber, and creates a zone of thermal ablation. Optical fibers are inherently MRI-conditional and can be extended such that the laser device can be situated outside the scanner room. Larger lesions can be treated with the use of either diffusing tips or a beam splitter and multiple fibers [43].
LITT has been used successfully for the treatment of benign fibroadenomata [44, 45] and breast cancer in a number of institutions [46–48]. Use of MRI systems at field strengths as low as 0.2T have been reported for targeting and monitoring [49]. Mumtaz et al. [46] correlated pre- and post-procedural MRI with histopathology in a study of 20 women with proven breast cancer. The non-enhancing area of ablated tissue correlated well with necrotic area seen histopathologically.
Harms et al. [45] Although no histopathology correlation was available, tumor sizes were observed to be reduced on follow-up imaging at five months. The same group also investigated MRI-guided LITT for treatment of breast cancer in 12 women with 22 breast lesions. Complete destruction was achieved in only three women; they had tumors with diameters of less than 3 cm. In the nine other patients, tumors larger than 3 cm were incompletely destroyed [50].
MR-guided High Intensity Focused Ultrasound (HIFU)
In MR-guided High Intensity Focused Ultrasound (HIFU), focal heating of target tissue is achieved via deposition of acoustic energy (1–2MHz) generated by a piezoelectric transducer array that is acoustically coupled to the breast of a prone patient via a water bath. A temperature elevation to 55°–90°C is produced during a 10–20 s sonication [51].
Preliminary data with MRI-guided HIFU have shown partial or complete coagulation of targeted benign fibroadenomas [52]. The first case report of HIFU used to treat cancer with a 1.5-T magnet was in a 56-year-old patient with a 22-mm invasive breast cancer Huber et al. [53]. Gianfelice et al. [54] used HIFU with a 1.5-T system to treat 24 patients who either refused surgery or were at increased risk for surgery. Each patient with no evidence of metastatic disease underwent one or two ablation procedures for a single lesion smaller than 25 mm. Of the 24 patients, 19 (79%) had negative percutaneous needle biopsy results following the procedures. One patient experienced a second-degree skin burn, and no other complications were reported.
Forty five of the 57 patients enrolled in treat and resect protocols at three centers [55–58] had 100% of the lesion included in the treatment field, but only 21 had complete ablation upon histological examination. Four patients experienced skin burns that were either healed or resected in the surgical approach to the lesion. All studies enrolled patients with a single invasive tumor smaller than 3.5 cm that was greater than 1 cm from the skin and chest wall and a 1.5-T MRI was used for guidance. Each patient underwent a standard lumpectomy within 5 weeks of ablation. Technical failures included the inability to target 100% of the tumor volume or failure to deliver 100% of the planned thermal energy to the targeted area.
Furusawa et al. [59] treated 50 patients who did not subsequently have lumpectomy. The purpose of this phase III study was to determine the efficacy and safety of HIFU followed by radiotherapy as a local treatment for early breast cancer. The patients had a single biopsy-proven invasive tumor greater than 1 cm from the skin and chest wall. The average tumor size was 11.0mm (6 -15mm). Forty-one of the patients had their lesions completely treated. There were no severe adverse events and no local recurrence. Hardware has now been developed by multiple vendors [60] and efforts are underway to develop temperature mapping techniques that can simultaneously monitor aqueous and fatty tissue [61].
MR: Role in Surgical Planning Evaluation of extent of disease
There are several objectives for MRI evaluation of the ipsilateral breast in patients with a recent cancer diagnosis: tumor and possible additional foci location within the breast and in relationship to chest wall, possible chest wall involvement, and detection of axillary nodes/masses or internal mammary chain nodes. Studies have shown that MRI has superior sensitivity to conventional imaging for detecting clinically occult cancer foci in women with breast cancer [62, 63]. According to a 2008 meta-analysis, MRI detects additional ipsilateral disease in in an average of 16 percent of women with a known breast cancer [64].
MR imaging detection rates of finding more than one cancer focus are consistent with prior studies of breast cancer: Holland et al.'s classical pathology studies on serial sectioning of mastectomy specimens in patients with presumed single breast cancer sites identified additional disease further than 2 cm from the index tumor in 43% of the cases [65].
MR studies have described multifocality (additional site of cancer within the same quadrant) in 4–9% of women, and multicentricity (additional site of cancer in different quadrant) in 7–10% of cases [62, 66–68] (Figure 6.). Criticisms of breast MRI include that the additional disease found has no clinical impact because it will be treated with radiation therapy. The clinical significance of additional foci is not clear as local recurrence rates following breast-conserving therapy are low: at less than 10% in ten years [69]. These data suggest that radiation with adjuvant therapy can control the additional tumor foci not detected clinically or by conventional imaging.
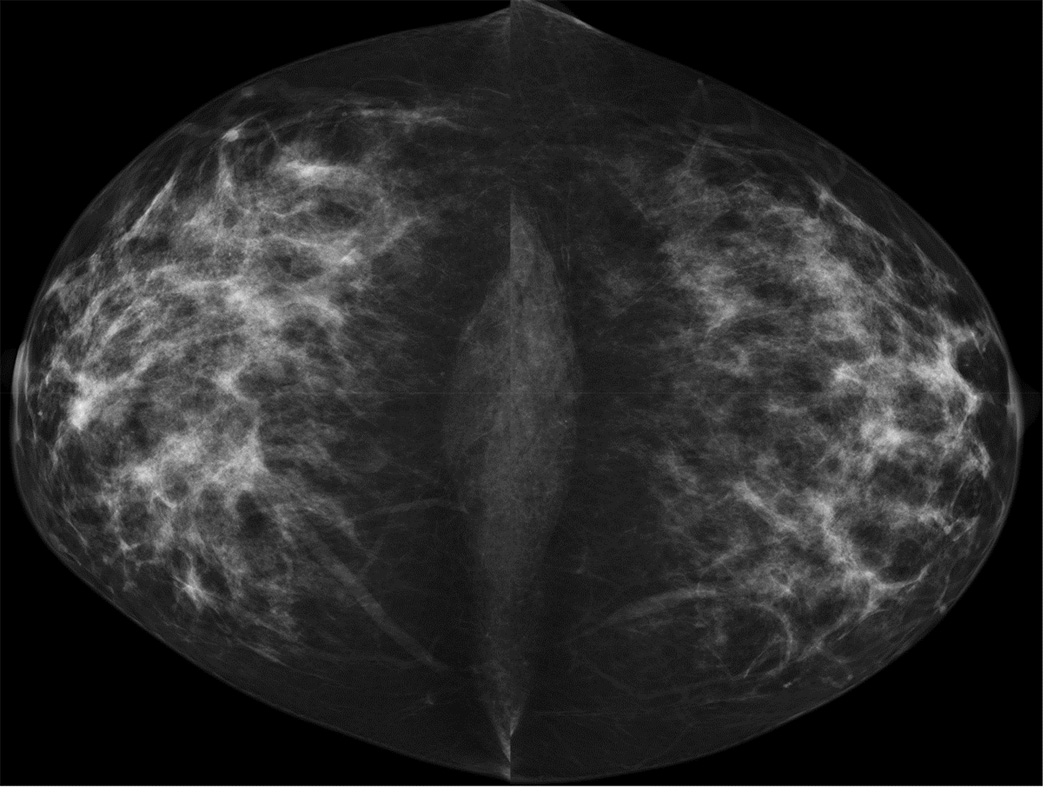
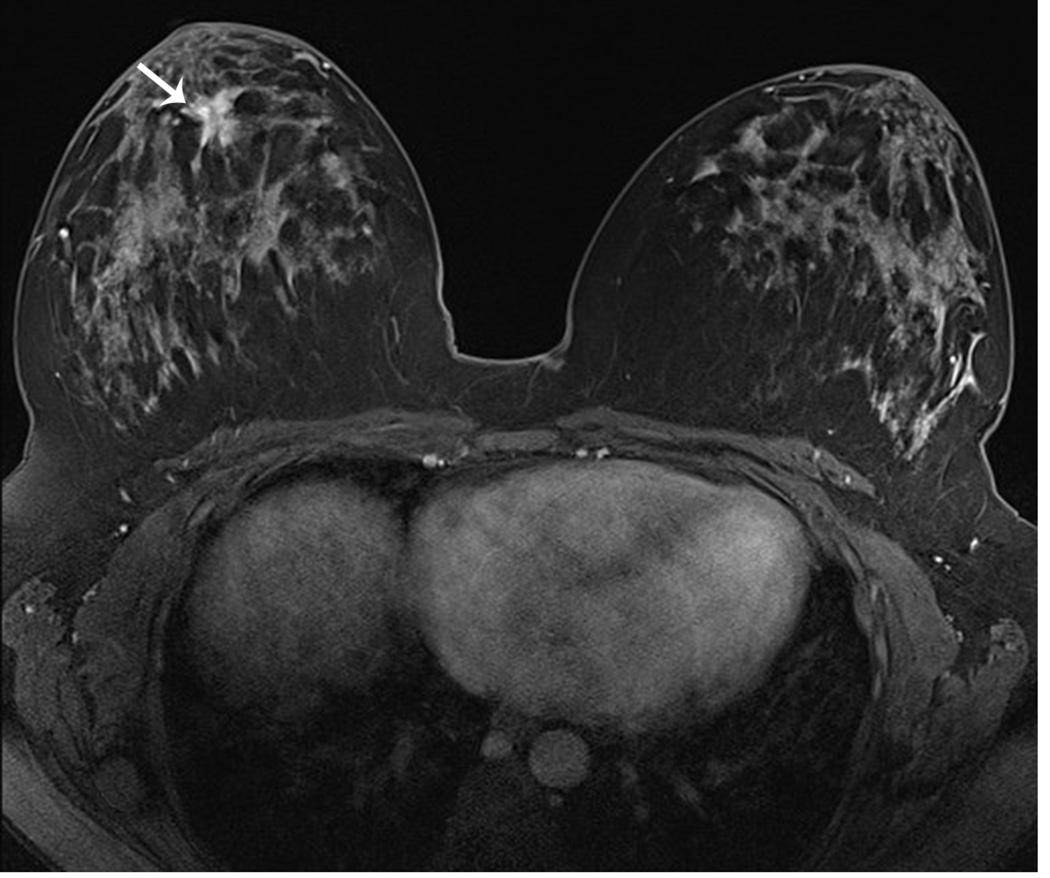
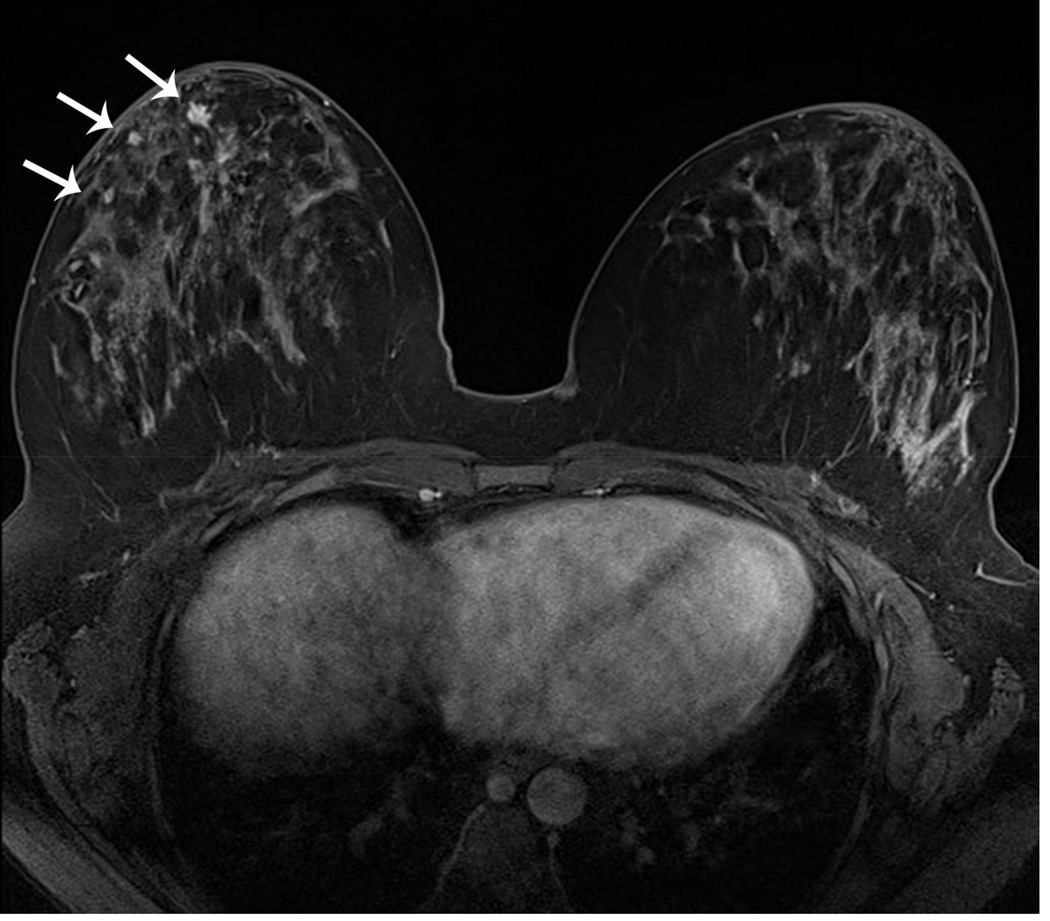
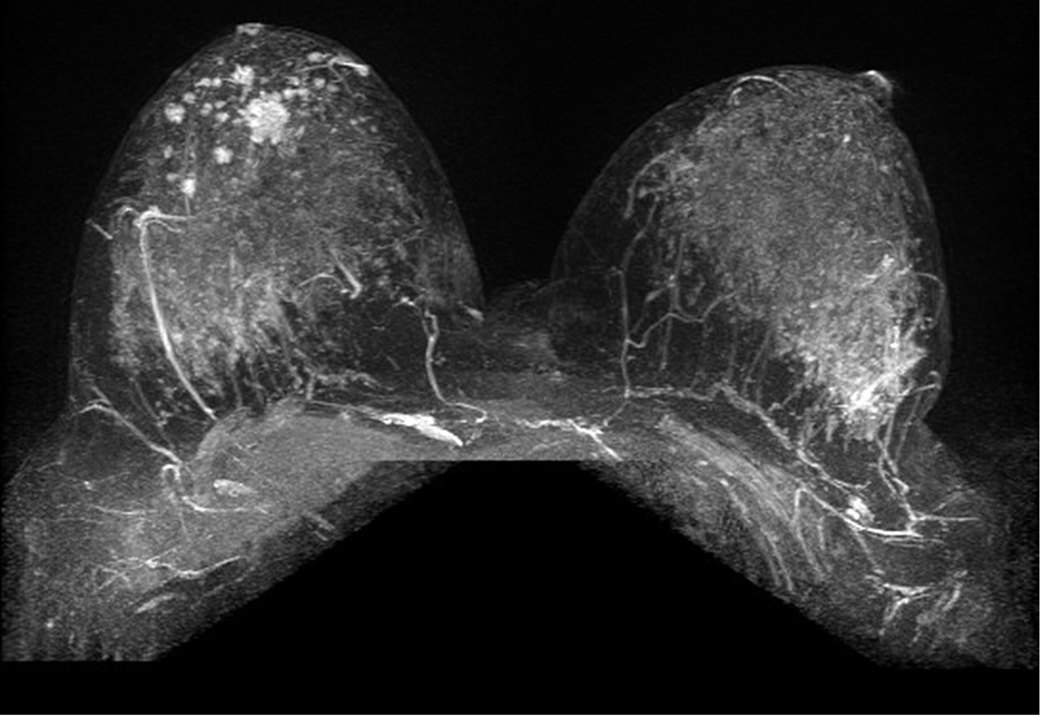
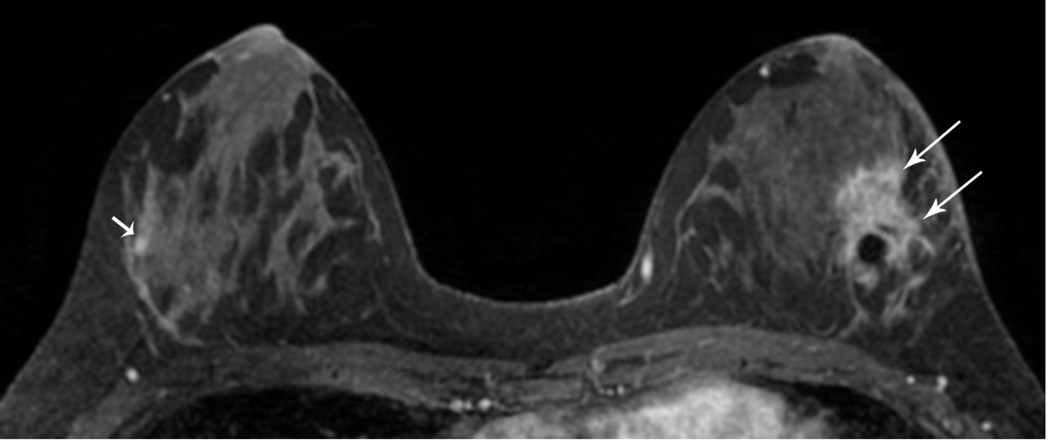
Figure 6. MRI may detect occult and multifocal disease.
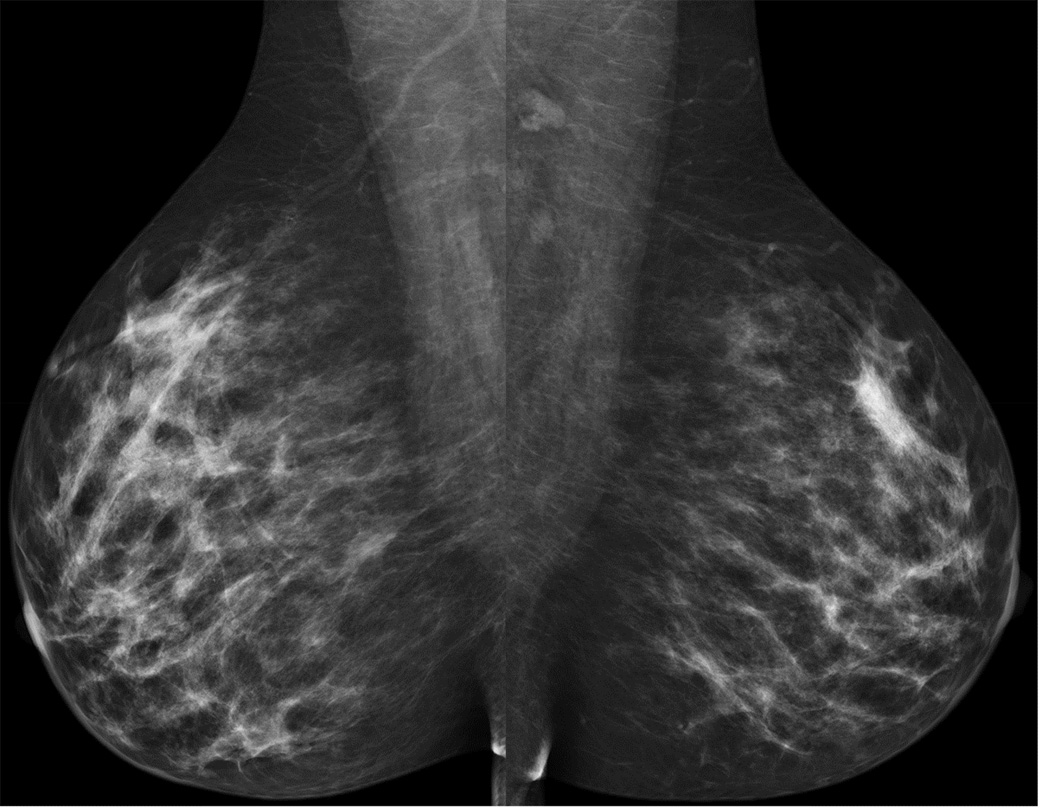
A 39 year-old asymptomatic woman with a strong family history and known BRCA2 mutation presented for screening studies by mammography (A and B) and MRI (C, D E) on the same day. Mammogram was interpreted as negative; however, MRI revealed a 2 cm lesion and multiple associated satellite lesions (arrows). Mastectomy confirmed multifocal disease.
Two prospective randomized trials studied the use of breast MRI for extent of disease evaluation. In the COMICE (Comparative Effectiveness of MR Imaging in Breast Cancer) trial, with a relatively short-term follow-up, no significant difference in re-excision rates was found (19% in each arm) and there were comparable local recurrence rates [70]. In the MONET (MR Mammography of Nonpalpable Breast Tumours) trial, a higher reoperation rate was found for the MRI group (34%) than for the non-MRI group (12%) [71]. The MONET study had a strong selection bias: 50% of compared cancer cases were mammographic calcifications and proved to be DCIS at excision. Both trials have been criticized for including use of a variety of equipment, technique, sequencing, and interpretation. Neither of these two studies included a strategy for managing data gained from MRI and consistently incorporating them into surgical planning.
The expectation from staging MR was that more accurate staging of extent of disease would probably decrease the number of surgeries required to achieve clear margins and could potentially reduce local recurrence and improve survival. However, the impact of breast MRI on frequency of positive margins was analyzed by a meta-analysis, examining the effect of preoperative MRI compared with standard preoperative assessment on surgical outcomes and found that preoperative MR did not have a positive effect on outcomes but patients with preoperative MRI had significantly increased mastectomy rates [63].
Selection of patients and surgical planning for breast conserving surgery (BSC)
BCS is performed with the goal of removing breast malignancy and adequate surrounding margin to preserve breast with good cosmesis. In appropriate candidates, survival rates are equivalent to those of mastectomy. It is considered when a satisfactory aesthetic result can be achieved with estimated low risk of in-breast recurrence. Mastectomy is preferred when a cosmetically acceptable outcome for the patient is unattainable by lumpectomy. Ineligibility for BCS includes multifocal and multicentric disease or the inability to achieve negative pathologic margins. Patients who are not able to receive or reject radiation treatment (e.g. previous radiation therapy in the area) are also excluded.
Pre-operative needle localization and wire placement with image guidance for nonpalpable lesions and/or to define radiologic extent of disease is performed to aid lesion removal. Larger extent of disease may require bracketing. New localization techniques being used or tested: intraoperative ultrasound [72], radioisotope seeds for lesion marking [73], and non-radioactive electromagnetic wave technology [74].
Up to 60% of patients undergoing BCS require re-excision with the mainstream re-excision rates approximately 20–40% [75–83]. Intraoperative margin assessment with frozen section histopathology analysis and imprint cytology provides useful information on margin status. It is crucial to achieve clear margins because presence of close or positive margins is associated with increased locoregional recurrence and a decrease in long-term survival [80, 84, 85]. Reoperations increase cost, delay completion of therapy, increase the potential for complications, including infection, diminished cosmetic outcomes, and have negative psychological impact on the patient [83, 86–88].
Intra-operative MRI for lumpectomy
The use of MRI scanners within the operating room has been shown to facilitate and refine the surgical approach, tumor localization, and detection of residual lesions in neurosurgery [89]. In the field of breast surgery, only a few intra-operative MRI-guided lumpectomy studies have been performed. Gould et al. used a 0.5T vertically open scanner and reported close agreement between maximum dimensions of MRI localization of benign breast lesions and histopathologic examination. All post-procedure scans demonstrated complete resection [90].
Hirose et al. at BWH, utilizing the same type of 0.5T vertically open scanner, reported that all the tumors in the 20 patients with invasive breast cancers were localized with MRI at the MR-guided lumpectomy [91]. Although MRI-guidance obviated the need for a second operation in four (20%) of the cases, the procedure was suspended. The suboptimal image quality at 0.5T made reliable detection of a residual tumor a challenge. Dynamic imaging with fat saturation was not possible due to low field strength. For these reasons, BWH chose to change the program to a 3T MRI scanner.
The Advanced Multimodal Image-Guided Operating (AMIGO) suite
The Advanced Multimodal Image-Guided Operating (AMIGO) suite was the first operating suite equipped with three sterile procedure rooms (MRI, OR, and PET-CT rooms). In the center of the MRI room is a high-field (3 Tesla) wide-bore (70 cm) Siemens Verio MRI scanner (Siemens AG, Erlangen, Germany) that is ceiling mounted and has the ability to move in and out of the surgical field.
BWH successfully demonstrated the feasibility of lumpectomy and intra-operative MRI in the AMIGO suite [5]. On the day of surgery, the patient undergoes wire localization of the tumor with an MRI conditional wire. A sentinel lymph node biopsy and a standard lumpectomy are performed in the AMIGO suite, followed by saline placement in the cavity and temporary closure of the breast to limit MRI artifact by air-tissue susceptibility mismatch. MRI visible fiducial markers are used to mark the superior and inferior margins of the surgical cavity. The MRI enters the operating room, and is positioned over the supine patient on the surgical table. The intra-operative MRI is done using a Siemens cardiac (32 channel) coil with foam cushion pads placed under the coil to prevent excessive pressure. The pre- and post-contrast VIBE images are obtained with the intubated patient in breath hold with the anesthesiologist's assistance. The contract-enhanced sequences are obtained with additional delay times to account for the reduced perfusion immediately following surgery.
Seven patients with breast cancer were evaluated with prone diagnostic and supine pre-procedural dynamic contrast enhanced MRI (DCE-MRI) as part of the Phase I clinical trial investigating intraoperative MRI for BCT margin assessment in the AMIGO suite. Sixty-five geometric, structural, and heterogeneity metrics were computed including volume, surface area, compactness, maximum 3D diameter, and sphericity. Distance of the tumor center from nipple, chest wall and skin were computed. The initial results suggest that there is a substantial difference in tumor deformity based on the patient's position -- prone vs. supine. Tumors measure larger in volume and surface area, and closer to the nipple and chest wall on supine than on prone images, underscoring the importance of a pre-operative supine MRI, which simulates the intraoperative position of the breast [92].
The AMIGO trial, demonstrated that there is no significant enhancement from bleeding vessels in an operative field if adequate hemostasis is obtained. However, fiducials are needed for accurate orientation of the margins [93] (Figure 7).
Figure 7. The first lumpectomy procedure in the AMIGO suite.
Diagnostic Imaging: (A) First post-contrast image showing the rapidly enhancing tumor; (B) CADstream output showing regions of enhancement with subsequent washout (red), plateau (yellow) and persistent (blue) signal intensity; (C) 3D volume rendered image showing the tumor and the breast outline. Pre-procedural Imaging: (D) First post-contrast image showing the tumor in the supine position; (E) CADstream output obtained intraoperatively showing the segmented tumor; (F) 3D volume rendered image showing the tumor in the supine surgical position. Post-procedural Imaging: (G) First post-contrast image showing the surgical cavity filled with saline immediately after BCS; (H) CADstream output showing no enhancing remnant tumor; (I) 3D volume rendered image showing the surgical cavity.
Summary
Breast MRI is the most sensitive examination for breast cancer detection and has become a well-established screening method supplementing mammography in high-risk women. MR imaging has been suggested as an adjunctive for identifying the extent of breast carcinoma and for guiding treatment planning. Currently, MR imaging for preoperative evaluation of disease extent in newly diagnosed breast cancer is controversial.
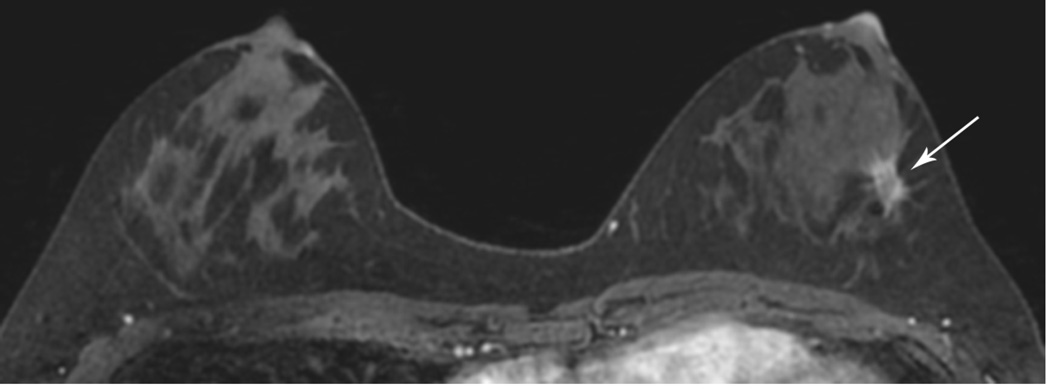
Figure 4. Underestimation of residual tumor size by MRI (ER positive cancers).
A 37 year-old woman with no known risk factors and left palpable lump. Ultrasound-guided CNB showed grade II/III IDC, ER/PR(+), ERBB2(Her-2/neu) (−)
(A). MRI for extent of disease shows a 2.7 cm irregular, spiculated mass with rapid washout (long arrows). In addition, a 0.5 cm enhancing mass was seen only by MR on the contralateral, right breast (short arrow). Right MRI-guided core biopsy showed a grade II/III IDC, ER/PR(+),ERBB2(Her-2/neu)(−) IDC. (B). MR at completion of NACT showed no residual lesion on the right and decreased size, minimal residual enhancement on the left (RECIST 37%, partial response) (long arrow). Bilateral mastectomy pathology showed a 0.1 cm residual IDC on the right and no pathologic response on the left (the residual carcinoma was 90% cellular and appeared viable).
Key points.
Breast MRI is the most sensitive imaging tool of detecting breast cancer and may reveal breast cancer that is occult to physical examination and by conventional imaging modalities (mammography and ultrasound)
MRI-guided tissue sampling. In cases in which a suspicious lesion is detected by MRI and no obvious correlative finding is found by other methods, MR-guided tissue sampling is needed to determine the underlying histopathology
Monitoring treatment response to neoadjuvant chemotherapy. Studies have shown advantages of breast MR imaging for predicting recurrence free survival and pathologic complete response over physical examination and conventional imaging
Lumpectomy planning. Anticipated benefits from higher sensitivity of preoperative MRI have not been clearly shown in large studies
Acknowledgments
Dr. Gombos discloses that she is receiving royalties for a book published by Amirsys, Inc.
Dr. Jagadeesan discloses that this project was supported by the National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through Grant Numbers P41EB015898 and P41RR019703.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Richman and Dr. Kacker have nothing to disclose.
Contributor Information
Eva C Gombos, Division of Breast Imaging, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, 617.732.6269, egombos@partners.org.
Jayender Jagadeesan, Surgical Planning Laboratory, Brigham and Women's Hospital, 75 Francis Street, Boston MA 02115, 617.278.0986, jayender@bwh.harvard.edu.
Danielle M Richman, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, 617.732.5938, dmrichman@partners.org.
Daniel F Kacher, Surgical Planning Lab, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, 617.732.7247, dkacher@partners.org.
References
- 1.Saslow D, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 2.Solin LJ, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26(3):386–391. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 3.Waljee JF, et al. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15(5):1297–1303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- 4.Menes TS, et al. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol. 2005;12(11):881–885. doi: 10.1245/ASO.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Golshan M, et al. Pilot study to evaluate feasibility of image-guided breast-conserving therapy in the advanced multimodal image-guided operating (AMIGO) suite. Ann Surg Oncol. 2014;21(10):3356–3357. doi: 10.1245/s10434-014-3926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACR Practice Parameter for the Performance of Contrast-Enhanced Magnetic Resonance Imaging (MRI) of the Breast. [March 20, 2015];2014 Available from: http://www.acr.org/~/media/2a0eb28eb59041e2825179afb72ef624.pdf. [Google Scholar]
- 7.Meissnitzer M, et al. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol. 2009;193(4):1025–1029. doi: 10.2214/AJR.09.2480. [DOI] [PubMed] [Google Scholar]
- 8.Abe H, et al. MR-directed ("Second-Look") ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. AJR Am J Roentgenol. 2010;194(2):370–377. doi: 10.2214/AJR.09.2707. [DOI] [PubMed] [Google Scholar]
- 9.Noroozian M, et al. Factors that impact the duration of MRI-guided core needle biopsy. AJR Am J Roentgenol. 2010;194(2):W150–W157. doi: 10.2214/AJR.09.2366. [DOI] [PubMed] [Google Scholar]
- 10.Schrading S, et al. MRI-guided breast biopsy: influence of choice of vacuum biopsy system on the mode of biopsy of MRI-only suspicious breast lesions. AJR Am J Roentgenol. 2010;194(6):1650–1657. doi: 10.2214/AJR.09.2550. [DOI] [PubMed] [Google Scholar]
- 11.Li J, et al. MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. AJR Am J Roentgenol. 2009;193(3):850–855. doi: 10.2214/AJR.08.2226. [DOI] [PubMed] [Google Scholar]
- 12.Han BK, et al. Outcome of MRI-guided breast biopsy. AJR Am J Roentgenol. 2008;191(6):1798–1804. doi: 10.2214/AJR.07.2827. [DOI] [PubMed] [Google Scholar]
- 13.Ghate SV, et al. MRI-guided vacuum-assisted breast biopsy with a handheld portable biopsy system. AJR Am J Roentgenol. 2006;186(6):1733–1736. doi: 10.2214/AJR.05.0551. [DOI] [PubMed] [Google Scholar]
- 14.van den Bosch MA, et al. MRI-guided needle localization of suspicious breast lesions: results of a freehand technique. Eur Radiol. 2006;16(8):1811–1817. doi: 10.1007/s00330-006-0214-5. [DOI] [PubMed] [Google Scholar]
- 15.Liberman L, et al. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol. 2005;185(1):183–193. doi: 10.2214/ajr.185.1.01850183. [DOI] [PubMed] [Google Scholar]
- 16.Yeh ED, et al. Positioning in breast MR imaging to optimize image quality. Radiographics. 2014;34(1):E1–E17. doi: 10.1148/rg.341125193. [DOI] [PubMed] [Google Scholar]
- 17.Lehman CD, et al. Clinical experience with MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2005;184(6):1782–1787. doi: 10.2214/ajr.184.6.01841782. [DOI] [PubMed] [Google Scholar]
- 18.Lee JM, et al. Imaging histologic discordance at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189(4):852–859. doi: 10.2214/AJR.07.2531. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, et al. Underestimation of DCIS at MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189(2):468–474. doi: 10.2214/AJR.07.2172. [DOI] [PubMed] [Google Scholar]
- 20.Liberman L, et al. Underestimation of atypical ductal hyperplasia at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;188(3):684–690. doi: 10.2214/AJR.06.0809. [DOI] [PubMed] [Google Scholar]
- 21.Carlson JW, et al. MRI-directed, wire-localized breast excisions: incidence of malignancy and recommendations for pathologic evaluation. Hum Pathol. 2007;38(12):1754–1759. doi: 10.1016/j.humpath.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 23.Chen AM, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 25.Wolmark N, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 26.Niu L, Wu B, Xu K. Cryosurgery for breast fibroadenomas. Gland Surgery. 2012;1(2):128–131. doi: 10.3978/j.issn.2227-684X.2012.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littrup PJ, et al. Cryotherapy for breast cancer: a feasibility study without excision. Journal of vascular and interventional radiology: JVIR. 2009;20(10):1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu L, et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology. 2013;67(1):17–22. doi: 10.1016/j.cryobiol.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Rabin Y, et al. A new cryosurgical device for controlled freezing. Cryobiology. 1996;33(1):93–105. doi: 10.1006/cryo.1996.0010. [DOI] [PubMed] [Google Scholar]
- 30.Sabel MS. Cryoablation as a Replacement for Surgical Resection in Early Stage Breast Cancer. Current Breast Cancer Reports. 2011;3(2):109–116. [Google Scholar]
- 31.Pfleiderer SOR, et al. Cryotherapy of breast cancer under ultrasound guidance: initial results and limitations. European Radiology. 2002;12(12):3009–3014. doi: 10.1007/s00330-002-1511-2. [DOI] [PubMed] [Google Scholar]
- 32.Morin J, et al. Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Canadian Journal of Surgery. Journal Canadien De Chirurgie. 2004;47(5):347–351. [PMC free article] [PubMed] [Google Scholar]
- 33.Pusztaszeri M, et al. Histopathological study of breast cancer and normal breast tissue after magnetic resonance-guided cryotherapy ablation. Cryobiology. 2007;55(1):44–51. doi: 10.1016/j.cryobiol.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Tuncali K, et al. MRI-guided percutaneous cryotherapy for soft-tissue and bone metastases: initial experience. AJR. American journal of roentgenology. 2007;189(1):232–239. doi: 10.2214/AJR.06.0588. [DOI] [PubMed] [Google Scholar]
- 35.Tozaki M, et al. Ultrasound-guided cryoablation of invasive ductal carcinoma inside the MR room. Magnetic resonance in medical sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine. 2010;9(1):31–36. doi: 10.2463/mrms.9.31. [DOI] [PubMed] [Google Scholar]
- 36.Rieke V, Butts Pauly K. MR thermometry. Journal of magnetic resonance imaging: JMRI. 2008;27(2):376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thrall DE, et al. Using units of CEM 43 degrees C T90, local hyperthermia thermal dose can be delivered as prescribed. International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2000;16(5):415–428. doi: 10.1080/026567300416712. [DOI] [PubMed] [Google Scholar]
- 38.Hynynen K, et al. Temperature monitoring in fat with MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;43(6):901–904. doi: 10.1002/1522-2594(200006)43:6<901::aid-mrm18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 39.Sprinkhuizen SM, et al. Temperature-induced tissue susceptibility changes lead to significant temperature errors in PRFS-based MR thermometry during thermal interventions. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;64(5):1360–1372. doi: 10.1002/mrm.22531. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, et al. A method for simultaneous RF ablation and MRI. Journal of magnetic resonance imaging: JMRI. 1998;8(1):110–114. doi: 10.1002/jmri.1880080122. [DOI] [PubMed] [Google Scholar]
- 41.van den Bosch M, et al. MRI-guided radiofrequency ablation of breast cancer: preliminary clinical experience. Journal of magnetic resonance imaging: JMRI. 2008;27(1):204–208. doi: 10.1002/jmri.21190. [DOI] [PubMed] [Google Scholar]
- 42.Lewin JS, et al. Phase II clinical trial of interactive MR imaging-guided interstitial radiofrequency thermal ablation of primary kidney tumors: initial experience. Radiology. 2004;232(3):835–845. doi: 10.1148/radiol.2323021351. [DOI] [PubMed] [Google Scholar]
- 43.van Hillegersberg R, et al. Interstitial Nd:YAG laser coagulation with a cylindrical diffusing fiber tip in experimental liver metastases. Lasers in Surgery and Medicine. 1994;14(2):124–138. doi: 10.1002/1096-9101(1994)14:2<124::aid-lsm1900140205>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Lai LM, et al. Interstitial laser photocoagulation for fibroadenomas of the breast. The Breast. 1999;8(2):89–94. [Google Scholar]
- 45.Harms S, et al. MRI directed interstitial thermal ablation of breast fibroadenomas. Hoboken, NJ.: Wiley &Sons, Inc; 1999. [Google Scholar]
- 46.Mumtaz H, et al. Laser therapy for breast cancer: MR imaging and histopathologic correlation. Radiology. 1996;200(3):651–658. doi: 10.1148/radiology.200.3.8756910. [DOI] [PubMed] [Google Scholar]
- 47.Dowlatshahi K, et al. Stereotactically guided laser therapy of occult breast tumors: work-in-progress report. Archives of Surgery (Chicago, Ill.: 1960) 2000;135(11):1345–1352. doi: 10.1001/archsurg.135.11.1345. [DOI] [PubMed] [Google Scholar]
- 48.Dowlatshahi K, Francescatti DS, Bloom KJ. Laser therapy for small breast cancers. American Journal of Surgery. 2002;184(4):359–363. doi: 10.1016/s0002-9610(02)00942-x. [DOI] [PubMed] [Google Scholar]
- 49.Vogl TJ, et al. MR-guided laser-induced thermotherapy with a cooled power laser system: a case report of a patient with a recurrent carcinoid metastasis in the breast. European Radiology. 2002;12(Suppl 3):S101–S104. doi: 10.1007/s00330-002-1602-0. [DOI] [PubMed] [Google Scholar]
- 50.Harms SE. Percutaneous ablation of breast lesions by radiologists and surgeons. Breast Disease. 2001;13:67–75. doi: 10.3233/bd-2001-13109. [DOI] [PubMed] [Google Scholar]
- 51.Hynynen K, et al. A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics: A Review Publication of the Radiological Society of North America, Inc. 1996;16(1):185–195. doi: 10.1148/radiographics.16.1.185. [DOI] [PubMed] [Google Scholar]
- 52.Hynynen K, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–185. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 53.Huber PE, et al. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Research. 2001;61(23):8441–8447. [PubMed] [Google Scholar]
- 54.Gianfelice D, et al. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. Journal of vascular and interventional radiology: JVIR. 2003;14(10):1275–1282. doi: 10.1097/01.rvi.0000092900.73329.a2. [DOI] [PubMed] [Google Scholar]
- 55.Gianfelice D, et al. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Research and Treatment. 2003;82(2):93–101. doi: 10.1023/B:BREA.0000003956.11376.5b. [DOI] [PubMed] [Google Scholar]
- 56.Zippel DB, Papa MZ. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer (Tokyo, Japan) 2005;12(1):32–38. doi: 10.2325/jbcs.12.32. [DOI] [PubMed] [Google Scholar]
- 57.Gianfelice D, et al. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness-- initial experience. Radiology. 2003;227(3):849–855. doi: 10.1148/radiol.2281012163. [DOI] [PubMed] [Google Scholar]
- 58.Furusawa H, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. Journal of the American College of Surgeons. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Furusawa H, Yasuda Y. Magnetic Resonance Image Guided Focused Ultrasound Surgery of Early Breast Cancer: Efficacy and Safety in Excisionless Study. Cancer Research. 2009;69(24) Supplimental-3. [Google Scholar]
- 60.Payne A, et al. Design and characterization of a laterally mounted phased-array transducer breast-specific MRgHIFU device with integrated 11-channel receiver array. Medical Physics. 2012;39(3):1552–1560. doi: 10.1118/1.3685576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Todd N, et al. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014;72(3):793–799. doi: 10.1002/mrm.24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liberman L, et al. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003;180(4):901–910. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 63.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257(2):249–255. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 64.Houssami N, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26(19):3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 65.Holland R, et al. Histologic multifocality of Tis, T1–2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56(5):979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 66.Schelfout K, et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol. 2004;30(5):501–507. doi: 10.1016/j.ejso.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Bilimoria KY, et al. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg. 2007;142(5):441–445. doi: 10.1001/archsurg.142.5.441. discussion 445–7. [DOI] [PubMed] [Google Scholar]
- 68.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrastenhanced MR imaging on the therapeutic approach. Radiology. 1999;213(3):881–888. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 69.Smitt MC, et al. The importance of the lumpectomy surgical margin status in longterm results of breast conservation. Cancer. 1995;76(2):259–267. doi: 10.1002/1097-0142(19950715)76:2<259::aid-cncr2820760216>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 70.Turnbull L, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375(9714):563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 71.Peters NH, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. Eur J Cancer. 2011;47(6):879–886. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 72.Harlow SP, et al. Intraoperative ultrasound localization to guide surgical excision of nonpalpable breast carcinoma. J Am Coll Surg. 1999;189(3):241–246. doi: 10.1016/s1072-7515(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 73.van Riet YE, et al. Localization of non-palpable breast cancer using a radiolabelled titanium seed. Br J Surg. 2010;97(8):1240–1245. doi: 10.1002/bjs.7097. [DOI] [PubMed] [Google Scholar]
- 74.Cox CE M, Whitworth P, Themar-Geck M, Prati R, Jung M, King J, Shivers SC. Pilot study of a passive non-radioactive electromagnetic wave technology to localize nonpalpable breast lesions. 2014 [Google Scholar]
- 75.Sanchez C, et al. Factors associated with re-excision in patients with early-stage breast cancer treated with breast conservation therapy. Am Surg. 2010;76(3):331–334. [PubMed] [Google Scholar]
- 76.Mullenix PS, et al. Secondary operations are frequently required to complete the surgical phase of therapy in the era of breast conservation and sentinel lymph node biopsy. Am J Surg. 2004;187(5):643–646. doi: 10.1016/j.amjsurg.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Boughey JC, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–470. doi: 10.1097/01.sla.0000234897.38950.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleming FJ, et al. Factors affecting metastases to non-sentinel lymph nodes in breast cancer. J Clin Pathol. 2004;57(1):73–76. doi: 10.1136/jcp.57.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bani MR, et al. Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol. 2009;35(1):32–37. doi: 10.1016/j.ejso.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 80.O'Sullivan MJ, et al. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007;14(11):3133–3140. doi: 10.1245/s10434-007-9523-4. [DOI] [PubMed] [Google Scholar]
- 81.Camp ER, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005;201(6):855–861. doi: 10.1016/j.jamcollsurg.2005.06.274. [DOI] [PubMed] [Google Scholar]
- 82.Kobbermann A, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol. 2011;18(5):1349–1355. doi: 10.1245/s10434-010-1420-6. [DOI] [PubMed] [Google Scholar]
- 83.Sabel MS, et al. Residual disease after re-excision lumpectomy for close margins. J Surg Oncol. 2009;99(2):99–103. doi: 10.1002/jso.21215. [DOI] [PubMed] [Google Scholar]
- 84.Cowen D, et al. Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys. 2000;47(2):305–312. doi: 10.1016/s0360-3016(99)00553-2. [DOI] [PubMed] [Google Scholar]
- 85.Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 86.Deutsch M, Flickinger JC. Patient characteristics and treatment factors affecting cosmesis following lumpectomy and breast irradiation. Am J Clin Oncol. 2003;26(4):350–353. doi: 10.1097/01.COC.0000020589.75948.E7. [DOI] [PubMed] [Google Scholar]
- 87.Cochrane RA, et al. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90(12):1505–1509. doi: 10.1002/bjs.4344. [DOI] [PubMed] [Google Scholar]
- 88.Heil J, et al. Do reexcisions impair aesthetic outcome in breast conservation surgery? Exploratory analysis of a prospective cohort study. Ann Surg Oncol. 2012;19(2):541–547. doi: 10.1245/s10434-011-1947-1. [DOI] [PubMed] [Google Scholar]
- 89.Risholm P, Golby AJ, Wells W., 3rd Multimodal image registration for preoperative planning and image-guided neurosurgical procedures. Neurosurg Clin N Am. 2011;22(2):197–206. viii. doi: 10.1016/j.nec.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gould SW, et al. Interventional MR-guided excisional biopsy of breast lesions. J Magn Reson Imaging. 1998;8(1):26–30. doi: 10.1002/jmri.1880080110. [DOI] [PubMed] [Google Scholar]
- 91.Hirose M, et al. Feasibility of MR imaging-guided breast lumpectomy for malignant tumors in a 0.5-T open-configuration MR imaging system. Acad Radiol. 2002;9(8):933–941. doi: 10.1016/s1076-6332(03)80464-6. [DOI] [PubMed] [Google Scholar]
- 92.J Jayender MG, Narayan V, Durgapal S, Mallory MA, Jolesz FA, Gombos EC. Clinical Impact and Quantification of Prone to Supine Position in Breast MRI. 2015 [Google Scholar]
- 93.Gombos E, et al. Intraprocedural High Field MRI Imaging In Breast Conserving Surgery: Initial Clinical Experience. 2012 [Google Scholar]