Abstract
Objective
HIMAL (hippocampal malrotation) is characterized by incomplete hippocampal inversion with rounded shape and blurred internal architecture. There is still debate whether or not HIMAL has pathological significance. We present findings from the FEBSTAT study on the frequency and risk factors for HIMAL.
Materials and Methods
FEBSTAT is a prospective multicenter study investigating consequences of febrile status epilepticus (FSE) in childhood. MR imaging studies of 226 FSE subjects were analyzed visually by two board-certified neuroradiologists blinded to clinical details and compared to MR imaging studies of 96 subjects with first simple febrile seizure (FS). Quantitative analysis of hippocampal volume was performed by two independent observers.
Results
HIMAL was present in 20 (8.8%), of FSE cases compared with 2 (2.1%) of controls (odds ratio 4.56; 95% CI=1.05, 19.9). HIMAL was exclusively left-sided in 18 (81.8%), and bilateral in the remaining 4 (18.2%). There was no case of exclusively right-sided HIMAL. HIMAL was more common in boys than in girls (OR 6.1, 95%CI = (1.7, 21.5) On quantitative volumetric MR imaging analysis, the left hippocampal volume in HIMAL cases was smaller than in simple FS controls (p=0.004), and the R/L hippocampal volume ratio was higher in the HIMAL group compared to the simple FS group (p<0.001).
Conclusion
HIMAL is a developmental malformation that predominantly affects the left hippocampus in males, and is more frequently found in children with prolonged FSE than in controls. These data provide further evidence that HIMAL represents a pathological error in brain development rather than a normal variant.
Keywords: HIMAL, Febrile Seizures, Status Epilepticus, MR Imaging
Introduction
HIMAL, a form of hippocampal malrotation with a normal corpus callosum, is characterized by incomplete inversion of the hippocampus with a rounded shape and blurred internal architecture, associated with a vertical collateral sulcus [1]. Unlike hippocampal sclerosis (HS), HIMAL is typically seen without evidence of abnormal signal intensity or visually evident volume loss of the hippocampus, HIMAL has been reported as present more frequently on magnetic resonance (MR) imaging examinations of patients with epilepsy, than those without epilepsy [2]. An increased incidence of unilateral HIMAL has been described in patients with chromosome 22q11.2 deletion syndrome (which itself is associated with a sevenfold increased risk of developing seizures) [3]. Otherwise, HIMAL is a rare finding in patients referred for conditions other than seizures [2]. However, the exact nature of the relationship between HIMAL and epilepsy has not been elucidated. HIMAL has not been considered as an epileptogenic lesion, and controversy exists as to whether it represents a cerebral abnormality or a developmental anatomic variant [4,5].
The Consequences of Prolonged Febrile Seizures in Childhood (FEBSTAT) study is a prospective multicenter study designed to address the relationship between febrile status epilepticus (FSE) and subsequent hippocampal sclerosis and mesial temporal lobe epilepsy (MTLE) [6–8]. Substantial attention has been paid to the presence of T2 signal abnormalities within the hippocampus in the acute stage of FSE; the results of acute neuroimaging findings of the main FEBSTAT cohort have been published [8], as has the baseline imaging from a control cohort of children with a first febrile seizure of all types [9,10]. In this report, we address the hypothesis that HIMAL is a pathological finding that predisposes to seizures and, in particular, to prolonged febrile seizures.
Materials and Methods
Patient recruitment methods
The FEBSTAT study is a prospective multicenter study designed to address the relationship between FSE, subsequent mesial temporal sclerosis, and MTLE. The methods of the study have been described elsewhere in detail [6,7]. Briefly, this study consists of a group of children age 1 month to 5 years who presented with an episode of FSE, defined as a seizure lasting a total of 30 minutes or more without fully regaining consciousness, which also met the definition of a FS [6,7,11–13]. Many of these children had an episode of FSE that lasted even longer than 60 minutes [6]. Children with known severe neurological disability prior to entry were excluded [6,7]. In addition to the main FEBSTAT cohort [6–8] of 191 cases with baseline MR studies, there are two additional cohorts which predate the main FEBSTAT cohort: a pilot cohort from Duke of 23 cases and 12 cases with FSE from the Columbia study of first febrile seizures [9,10]. The Columbia study also provided the 96 cases of children with first simple FS and normal baseline MRI that formed the control group for FEBSTAT. Controls with simple FS were used because it was not feasible to obtain sedated MR imaging studies in a similarly aged sample of children who had no prior history of any neurological event. A detailed description of the cohort characteristics, recruitment process and the procedures used has been published [7]. All study procedures were approved by the Institutional Review Boards for the Protection of Human Subjects at all participating institutions.
MRI Procedures
An MR imaging study was performed within 72 hours of the FSE whenever possible or very shortly thereafter using a standard protocol. The MR imaging studies, standardized through use of phantoms at each site, included sequences that focused on the hippocampus and were designed to allow both visual and quantitative measurements of volume and of T2 signal, as well as quantitative measurement of T2 signal relaxation times and apparent diffusion coefficients [7,8]. Visual readings were undertaken by two neuroradiologists examining hippocampal volume, hippocampal T2 signal, and other hippocampal and extrahippocampal abnormalities. The earlier MR imaging studies from Duke and Columbia utilized a similar, but not identical, MR imaging protocol (including T1-weighted SPGR coronal images of the brain and T2-weighted coronal oblique images of the temporal lobes). However, no geometric phantoms had been used for imaging of children with febrile seizures at these institutions prior to the FEBSTAT study [9,14]. Neuroradiologists were blinded to all other clinical and research details, except the age of the subject.
Imaging methods
MR imaging studies were performed on GE and Siemens 1.5 T MRI systems with standard T1-weighted sagittal, diffusion-weighted axial, and T2-weighted axial and/or FLAIR axial images of the whole brain. In addition, T1-weighted coronal images of the whole brain and T2-weighted coronal images of the temporal lobes were obtained. The following specific parameters for the imaging pulse sequences in the main FEBSTAT cohort were as follows:
GE systems
A) coronal oblique (slices perpendicular to the temporal lobe axis) T2 weighted fast spin echo, TR/TE=4500/96, ETL=8 (echo train length), 20 cm × 15 cm FOV, 3mm slice, 0mm gap, 256×256 matrix and 4 NEX. B) 3D coronal fast T1-weighted SPGR of the whole head TR/TE/flip = 2/5/30°, (full echo), 20cm FOV, 1.5 mm slice, 124 slices, 256×192 matrix, 2 NEX.
Siemens systems
A) coronal oblique (slices perpendicular to the temporal lobe axis) T2 weighted turbo spin echo with pulse sequence parameters: TR/TE=4500/101, turbo factor 7, 20cm × 15cm FOV, 3mm slice, distance factor=0% (no gaps), 256×256 matrix (3/4 FOV), and 4 NEX. B) 3D coronal T1-weighted spoiled TurboFLASH of the whole head, 3D, TR/TE/flip=12/5/20°, (full echo), 20cm FOV, 1.5 mm slice, 124 slices, 256×192 matrix, 2 NEX protocol.
Hippocampal volumetric analysis
Hippocampal regions of interest (ROIs) were traced using SnAP:IRIS and previously described boundaries [15,16] by a trained, independent observer blinded to the clinical data. (Technical details of the volumetric tracing technique are included in the appendix.) Slices posterior to and including the anterior commissure were summed for each hippocampal volume. In order to ascertain the presence or absence of hippocampal volume asymmetry, the right hippocampal volume and the left hippocampal volume of each individual subject were obtained separately. Then, the right to left (R/L) volume ratio for each individual subject was calculated as the volume of the right hippocampus divided by that of the left hippocampus. Comparisons of the mean R/L volume ratios were made between (1) the group of FSE cases with HIMAL (excluding those with abnormal T2 signal intensity in the hippocampus) and the simple FS control group and (2) the FSE cases with HIMAL and FSE controls without HIMAL. For further assessment of R/L volume asymmetry, the mean volumes of the left hippocampus and right hippocampus in the HIMAL group were compared to the corresponding mean volumes of the left hippocampus and right hippocampus of the simple FS control group and to the volumes of the FSE controls.
Consistency of volumetric measurements was assessed using the intraclass correlation coefficient (ICC) [17]. Ten MRIs randomly selected every two months from 33 subjects representing the age range of subjects were analyzed independently by two independent observers. ICC was 0.95 for the left hippocampus and 0.97 for the right hippocampus.
HIMAL
We defined HIMAL as incomplete rotation of the hippocampus with an abnormally rounded shape, typically associated with an atypical, vertically oriented, collateral sulcus angle and an atypical position and size of the fornix [1] (Figure 1). Although “normal” signal intensity of the hippocampus has been used to distinguish HIMAL from hippocampal sclerosis (HS), we did not use the presence of increased T2 hippocampal signal intensity to exclude the diagnosis of HIMAL on the baseline study, since FSE is known to be associated with hippocampal hyperintensity. Since hippocampal hyperintensity is likely a consequence of the prolonged FS, we did not consider it an exclusion criterion in this study [9]. In addition, blurred internal structure has been described as a typical finding of HIMAL [1]. However, this was not a requirement for diagnosing HIMAL, especially since it is often difficult to discern the internal architecture of the hippocampus clearly on T2-weighted coronal images of subjects below the ages of 18 months when scanning at 1.5 Tesla. In instances of an isolated finding of an abnormally rounded hippocampus without other findings typically associated with HIMAL (such as abnormal collateral sulcus angle or abnormal position of the fornix), we coded this as a “dysmorphic hippocampus”, but did not consider it to be HIMAL. In cases where there was disagreement about hippocampal findings between the two neuroradiologist readers, those study were re-reviewed together, with a consensus reading decided in the presence of a third investigator. The diagnosis of HIMAL was made on the basis of visual readings alone. While we performed quantitative hippocampal volume measurements as part of the FEBSTAT study, they were not used for the diagnosis of HIMAL, since no such quantitative criterion exists in the neuroimaging literature.
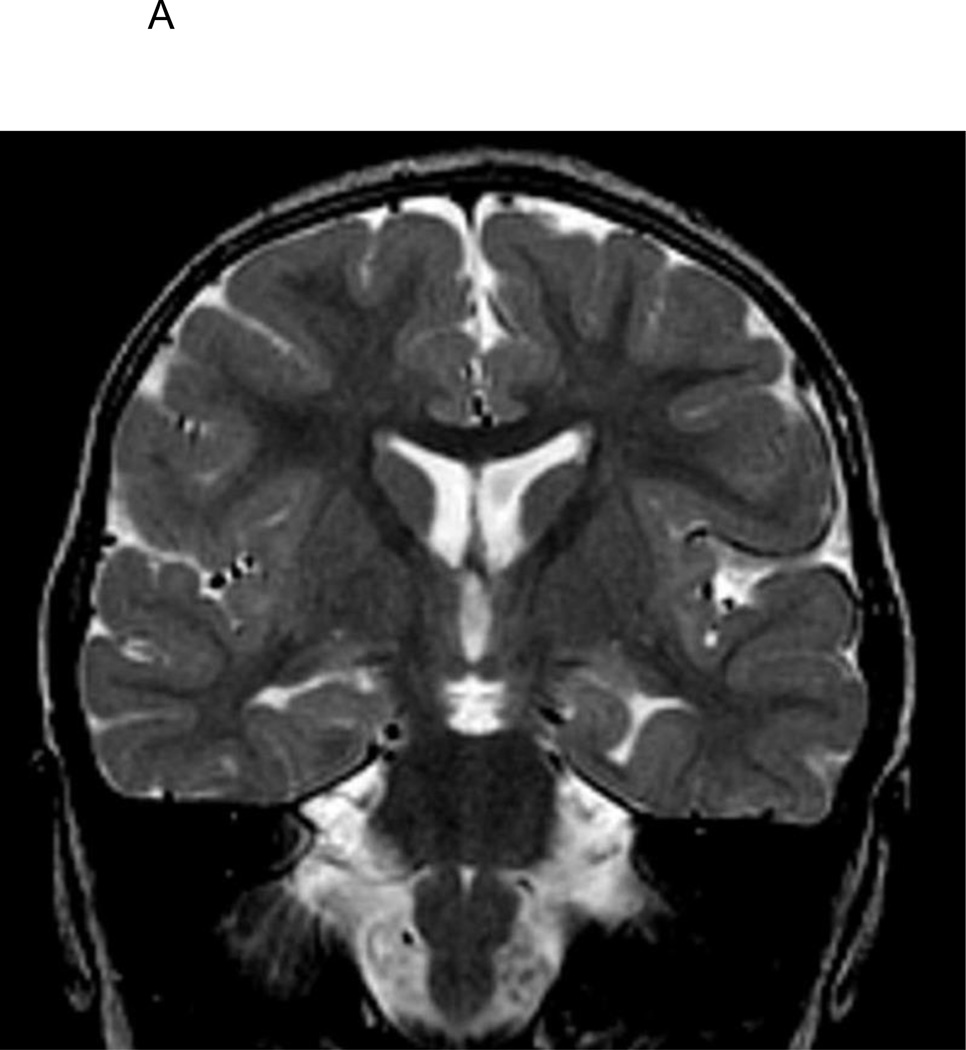
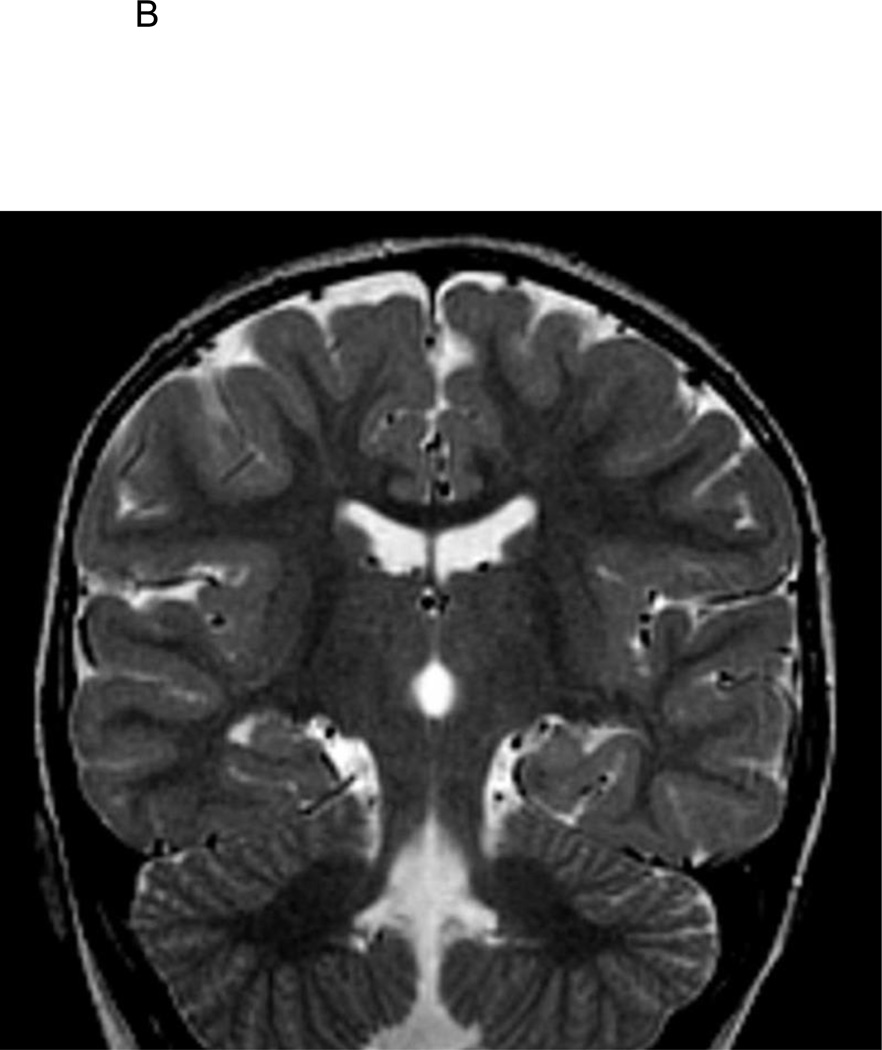
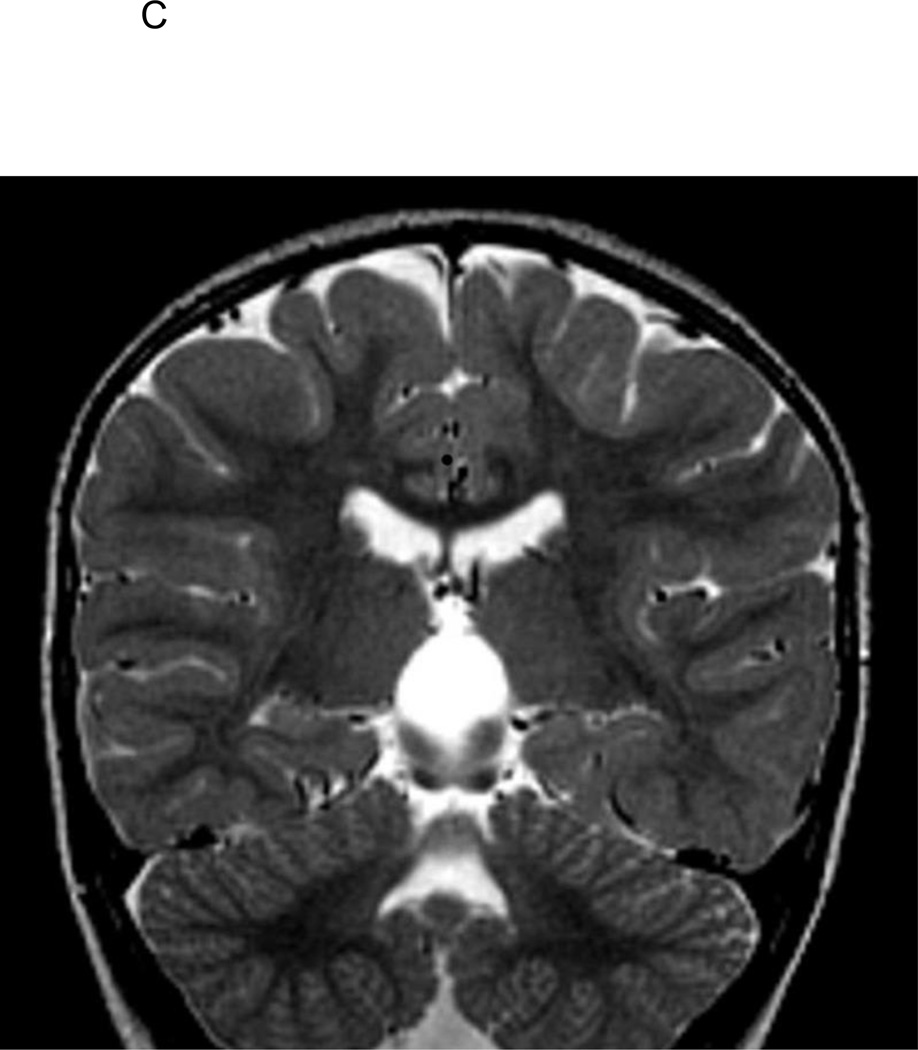
Figure 1.
A. T2-weighted coronal image of 2 1/2-year old FSE patient demonstrating medial positioning and globular shape of the left hippocampus.
B. T2-weighted coronal image of 2 1/2-year old FSE patient demonstrating medial positioning and globular shape of the left hippocampus, with associated vertically orientation of the left collateral sulcus.
C. T2-weighted coronal image of 2 1/2-year old FSE patient demonstrating medial positioning and globular shape of the left hippocampus, with associated inferior positioning of the posterior left crus of the fornix.
Statistical methods
Data were summarized as frequencies and percentages, and the chi-2 test and Fisher’s exact tests were used to compare frequencies [18]. Hippocampal volumes were compared using one-way analysis of covariance controlling for age. Significant omnibus F tests were followed by post-hoc t tests with Bonferroni corrections for the pre-determined number of comparisons. The odds of HIMAL in FSE were compared to simple FS controls. Logistic regression was used to analyze risk factors for HIMAL in FSE compared to FSE controls with normal baseline MRI [19]. A two-sided alpha level of 0.05 was used for all analyses.
Results
Among the 226 cases with FSE, there were 20 (8.8%) whose MR examinations met the criteria for HIMAL (15 from the FEBSTAT main cohort, 3 from the Duke pilot cohort, and 2 from the Columbia cohort) compared with 2 (2.1%) of 96 controls with simple FS (Table 1). The odds ratio of having HIMAL in FSE cases versus simple FS controls was 4.56 (95%CI 1.05 – 19.92). HIMAL was a predominantly left-sided finding. It occurred solely on the left side in 18 (82%) of 22 children, including 17 of 20 children with FSE and 1 of 2 simple FS controls, and was bilateral in 4 (18%), including 3 FSE cases and 1 simple FS control. There was no case of solely right-sided HIMAL among the FSE cases or simple FS controls. Inter-rater agreement was generally high between the two raters (Kappa=0.87 [95%CI: 0.82 – 0.92]).
Table 1.
Proportion of HIMAL cases in FSE versus Controls
| Factor | HIMAL N (%) |
No HIMAL N (%) |
|---|---|---|
| FSE | 20 (8.8%) | 206 (91.2%) |
| Control | 2 (2.1%) | 94 (97.9%) |
Volumetric analysis
The mean right to left (R/L) ratio of hippocampal volumes was greater for the HIMAL group compared to the simple FS controls (1.14 for HIMAL without T2 signal abnormality versus 1.01 for the simple FS (p<.001). The mean volume of the left hippocampus was smaller in HIMAL cases (2223.1 mm3; sd=418.0) compared to simple FS controls (2532.6 mm3; sd=402.9) (p=0.004). The mean volume of the right hippocampus did not differ in cases compared to controls (2493.4 mm3; sd=341.3 for HIMAL cases vs. 2549.4 mm3 sd=378.7 for simple FS controls; p=0.6) (Table 2). While the asymmetric mean R/L ratio among HIMAL cases by itself does not indicate whether the right or left side is affected, the significant difference in mean left hippocampal volume between HIMAL and simple FS (and the absence of a difference in mean right hippocampal volumes) suggest that the hippocampal volume in the HIMAL group was decreased on the affected left side, thus increasing the R/L ratio.
Table 2.
Mean Hippocampal Volumes in HIMAL vs simple FS Controls (in cubic mm)
| Factor | Right hippocampal volume |
Left hippocampal volume |
|---|---|---|
| HIMAL cases | 2493.4 | 2223.1 |
| Simple FS controls | 2594.4 | 2532.6 |
We have previously reported that in the FSE cases with baseline MRIs visually interpreted as normal, both the left and right sided hippocampal volumes were smaller than the respective hippocampal volumes in simple FS controls with the right side more affected. [20]. We compared the hippocampal volumes measured in the baseline MRIs of the FSE cases visually read as normal (n=155) to the respective volumes of the HIMAL cases. There were no differences in the left hippocampal volumes (2,223.1 mm3 (sd=418.0) vs. 2,272 mm3 (sd=354.8); p=0.174) between the two groups. After adjusting for age, there were no differences in the right hippocampal volumes between the two groups (2,219.4 mm3 (sd=381.5) vs 2,493.4 mm3 (sd=341.3); p=0.14). The R/L ratio however was higher in the HIMAL cases than in the FSE cases (1.14 (sd=0.15) vs. 0.98 (sd=0.10); p<0.001) after adjustment for age.
T2 signal abnormalities in HIMAL cases
On baseline MR imaging, abnormal hippocampal T2 signal was present in 4 of the 20 FSE subjects with HIMAL, and in none of the 2 simple FS subjects with HIMAL (Table 3). Among the 4 FSE cases with both abnormal T2 signal and HIMAL, the abnormal hippocampal T2 signal was found in the left hippocampus on 3 cases (2 with left HIMAL, and 1 with bilateral HIMAL), and in the right hippocampus on 1 case (1 with left HIMAL). In contrast, in the 18 FSE cases with abnormal hippocampal T2 signal and no HIMAL, the abnormal T2 signal was right-sided in 14 cases. Although abnormal T2 signal intensity in the left hippocampus was observed in a higher proportion of FSE subjects with HIMAL than FSE subjects without HIMAL, this difference is of borderline statistical significance (p=.08), given the small number of subjects with HIMAL and abnormal hippocampal T2 signal.
Table 3.
Proportion of Cases with T2 signal abnormality in HIMAL cases vs non-HIMAL cases of FSE
| Factor | Abnormal T2 signal N (%) |
Normal T2 signal N (%) |
|---|---|---|
| FSE with HIMAL | 4 (20.0%) | 16 (80.0%) |
| FSE without HIMAL | 18 (8.7%) | 188 (91.3%) |
Risk Factors for HIMAL
Risk factors for HIMAL in children with FSE are summarized in Table 4. HIMAL was much more common in boys, and in children with FSE lasting more than 60 minutes. In the adjusted analysis, both factors remained statistically significant (Table 4). There was no association between HIMAL and abnormal development, prematurity, peak temperature of the febrile episode, or age at FSE. By definition, no FSE cases had a prior afebrile seizure, and only 18.6% had prior episodes of FS. Of the HIMAL cases, 26% had prior episodes of FS (p=0.45).
Table 4.
Factors associated with HIMAL in children with FSE
| Factor | FSE with normal baseline MRI N (%) |
FSE with HIMAL N (%) |
Crude OR OR (95% CI) |
Adjusted OR OR (95% CI) |
|---|---|---|---|---|
| Female | 78 (96.3%) | 3 (3.7%) | 1.0 (Referent) | 1.0 (Referent) |
| Male | 77 (81.91%) | 17 (18.09%) | 5.74 (1.6 – 20.4) | 5.36 (1.5 – 19.4) |
| < 18 mo at FSE | 100 (91.74%) | 9 (8.26%) | 1.0 (Referent) | |
| ≥ 18 mo at FSE | 55 (83.33%) | 11 (16.67%) | 2.22 (0.9 – 5.7) | |
| Abnormal/suspect Prior Development | 17 (80.95%) | 4 (19.05%) | 1.0 (Referent) | |
| Normal Prior Development | 138 (89.61%) | 16 (10.39%) | 0.49 (0.1 – 1.6) | |
| Not Premature | 96 (92.31%) | 8 (7.69%) | 1.0 (Referent) | |
| Premature | 38 (84.44%) | 7 (15.56%) | 2.21 (0.7 – 6.5) | |
| < 104F Temp at ED | 131 (87.33%) | 19 (12.67%) | 1.0 (Referent) | |
| ≥ 104F Temp at ED | 24 (96%) | 1 (4%) | 0.29 (0 – 2.2) | |
| Focal Seizure | 108 (87.8%) | 15 (12.2%) | 1.0 (Referent) | |
| non-Focal Seizure | 47 (90.38%) | 5 (9.62%) | 0.77 (0.3 – 2.2) | |
| Continuous | 86 (87.76%) | 12 (12.24%) | 1.0 (Referent) | |
| Intermittent | 69 (89.61%) | 8 (10.39%) | 0.83 (0.3 – 2.1) | |
| ≤ 60 min Duration FSE | 69 (97.18%) | 2 (2.82%) | 1.0 (Referent) | 1.0 (Referent) |
| > 60 min Duration FSE | 86 (82.69%) | 18 (17.31%) | 7.22 (1.6 – 32.2) | 6.73 (1.5 – 30.5) |
37 missing information on prematurity;
2 missing temperature
Discussion
In our prior report on the baseline findings in the main FEBSTAT cohort [8] we found a higher rate of HIMAL in FSE group compared with the simple FS cases but the results were of borderline statistical significance. The focus of that report was on evidence of hippocampal injury. In this report on the enlarged FEBSTAT cohort, we focus on the significance of HIMAL and have a sufficient sample size to allow analysis of risk factors as well as the formal quantitative volumetrics. We confirmed that HIMAL is more frequently found on MR imaging among children with FSE than among children with simple FS. For reasons that are not well understood, it appears to be a left-sided phenomenon. In addition, quantitative volumetric analysis revealed smaller left hippocampal volumes in the HIMAL cases compared to simple FS controls. This volume difference is not readily apparent on visual analysis due to difficulties in comparing the volume of the rounded left hippocampus with that of the typical oblong shape of the non-affected right hippocampus. Smaller hippocampal volumes at baseline in children with FSE with visually normal-appearing hippocampi on MR imaging have been previously reported on quantitative analysis of the FEBSTAT cohort [20], suggesting subtle preexisting abnormality. In this study, the smaller volumes of affected hippocampi and the longer duration of FSE provide further evidence that HIMAL is a pathological finding rather than a variant of unclear significance.
HIMAL has been detected more frequently among patients with epilepsy than in patients without epilepsy, but controversy has existed as to whether or not this lesion is related to the pathogenesis of epilepsy [1–6]. The evidence increasingly implicates HIMAL as a pathological lesion. A recent study reported that HIMAL in pediatric patients with epilepsy was associated with complex prefrontal dysfunction on formal neuropsychological testing [21]. Another case-control study found it to be uncommon in cases without known epilepsy [2]. HIMAL was found in 9 of 14 adult patients with chromosome 22q11.2 deletion syndrome, but only a subset had also developed epilepsy [3]. These results suggest that the clinical manifestations of HIMAL may be expressed in different ways, and that HIMAL may not necessarily be the proximate cause of epilepsy in all individuals who harbor this finding.
Most surgical series of hippocampal sclerosis do not describe any obvious asymmetry in the side of hippocampal involvement [22–26]. The exception is one series which reported a preponderance of right sided hippocampal sclerosis in those patients who had a history of prior prolonged febrile seizures [27]. This is consistent with our finding [8] that abnormal T2 hippocampal signal is more common in the right hippocampus, especially in those without HIMAL. The finding that the hippocampus is more often involved by abnormal T2 hyperintensity at the time of the initial episode of FSE [8,27] lends support to the hypothesis that “excitotoxic injury” causes injury to a previously normal hippocampus, ultimately leading to the development of hippocampal sclerosis. Previous case reports and case series have provided examples of this mechanism of pathogenesis [28–31]. If it is true that FSE tends to involve the right hippocampus more frequently than the left hippocampus in patients without HIMAL, then the possibility of disproportionate involvement of the left hippocampus in patients with HIMAL suggests an alternative, countervailing mechanism by which the left hippocampus is damaged by FSE. This possibility is supported by prior work suggesting that duration of febrile seizures is a predictor of temporal lobe epilepsy [32,33]. In our study, we observed that HIMAL was associated with longer duration of the seizure episode of FSE, thereby increasing the likelihood of hippocampal injury, more frequently on the left side. The higher likelihood of left-sided hippocampal damage among HIMAL patients may help to explain why most surgical series of temporal lobe resections for medically refractory mesial temporal lobe epilepsy do not report preponderance of one side or the other.
Further support for the role of HIMAL in the evolution of temporal lobe epilepsy comes from MR findings in 2 families with familial epilepsy [34]. Each family was related to a single proband with left-sided HS, with both families including several members who harbored HIMAL, mainly affecting the left hippocampus. The fact that only one member of each family developed HS, even though several individuals had HIMAL, suggests that HIMAL serves as a risk factor for FSE, which, in turn, may lead to HS.
Most of the literature describes cases of HIMAL in children older than age 18 months. Our work suggests that it is feasible to make the diagnosis of HIMAL on brain MR imaging during the first 18 months of life, though it is slightly more difficult to do so than in older children. This is due to the combination of the smaller size of the hippocampi, and the decreased extent of myelination in the temporal lobes. However, if the specific signs of rounded hippocampal shape, medial positioning of the hippocampus, vertical orientation of the ipsilateral collateral sulcus towards the superior-inferior plane, and inferior positioning of the ipsilateral fornix, are assiduously sought by experienced, trained neuroradiologists, then identification of HIMAL, even in the first two years of life, is facilitated. In order not to miss the diagnosis of HIMAL, it is critical not to limit hippocampal inspection to the detection of T2 signal abnormalities and volume loss. In our study, we did not use the sign of blurring of internal architecture of the hippocampus as a sign of HIMAL, simply because the small size of the hippocampus in the infant brain already rendered internal detail of the hippocampus more difficult to visualize in many cases. In addition, more recent work on high-resolution MR imaging at 7 Tesla suggests that the internal architecture of the hippocampus is actually not blurred in patients with HIMAL [35].
Conclusion
As reported in prior studies, we found that HIMAL is a developmental malformation that predominantly affects the left hippocampus, and is found much more frequently among boys. Our data show that it is associated with smaller hippocampal volumes on the affected side and with a predisposition to prolonged febrile seizures. These findings provide support to the hypothesis that HIMAL represents a pathologic finding. Long-term follow-up of this cohort is in progress and will ultimately provide more definitive data on the relationship between HIMAL, FSE and subsequent hippocampal sclerosis.
Acknowledgments
This investigation was supported by two grants from the National Institutes of Health, R01-NS-043209 (Shlomo Shinnar, P.I.) and RO1-HD-036867(Dale C. Hesdorffer, P.I.)
Abbreviation Key
- HIMAL
hippocampal inversion with malrotation
- FSE
febrile status epilepticus
- HS
hippocampal sclerosis
- MTLE
mesial temporal lobe epilepsy
- FS
febrile seizure
FEBSTAT Study Team
Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY: Shlomo Shinnar MD PhD, Jacqueline Bello MD, Evelyn Berman MD, William Gomes MD PhD, James Hannigan RT, Sharyn Katz R-EEGT, FASET, Ann Mancini MA, David Masur PhD, Solomon L. Moshé MD, Ruth Shinnar RN MSN, Yoshimi Sogawa MD, Erica Weiss PhD.
Columbia University, New York, NY: Dale Hesdorffer PhD, Stephen Chan MD, Claire C Litherland MS.
Duke University Medical Center, Durham, NC: Darrell Lewis MD, Melanie Bonner PhD, Karen Cornett BS, MT, William Gallentine DO, James MacFall PhD, James Provenzale MD, Allen Song PhD, James Voyvodic PhD, Yuan Xu BS.
Eastern Virginia Medical School, Norfolk, VA: L. Matthew Frank MD, Joanne Andy RT, Terrie Conklin RN, Susan Grasso MD, Connie S. Powers R-EEG T, David Kushner MD, Susan Landers RT, Virginia Van de Water PhD.
International Epilepsy Consortium at Department of Biostatistics, Virginia Commonwealth University, Richmond, VA: Shumei Sun PhD, John Pellock MD, Brian J Bush MSMIT, Lori L Davis BA, Xiaoyan Deng MS, Christiane Rogers, Cynthia Shier Sabo MS.
Ann & Robert Lurie Children’s Hospital, Chicago, IL: Douglas Nordli MD, John Curran MD, Leon G Epstein MD, Andrew Kim MD, Diana Miazga, Julie Rinaldi PhD.
Mount Sinai Medical Center: Emilia Bagiella PhD.
Virginia Commonwealth University, Richmond, VA: John Pellock MD, Tanya Brazemore R-EEGT, James Culbert PhD, Kathryn O’Hara RN, Syndi Seinfeld DO, Jean Snow RT-R.
Appendix
Hippocampal volumetric tracing methodology: Hippocampal regions of interest (ROIs) were traced using SnAP:IRIS by a trained, independent observer blinded to the clinical data. Tracings were performed on the T1-weighted SPGR brain images reformatted using AnalyzeTM to assure that the slice planes were perpendicular to the long axis of each hippocampus and parallel with a line joining the symmetric portions of the 8th or 5th nerves as they exit the brain stem. Determination of the anatomical boundaries of the hippocampal formation - including the alveus and subiculum - were based on protocols developed by Watson et al, and Jack et al [15,16]. For boundaries of the body of the hippocampus, CSF defined the lateral and superior boundaries in the ventricle and choroid fissure. CSF formed the superior portion of the medial boundary in the ambient cistern. The boundary between fimbria and hippocampal tissue was defined by a smoothly arcing line following the curve of the alvear surface of the hippocampus. The inferior boundary included the subiculum and was defined inferiorly by the interface of the subicular gray matter and the white matter underlying the collateral sulcus and medially by a line drawn from the most medial and superior white matter across the subicular gray to the cistern. The most anterior slice on which the hippocampus was traced was that containing the maximum width of the anterior commissure. The limit of the anterior upper hippocampal surface was the interface of the ventricular CSF with the hippocampal or alvear tissue. The lateral-inferior limit was the junction of gray hippocampal tissue or alveus and the collateral white matter. The uncinate gyrus is often seen as it turns superiorly to join the amygdala in several anterior slices and contains hippocampal tissue. The uncinate gyrus was included in the slice up to the medial extension of a line drawn parallel to the alvear surface of the hippocampus. The most posterior slice selected was that slice upon which recognizable portions of both the crus of the fornix and hippocampal tissue remained clearly visible.
Footnotes
Presented in part at the Annual Meeting of the American Society of Neuroradiology, April 21–26, 2012; New York, New York.
References
- 1.Barsi P, Kenez J, Solymosi D, et al. Hippocampal malrotation with normal corpus callosum: a new entity? Neuroradiology. 2000;42:339–345. doi: 10.1007/s002340050895. [DOI] [PubMed] [Google Scholar]
- 2.Gamss RP, Slasky SE, Bello JA, et al. Prevalence of hippocampal malrotation in a population without seizures. AJNR Am J Neuroradiol. 2009;30:1571–1573. doi: 10.3174/ajnr.A1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade DM, Krings T, Chow EW, Kiehl TR, Bassett AS. Hippocampal malrotation is associated with chromosome 22q11.2 microdeletion. Can J Neurol Sci. 2013;40:652–656. doi: 10.1017/s0317167100014876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajic D, Kumlien E, Mattson P, et al. Incomplete hippocampal inversion – is there a relation to epilepsy? Eur Radiol. 2009;19:2544–2550. doi: 10.1007/s00330-009-1438-y. [DOI] [PubMed] [Google Scholar]
- 5.Bajic D, Canto Moreira N, Wikström J, et al. Asymmetric development of the hippocampal region is common: a fetal MR imaging study. AJNR Am J Neuroradiol. 2012;33(3):513–518. doi: 10.3174/ajnr.A2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinnar S, Hesdorffer DC, Nordli DR, Jr, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71:170–176. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Shinnar S, Lewis DV, et al. Design and phenomenology of the FEBSTAT Study. Epilepsia. 2012;53:1471–1480. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinnar S, Bello JA, Chan S, et al. MR imaging abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology. 2012;79:871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesdorffer DC, Chan S, Tian H, Hauser WA, Dayan P, Leary L, Hinton V. MRI-detected brain abnormalities among children with first febrile seizure. Epilepsia. 2008;49:765–771. doi: 10.1111/j.1528-1167.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 10.Hesdorffer DC, Benn EK, Bagiella E, et al. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70:93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commission on Epidemiology and Prognosis of the ILAE. Guidelines for epidemiological studies on epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Febrile Seizures: Consensus Development Conference Summary. 2. Vol. 3. Bethesda, MD: National Institutes of Health; 1980. [Google Scholar]
- 13.Dodson WE, DeLorenzo RJ, Pedley TA, et al. The treatment of convulsive status epilepticus: Recommendations of the Epilepsy Foundation of America’s working group on status epilepticus. JAMA. 1993;270:854–859. [PubMed] [Google Scholar]
- 14.VanLandingham KE, Heinz ER, Cavazos JE, et al. MRI evidence of hippocampal injury after prolonged, focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 15.Jack Jr. CR. MRI based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(Suppl. 6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 16.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurol. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B. Fundamentals of Biostatistics. fourth edition. New York: Duxbury Press; 1995. [Google Scholar]
- 19.Fleiss JL. Statistical Methods for Rates and Proportions. second edition. New York: John Wiley & Sons; 1981. [Google Scholar]
- 20.Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, et al. Hippocampal sclerosis after febrile status epilepticus: The FEBSTAT Study. Ann Neurol. doi: 10.1002/ana.24081. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiers P, Fonteyne A, Wouters H, et al. Hippocampal malrotation in pediatric patients with epilepsy is associated with complex prefrontal dysfunction. Epilepsia. 2010;51:546–555. doi: 10.1111/j.1528-1167.2009.02419.x. [DOI] [PubMed] [Google Scholar]
- 22.Falconer MA. Genetic and related etiological factors in temporal lobe epilepsy: a review. Epilepsia. 1971;12:13–31. doi: 10.1111/j.1528-1157.1971.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 23.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy. I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 24.Williamson PD, French JA, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 25.Bruton CJ. The neuropathology of temporal lobe epilepsy. New York: Oxford; 1988. [Google Scholar]
- 26.Sloviter RS, Pedley TA. Editorial: Subtle hippocampal malformation; importance in febrile seizures and development of epilepsy. Neurology. 1998;50:846–849. doi: 10.1212/wnl.50.4.846. [DOI] [PubMed] [Google Scholar]
- 27.Janszky J, Woermann FG, Barsi P, et al. Right hippocampal sclerosis is more common than left after febrile seizures. Neurology. 2003;60(7):1209–1210. doi: 10.1212/01.wnl.0000052823.29467.a0. [DOI] [PubMed] [Google Scholar]
- 28.Sokol DK, Demyer WE, Edwards-Brown M, et al. From swelling to sclerosis: acute change in mesial hippocampus after prolonged febrile seizure. Seizure. 2003;12:237–240. doi: 10.1016/s1059-1311(02)00195-4. [DOI] [PubMed] [Google Scholar]
- 29.Tsuchida TN, Barkovich AJ, Bollen AW, et al. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr Neurol. 2007;36:253–257. doi: 10.1016/j.pediatrneurol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Provenzale JM, Barboriak DP, VanLandingham K, et al. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol. 2008;190:976–983. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez G, Effenberger O, Vinz B, et al. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis. Neurology. 1998;50:909–917. doi: 10.1212/wnl.50.4.909. [DOI] [PubMed] [Google Scholar]
- 32.Maher J, McLachlan RS. Febrile convulsions: Is seizure duration the most important predictor of temporal lobe epilepsy? Brain. 1995;118:1521–1528. doi: 10.1093/brain/118.6.1521. [DOI] [PubMed] [Google Scholar]
- 33.Lewis DV, Barboriak DP, MacFall JR, et al. Do prolonged febrile seizures produce medial temporal sclerosis? Hypothesis, MRI evidence and unanswered questions. Prog Brain Res. 2002;135:263–278. doi: 10.1016/s0079-6123(02)35025-8. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez G, Effenberger O, Vinz B, et al. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis. Neurology. 1998;50:909–917. doi: 10.1212/wnl.50.4.909. [DOI] [PubMed] [Google Scholar]
- 35.Henry TR, Chupin M, Lehéricy S, et al. Hippocampal sclerosis in temporal lobe epilepsy: findings at 7 T. Radiology. 2011;261:199–209. doi: 10.1148/radiol.11101651. [DOI] [PMC free article] [PubMed] [Google Scholar]