Abstract
Introduction
A dysregulated immune response leading to sepsis is the most frequent cause of late post-traumatic deaths. We have found a novel anti-inflammatory pathway that is initiated by the acute phase protein, C-reactive protein (CRP), interacting with Fcγ receptor (FcγR) on monocytes. This pathway is protective in animal models of endotoxin shock. We hypothesized that genetic polymorphisms in the FcγR might contribute to monocyte responses and susceptibility to infectious complications after severe trauma.
Methods
We conducted an observational study on a prospectively identified cohort of adult patients with convenience enrollment admitted after severe trauma. We enrolled 66 patients and collected blood samples at enrollment and again at 48 and 72 hrs. Patients were followed through their hospital stay and any septic events before 14 days were recorded. Cytokine and CRP levels were determined in the plasma from all three blood draws. Additionally, DNA was extracted from blood and analyzed for the 131 H/R FcγRIIa polymorphism that strongly affects the binding of IgG and CRP to this receptor.
Results
Elevated levels of IL-8, IL-6, IL-10 MCP-1 as well as CRP were associated with reduced time to post-traumatic sepsis in Cox regression analysis. Expression of monocyte HLA-DR below 45% on patient monocytes was also associated with sepsis (HR = 3.15, 95% CI 1.45-6.93). Genetic analysis found that individuals with the polymorphism of the FcγRIIa receptor that binds CRP poorly were also more likely to have decreased monocyte HLA-DR and post-traumatic sepsis. In vitro studies showed that CRP could attenuate monocyte deactivation in volunteers with the polymorphism of the FcγRIIa receptor that binds CRP.
Conclusions
Our findings suggest that a common genetic variation in the FcγRIIa receptor may contribute to infectious susceptibility in trauma patients. In vitro experiments suggest that this association is related to the inability of CRP to bind to this FcγRIIa receptor variant.
Level of Evidence
Prognostic study, level III
Keywords: post-traumatic sepsis, monocyte deactivation, C reactive protein, FcγRIIa receptor
Introduction
Traumatic injury is a leading cause of death in persons under 44 years of age. Most deaths are secondary to massive hemorrhage or severe brain injury; however, victims who survive the initial trauma and resuscitation remain at risk for sepsis and sepsis-associated multi-organ failure. In fact, 17-23% of all trauma-related deaths are associated with post-traumatic sepsis acquired during the hospital in the days and weeks following a severe injury (1-3). Although the patterns and severity of injury help identify patients at high risk for developing sepsis, clinical factors alone do not account for the development of post-traumatic sepsis.
Post-traumatic sepsis is responsible for prolonged length of ICU stay, increased rates of organ failure and remains the leading cause of death in patients who survive the initial resuscitation (1-3). A large retrospective analysis of severely injured patients in Germany between 1993 and 2008 revealed an overall incidence of post-traumatic sepsis in 10.2% of severely injured patients. While the overall mortality for trauma patients decreased over the study period, the mortality rates in the subgroup of septic trauma patients did not significantly decrease (4). Angus et al estimated average costs per case were $22,100 with annual total costs of $16.7 billion nationally in a 1995 review of national cases (5). Consequently, post-traumatic sepsis remains a significant problem that requires further research. Early identification of patient-specific characteristics associated with its development may help in early identification of this complication and timely intervention to improve clinical outcomes.
The causes of sepsis are multi-factorial. Acute injury is a risk factor, but so are the many interventions that doctors and nurses use to treat acutely injured patients. Preventative interventions and quality control programs in hospital care, such as the Surviving Sepsis Campaign and CDC monitoring efforts, have done much over the last ten years to increase awareness about sepsis. These efforts target early identification of at risk patients, since early aggressive treatment of sepsis increases the chance of survival (6). Through these efforts, mortality rates from sepsis have declined, yet the risk of sepsis remains high, particularly for trauma patients with severe injuries (3,4,6). Identifying predictive factors that are associated with post-traumatic sepsis may be the next step for improving care for trauma patients.
We and others have found a correlation between post-traumatic sepsis in individuals with significant monocyte deactivation following their traumatic injury (7-9). Monocyte deactivation in these patients is characterized by 1) a lack of tumor necrosis factor (TNF)-α production upon LPS challenge in vitro, 2) increased production of anti-inflammatory cytokines, and 3) markedly reduced HLA-DR expression resulting in a loss of antigen-presenting capacity. Monocytes with reduced HLA-DR expression have been described in trauma patients, and failure to return to normal levels of HLA-DR expression has been correlated with higher mortality in severely injured trauma patients (8,9), but the mechanisms surrounding monocyte deactivation and its reversal are not fully recognized (10).
Fcγ receptors (FcγR) are the family of receptors expressed on innate immune effector cells and set a threshold for cell activation by immune complexes. They consist of one inhibitory and several activating receptors that differ in their affinity and specificity for immunoglobulin (Ig) subclasses. FcγR-induced leukocyte functions, including antibody-dependent cellular cytotoxicity, phagocytosis, superoxide generation, degranulation, cytokine production and regulation of antibody production, are essential for host defense and immune regulation (11,12). The ligand– FcγR interaction is influenced by several factors that may affect the expression levels of activating and inhibitory FcγRs or change the affinity of the receptor (13,14).
C-reactive protein (CRP) is an acute phase serum protein that shares several functions with IgG, including complement activation and binding to Fc receptors on monocytes and neutrophils (15). CRP binds to microbial antigens and damaged cells, opsonizes particles for phagocytosis and regulates the inflammatory response by the induction of cytokine synthesis. These activities of CRP depend on its ability to activate complement and to bind to FcγR (15,16,17). CRP binds to all three types of FcγR and to the IgA receptor, FcγRI (18). Freshly isolated monocytes primarily bind CRP through FcγRIIa (16,19). The FcγRIIa plays a central role in the regulation of immunity and autoimmunity and the initiation of local inflammation.
The FcγRIIa gene contains a functional single nucleotide polymorphism (SNP) with a G→A point mutation resulting in an arginine (R) or histidine (H) at position 131 in the Ig-binding domain. This polymorphism is known to affect receptor affinity and specificity. Interestingly, the R 131 variant shows strong binding to CRP and weak binding to IgG, whereas the H 131 variant shows weak binding to CRP and strong binding to IgG (16,17). This polymorphism has clinical implications and may represent a risk factor for certain diseases, either at the level of disease susceptibility or at the level of disease severity (20). Patients with the R allele were found to have a more severe case of Severe Acute Respiratory Syndrome infection and were more likely to be susceptible to encapsulated organism infection, which was attributed to poor IgG2 binding to the R variant of FcγRIIa (11,20). The FcγRIIa polymorphism has been described as a heritable risk factor for autoimmune and infectious diseases (11,20). We hypothesized this common genetic variation in the receptor for CRP might contribute to individual differences in monocyte responses and susceptibility to infectious complications after severe trauma.
Patients and Methods
Patients
The Human Research Review Committee at the University of New Mexico Health Sciences Center approved all protocols prior to sample collection. Patients eligible for enrollment included severely injured trauma patients defined by an ISS of 16 or greater or requiring ICU admission, with age greater than 18 years, and negative pregnancy test. No patients were enrolled that were admitted to the ICU for merely observation status. No patient was treated with any immunosuppressant or steroids. Patients were followed through their hospital stay and any septic events occurring within 14 days of admission were recorded, as defined by systemic inflammatory response syndrome (SIRS) criteria with confirmed positive cultures and clinical confirmation of infection. Severe sepsis was defined as documented infection with acute organ dysfunction. A subset of this study's patient population was previously characterized (10,21,22). The subjects characterized in this study are part of an ongoing effort to better understand the changes in immune function after severe trauma.
Methods
Sample Collection
Study design was a convenience sample (patient enrollment was limited to weekdays due to personnel constraints) of prospectively identified patients who met enrollment criteria. Blood samples were collected from 66 eligible patients at enrollment (day 1) and again at approximately 24 hours (day 2) and 48 hours (day 3) who provided informed consent or had consent from a legally authorized representative. Whole blood was collected each day in heparinized tubes for determination of plasma cytokine levels and in EDTA tubes on day 2 for determination of monocyte markers by flow cytometry and for DNA extraction. Septic events were recorded for enrolled patients up until day 14 of hospitalization and were defined as presence of 2 or more SIRS criteria with confirmed positive culture, combined with the appropriate clinical findings (ie. documented course of antibiotic treatment for clinically presumed infection). Only positive cultures were considered in the sepsis group. The EMR was evaluated to determine if the appropriate clinical criteria were present, ie- tachycardia, fever, leukocytosis and/or tachypnea to determine presence of SIRS criteria as well as documentation from the treating physician of clinical infection. Multiple organ dysfunction syndrome (MODS) was defined as organ failure for at least two organ systems. Organ failure was defined by the Sequential Organ Failure Assessment criteria (SOFA score > or = 3 points) during the ICU stay (23). A subset of this study's patient population was previously characterized (10,21,22). We have previously described the association of heme-oxygenase with monocyte deactivation and showed that HO-1 did not correlate with monocyte deactivation in a subset of this cohort (10). We identified the expansion of a subpopulation of monocytes in a portion of this cohort (21), and finally, we described a correlation between lower apolipoproteins B and AII levels and nosocomial infections in a portion of this cohort (22).
Plasma Cytokine Analysis
Heparinized blood samples from each day were centrifuged at 1000 rpm for 10 minutes and plasma was collected and stored at -80°C for later determination of cytokine concentrations and factor levels. Plasma cytokine concentrations were determined with Milliplex Human Cytokine Immunoassay according to the manufacturer's instructions (Millipore, Billerica, MA). The cytokines measured by this technique included TNF-alpha, IL-1RA, IL-6, IL-8, IL-10, and MCP-1. CRP, TGF-β, M-CSF and soluble CD163 concentrations were determined separately by ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN for CRP, M-CSF and soluble CD163; BD Bioscience, San Jose, CA for TGF-β).
DNA Extraction and Determination of FcγR polymorphism
Whole blood was collected from patients at enrollment and control volunteers and stored at -80°C for future DNA extraction. DNA extraction was performed using Qiagen DNeasy Blood Kit according to manufacturer's instructions (Qiagen Inc, Valencia, CA). Determination of FcγRIIa polymorphism Genomic DNA was extracted from EDTA anticoagulated whole blood (Gentra Systems Inc., Plymouth, MN). For FcγRIIa genotype determinations, we used allele-specific PCR reactions to characterize the presence or absence of the FcγRIIa-131 histidine (H)/arginine (R) alleles (16). For the FcγRIIa alleles, a forward PCR primer was used to bind to a sequence in intron 4 that is unique to the FcγRIIa gene together with either H-131– or R-131–specific reverse primers as previously reported (16,24). For the FcγRIII polymorphism, Genomic DNA was extracted as described above. FcγRIIIa-158 valine (V)/phenylanaline (F) polymorphisms were evaluated by directly sequencing, and DNA was prepared with commercially purchased primers (Sigma, St. Louis, MO) according to Genewiz (Genewiz, Inc, South Plainfield, NJ) sample submission guidelines.
Monocyte Surface Marker Expression
Expression of CD-14 and HLA-DR on patient monocytes and volunteer monocytes was determined by flow cytometry, as previously described (10). Briefly, whole blood samples were collected from donors in EDTA-treated tubes at 48 hours, and 200 μl was used for measurements. The following mAb were used: FITC anti-CD14 (Miltenyi Biotec, San Diego, CA) and PerCP-Cy5.5 anti-HLA-DR (BD Bioscience, San Jose, CA) and corresponding isotype controls. Cells were incubated with the mAb for 10 minutes in the dark. RBC were lysed with 2 ml of RBC lysis solution (eBioscience, San Diego, CA) at room temperature for 10 minutes. Cells were washed once with phosphate buffered saline (PBS) and twice with 3 ml of PAB (0.1% BSA/0.05% sodium azide in PBS) staining buffer. Cells were then fixed with 2% paraformaldehyde and the fluorescence of each sample was analyzed with a flow cytometer (BD Bioscience, San Jose, CA) and FlowJo Software (Tree Star, Inc, Ashland, OR). Results are expressed as the percentage HLA-DR expressed on CD-14+ cells after subtracting the corresponding isotype control.
Deactivation of Control Monocytes
The use of human peripheral blood monocytes from healthy donors was approved by the Human Research Review Committee of the University of New Mexico Health Sciences Center. Blood from healthy volunteers was drawn into heparinized tubes. Peripheral blood mononuclear cells (PBMC) were obtained by gradient separation using Ficoll Paque Plus (GE Healthcare, Pittsburgh, PA). Briefly, 5 ml of whole blood was diluted 1:1 with PBS and carefully layered over 3 ml of Ficoll Paque Plus. Samples were then centrifuged at 1400 rpm for 30 minutes and the mononuclear cell layer was collected. PBMC were washed two times in PBS and resuspended in RPMI-1640 medium (containing 10% heat-inactivated FBS-HyClone, Sigma, St. Louis, MO). Control PBMC were incubated with IL-10 (20 ng/μl) and TGF-β (20 ng/μl) for 22 hours and then stimulated with LPS (100 ng/ml) for 4 hours. Acute phase levels of heat-aggregated CRP (100 μg/ml) were added at the same time as LPS to the appropriate wells. After an additional 4 hours of incubation, supernatants were collected and stored at -80°C for future determination of TNF-α production by ELISA (BD Bioscience, San Jose, CA) according to manufacturer's instructions. Cells were removed and collected with multiple washes of PBS. HLA-DR and CD-14 expression was determined by flow cytometry on cultured control PBMCs as described above for patient monocytes.
Statistical Analysis
Graphical and statistical analyses were performed using Prism software version 5.0 (GraphPad, La Jolla, CA) and SAS version 9.3 (Statistical Analysis Software, Cary, NC). Univariate analyses were conducted for each biomarker measured and septic complications in patients. Mann-Whitney U tests were used to compare continuous variables and Fisher exact tests were used to compare categorical variables. Unadjusted and adjusted Cox model regression analyses were used to assess the association between biomarkers and time until sepsis. Adjusted models included ISS scores and transfusion in addition to biomarkers. ISS was modeled as a quadratic polynomial effect centered at ISS = 27 (ISS* = (ISS - 27) and transfusion was 1 if the patient was transfused and 0 otherwise. Patient follow up was censored after 14 days for patients that did not develop sepsis. Biomarkers had skewed distributions and were transformed before analyses using the base 2 logarithm. With this transformation the hazard ratio for a one unit increase in log2(biomarker) corresponds to the effect size for a doubling of the biomarker concentration. Longitudinal changes in biomarker concentration were assessed by mixed model regression analyses with terms for days from initial blood draw, ISS and whether the patient developed sepsis within 14 days. A patient-level random effect was used to account for repeated measures. Mann-Whitney U test was used to compare values of volunteer monocyte TNF-α production and HLA-DR expression. Association between FcγRIIa genotype and sepsis was assessed using Cochran-Armitage exact trend test and with Cox model regression. Genetic models in Cox regressions used the number of H copies (0, 1, or 2) or HH/RH versus RR to assess association with sepsis. Statistical significance was determined at p < 0.05. Adjustment for multiple hypotheses tests was made to control the false discovery rate to <5% (25).
Results
Clinical Characteristics Correlate with Post-Traumatic Sepsis
Thirty patients developed sepsis during the study period. Characteristics of the study population are noted in Table 1. The average ISS was 30 (range 9-55). The average age was 39 years (range 18-79). There was no significant difference in the incidence of sepsis and the mechanism of trauma (i.e. blunt vs. penetrating). Patients who developed post-traumatic sepsis were significantly more likely to have received a blood transfusion in the first 24 hours of resuscitation than patients that did not develop sepsis during the study period (Table 1). Fifty-seven percent of transfused patients developed sepsis vs. 25% of patients who were not transfused (p=0.02).
Table 1.
Demographics and characteristics of severely injured trauma patients with and without sepsis.
| Patient Characteristics | All Patients (n = 66) | Sepsis (n = 30) | No Sepsis (n = 36) | P value |
|---|---|---|---|---|
|
| ||||
| Age (mean ± SEM) | 39 ± 2 | 41 ± 3 | 38 ± 3 | 0.99 |
|
| ||||
| Male, n (%) | 44 (67%) | 21 (70%) | 23 (64%) | 0.79 |
|
| ||||
| Blunt/Penetrating, n (%) | 56/10 (85/15) | 29/2 (94/6) | 27/8 (77/23) | 0.089 |
|
| ||||
| Injury Severity Score (median, interquartile range Q3,Q1) | 29 (41,20) | 37 (41,28) | 24 (31,18) | < 0.001 |
|
| ||||
| ICU LOS (days, median, interquartile range Q3,Q1) | 5.5 (17,3) | 17 (22,10) | 3 (4,2) | < 0.001 |
|
| ||||
| Ventilator days (median, interquartile range Q3,Q1) | 3 (14,0) | 14 (19,7) | 0.5 (2,0) | < 0.001 |
|
| ||||
| Initial Systolic Blood Pressure < 90 mmHg, n (%) | 20 (30%) | 15 (50%) | 5 (14%) | 0.003 |
|
| ||||
| Antibiotic use n (%) | 35 (53%) | 20 (67%) | 15 (42%) | 0.051 |
|
| ||||
| Blood transfusion within 24 hours of admission, n (%) | 42 (64%) | 24 (80%) | 18 (50%) | 0.02 |
|
| ||||
| SNP label here, n (column %) | 0.049 | |||
| RR | 11 (17%) | 9 (25%) | 2 (7%) | |
| RH | 40 (61%) | 21 (58%) | 19 (63%) | |
| HH | 15 (23%) | 6 (17%) | 9 (30%) | |
Statistical significance was determined using Mann Whitney U test for continuous variables, Fisher's exact test for categorical data and the exact Cochran-Armitage trend test for FcγRIIa polymorphism.
Forty-seven patients (71 percent) were intubated, and 34 of these patients (51 percent) were intubated for more than 48 hours (range 3-34 days). Severe sepsis was documented in 23 patients and seven patients developed MODS. Three patients died during the study period. The median day of infection was hospital day 7, with 40% occurring early (≤ hospital day 5). The specific foci of infection were pneumonia (n=20), urinary tract infection (n=8), and bacteremia (n=5), Clostridium dificile colitis (n=1), intraabdominal abscess (n=1), with four patients developing infections in multiple sites. Causative bacteria were primarily Gram-negative (n=20), with one patient having a mixed bacterial infection. Fourteen (21%) were treated with empiric antibiotics and seven (11%) were treated with perioperative prophylactic antibiotics. Although approaching significance, we did not find a statistically significant difference in the incidence of sepsis and the use of antibiotics in our patients (67% in those receiving antibiotics vs. 42% in those not receiving antibiotics, p = 0.051). The length of antibiotic administration prior to the septic event varied, but the majority were given > 48 hours before the septic event occurred. In four patients, the diagnosis of sepsis was made while the patient was receiving empiric antibiotic therapy.
Inflammatory Markers Correlate with Post-Traumatic Sepsis
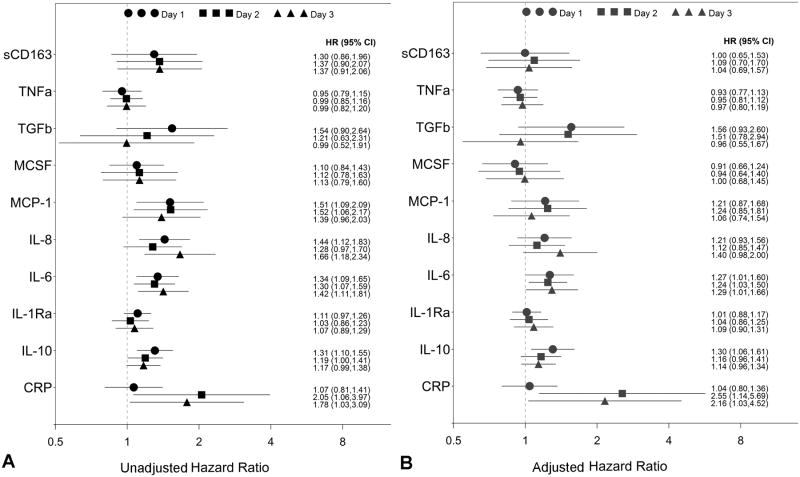
Median levels of cytokines and other biomarkers measured on all 3 days of blood draw are listed in Table 2. We performed separate unadjusted and adjusted Cox model regression analyses of cytokines measured on days 1, 2 and 3 with presence of septic complication prior to day 14 of hospitalization (Figure 1). For adjusted analyses, we chose to adjust for injury severity score and need for blood transfusion as these were each significant factors in development of post-traumatic sepsis when analyzing clinical factors. We did not include hypotension in the adjustment since the transfusion model predicted sepsis as well as hypotension in our model. The anti-inflammatory cytokine IL-10 was associated with an increased risk of developing sepsis. Similarly, the pro-inflammatory cytokines IL-8 and IL-6 and the chemokine MCP-1 as well as the acute phase protein CRP were associated with increased risk of developing sepsis in the unadjusted analysis. When we adjusted for the clinical factors ISS and need for transfusion, only IL-6, IL-10 and CRP remained associated with sepsis (Figure 1). After controlling for false discovery, the association between time to sepsis and these three biomarkers was not significant. We also explored the time course of biomarker concentrations using mixed model regression analyses. Patients that ultimately developed sepsis had significantly higher concentrations of IL-6, IL-10 and CRP in their initial measurement compared to those that did not develop sepsis. CRP concentrations continued to increase over time (p < 0.001) with trends being similar for sepsis and non-sepsis patients (p = 0.53). IL-6 and IL-10 decreased over time (p < 0.001 for each), and the rate of IL-10 decrease was faster in sepsis patients than on non-sepsis patients (p = 0.03).
Table 2.
Median and Interquartile Range Plasma Levels of Measured Biomarkers.
| All Patients | Septic Patients | Non-Septic Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| sCD163 | 281 (376,196) | 219 (279,153) | 176 (266,110) | 285 (403,204) | 221 (349,168) | 198 (359,121) | 272 (353,183) | 219 (264,124) | 160 (226,96) |
| TNF-α | 4.3 (7.6,0.9) | 5.6 (9.2,2.3) | 4.7 (9.4,1.6) | 4.2 (7.1,0.9) | 5.8 (8.2,3.3) | 4.8 (11.9,2.3) | 4.0 (8.2,0.9) | 4.9 (9.7,1.9) | 4.7 (7.9,1.3) |
| TGF-β | 4.3 (6.2,3.2) | 3.3 (4.2,2.4) | 3.0 (4.2,2.2) | 5.2 (6.8,3.7) | 3.3 (4.3,2.5) | 2.9 (4.2,2.2) | 3.5 (5.5,2.9) | 3.3 (4.2,2.4) | 3.1 (3.7,2.3) |
| MCSF | 479 (1071,190) | 693 (1216,476) | 434 (870,267) | 555 (1104,269) | 713 (1248,577) | 494 (1100,299) | 331 (975,173) | 690 (1113,411) | 429 (802,254) |
| MCP-1 | 873 (1426,458) | 784 (1365,505) | 757 (1256,449) | 1119 (2037,784) | 976 (1678,625) | 844 (1637,502) | 775 (991,415) | 703 (957,472) | 546 (994,411) |
| IL-8 | 32 (61,14) | 22 (39,10) | 18 (40,10) | 50 (113,23) | 34 (54,13) | 36 (54,15) | 25 (41,11) | 18 (26,8) | 12 (22,8) |
| IL-6 | 95 (239,44) | 73 (139,31) | 46 (111,20) | 158 (426,64) | 117 (309,61) | 59 (172,27) | 53 (145,31) | 48 (109,21) | 29 (69,16) |
| IL-1RA | 18 (74,3) | 10 (26,1) | 12 (24,4) | 29 (88,12) | 16 (43,2) | 14 (44,4) | 8.9 (24,2) | 7.0 (18,0) | 11 (21,3) |
| IL-10 | 36 (102,13) | 17 (51,7) | 13 (29,2) | 61 (213,28) | 26 (68,12) | 18 (74,8) | 21 (49,4) | 15 (49,2) | 10 (25,1) |
| CRP | 42.6 (59,17) | 73.7 (104,52) | 76.8 (108,47) | 46.3 (65,16) | 84.9 (112,64) | 91.5 (118,74) | 37.2 (56,19) | 60.6 (87,48) | 61.2 (89,35) |
| HLA-DR | 46 (61,27) | 33 (52,24) | 54 (67,35) | ||||||
Biochemical markers are expressed as median (interquartile range Q3,Q1). Cytokines and chemokines are expressed as pg/ml, HLA-DR is percentage positive expression on monocytes.
Figure 1.
Hazard ratios and 95% confidence intervals for predicting post-traumatic sepsis of each inflammatory marker with A) unadjusted COX regression analysis and B) COX regression analysis adjusted for confounders, injury severity score and blood transfusion. Values for days 1-3 for each biomarker are shown.
In our severely injured patient cohort, we found that HLA-DR expression on monocytes, measured on day 2 of admission, inversely correlated with infectious complications (52 ± 4 vs. 38 ± 3, p< 0.005) as previously found (7,8). Expression of monocyte HLA-DR below 45% on patient monocytes was also associated with sepsis (HR = 3.15, 95% CI 1.45-6.93). Decreased HLA-DR expression is an indicator of decreased monocyte responsiveness known as monocyte deactivation.
Genetic Factors Correlate with Post-Traumatic Sepsis
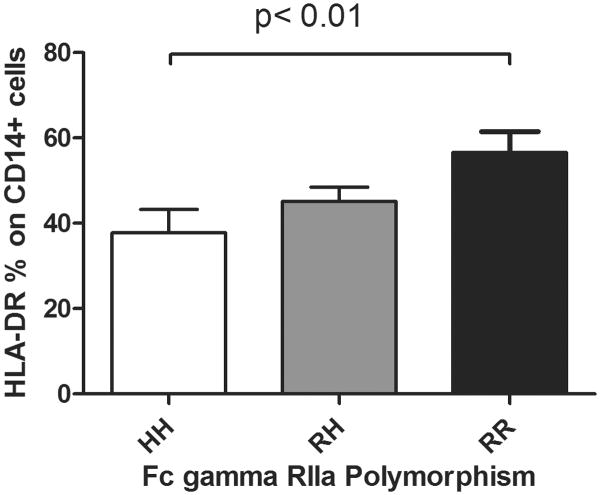
We also found that for each H allele of the FcγRIIa polymorphism, there was an increased risk of sepsis (Table 1, Cochran-Armitage Trend Test p = 0.049). The association between time to sepsis and the number of H alleles was assessed using Cox model regression with a hazard ratio equal to 1.69 per added H allele (95% CI 0.96 – 3.02). When we added ISS and CRP (day 3) to the model the hazard ratio for sepsis was 2.90 (95% CI 1.38 – 6.26) per added H allele. HH or HR patients had a higher risk of sepsis than RR patients (HR = 3.77, 95% CI 0.91 – 26.74, p = 0.07) after adjusting for ISS and CRP on day 3. The H allotype of the FcγRIIa receptor also correlated with the percentage of monocyte HLA-DR; HH variants were more likely to have lower HLA-DR expression when compared to RR variants (Figure 2). We found no association with FcγRIII polymorphisms on monocyte HLA-DR expression or TNF-α production (data not shown).
Figure 2.
HLA-DR expression in monocytes in severely injured trauma patients is significantly diminished in patients with the HH FcγRIIa receptor polymorphism compared to patients with RR polymorphism of the FcγRIIa receptor, n=15 for HH, n=40 for RH, and n=11 for RR, * p < 0.05 in HH vs. RR patients
CRP Reverses Monocyte Deactivation in Volunteers with R allele of FcγRIIa
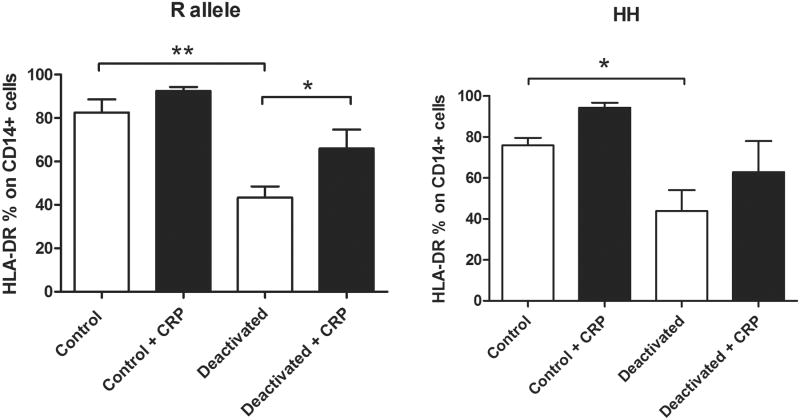
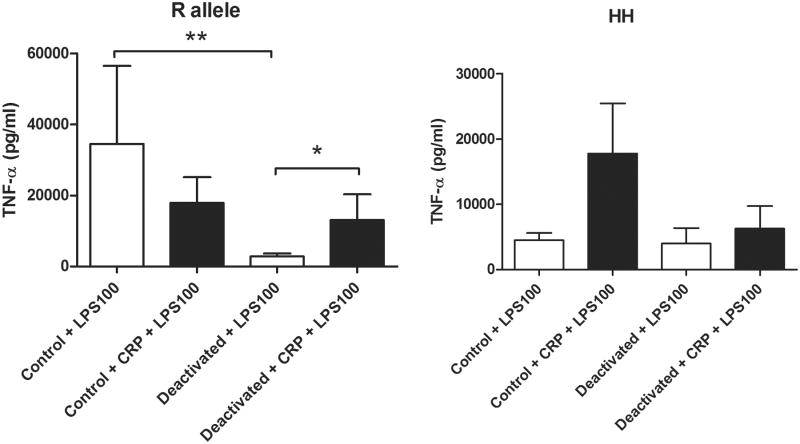
Because of our findings in the severely injured cohort of patients, we hypothesized that the polymorphism of the FcγRIIa receptor that has a higher affinity for CRP could reverse monocyte deactivation. We designed an in vitro experiment looking at an experimental model of monocyte deactivation and the effects of the allelic polymorphism of FcγRIIa in the presence of acute phase levels of CRP. In volunteers with the R allotype of the FcγRIIa receptor, a 22-hour incubation with IL-10 and TGF-β significantly decreased HLA-DR expression on CD14+ monocytes and decreased the production of TNF-α following LPS stimulation when compared to control. In volunteers with an R allele, acute phase levels of CRP were able to reverse the monocyte deactivation by increasing HLA-DR expression on CD14+ (Figure 3) and by increasing the production of TNF-α in response to subsequent stimulation with LPS when compared to controls (Figure 4). In volunteers with the H allele, CRP was unable to attenuate monocyte deactivation.
Figure 3.
Acute phase levels of CRP (100 μg/ml) reversed monocyte deactivation in volunteers with R allele of the FcγRIIa receptor as evidenced by increased HLA-DR expression on monocytes, but did not increase HLA-DR expression on monocytes in volunteers without an R allele (HH genotype) n=6 for R allele volunteers, n=5 for HH volunteers, * p < 0.05, ** p < 0.01.
Figure 4.
Acute phase levels of CRP (100 μg/ml) increased production of TNF-α following LPS stimulation (100 ng/ml) in deactivated monocytes from R allele volunteers but failed to affect TNF-α production following LPS stimulation in HH volunteers. n=6 for R allele volunteers, n=5 for HH volunteers, * p < 0.05, ** p < 0.01.
Discussion
Trauma patients who survive their initial injuries and resuscitation remain at an elevated risk of morbidity and mortality. This is characterized by a relative immunosuppression that can increase the susceptibility to sepsis. The overall incidence of sepsis in post-traumatic patients is reported between 2 and 10% (1,4,26). The incidence of sepsis in our patient cohort was much higher at 45%; likely reflecting the higher severity of injury with a mean ISS of 30 ± 12. These findings are similar to the rates described by Gouel-Chéron et al. whose cohort had a 37% incidence of post-traumatic sepsis with a mean ISS of 37 ± 10 (27) and Gu et al. who described an incidence of 48.4% with a mean ISS of 25.5 ± 8.0 (28).
The majority of the patients whom developed sepsis had pneumonia as the specific foci of infection (65%). This is in spite of the routine use of the ventilator bundle in our intubated trauma patients, similar to the findings of Croce et al (29). Although pneumonia was the most common infectious site for septic complications, no specific cytokine measured in our cohort was associated with pneumonia in independent analysis (data not shown).
We found clinical variables, biochemical factors and genetic factors that correlated with post-traumatic sepsis in our severely injured cohort of patients. We found that blood transfusion within the first 24 hours of presentation was associated with the development of post-traumatic sepsis. This finding was recently confirmed and further investigated by Torrance, et al (30). They found that transfusion of blood products was independently associated with altered patterns of gene expression. Their conclusion was that the immunosuppressive response to trauma may be exacerbated by blood transfusions, and this was associated with an increased susceptibility to nosocomial infections. They demonstrated no difference in the overall injury severity score between patients receiving transfusions and those that did not. However, there were significantly higher numbers of traumatic brain injury patients in the non-transfused group, which may account for the equivalent ISS between the two groups, since head trauma is not usually associated with a high transfusion requirement (30). When clinical variables were used to predict sepsis in a multivariable model, we found that ISS (HR = 1.65 per 10 unit increase, 95% CI 1.21 – 2.28) and transfusion (HR = 2.57 95% CI 1.12 – 6.96) were jointly associated with sepsis. Neither age nor gender were significantly associated with sepsis, thus our adjusted models used ISS and transfusion in these analyses.
We found that elevated levels of the anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokines IL-8 and IL-6 as well as the chemokine MCP-1 were associated with increased odds of developing sepsis. Elevated levels of the acute phase protein CRP were also associated with post-traumatic sepsis. Interestingly, soluble CD163 was not associated with sepsis in our population, although it has been shown to be a predictor in burn patients by Piatkowski et al (31).
Monocyte deactivation as measured by HLA-DR expression is an easily measured marker of immune function that has been well characterized in septic and trauma patients (8,9). We measured monocyte HLA-DR expression on hospital day 2 for our cohort, and confirmed that day 2 levels of HLA-DR expression had a strong inverse correlation with septic complications (7,27). We also found that the FcγRIIa allele that does not bind CRP was associated with septic complications in the severely injured cohort. We hypothesized that this receptor, which is known to regulate monocyte activation and function in host defense, could contribute to either reversal or prolongation of monocyte deactivation in these patients. In order to further evaluate this hypothesis, we examined monocyte responses from FcγRIIa polymorphism-typed donors in an experimental model of monocyte deactivation in the presence and absence of acute phase levels of CRP.
Single nucleotide polymorphisms are single base changes in a gene that occur at a significant frequency (often defined as more than 1%) in a population. SNPs in the coding region of a gene often result in amino acid changes that may alter the functioning of the affected proteins. Single nucleotide polymorphisms in the coding regions of FcγRIII and FcγRIIa genes appear to have clinical significance (32). The FcγRIIa receptor contains a SNP resulting in a functional allelic difference in the binding domain. The H allele binds IgG with micromolar affinity while the R allele binds CRP with micromolar affinity. These functional differences in the receptor have been shown to alter phagocytic function of host monocytes and clinically to result in differences in susceptibility and severity of disease (11-14).
We found that elevated levels of CRP in our severely injured patient cohort were associated with infectious complications. This is likely a reflection of the severity of injury. However, since CRP levels are known to be elevated after trauma, and we found that the patients with the H allele of the FcγRIIa receptor had more infectious complications when compared to patients with the R allele, we evaluated the role of acute phase levels of CRP in our in vitro experiments. In the in vitro experiments, we were able to attenuate monocyte deactivation in volunteers with an R allele when treated with acute phase levels of CRP.
Our findings suggest that a common genetic variation in the FcγRIIa receptor may contribute to infectious susceptibility in trauma patients through monocyte deactivation. Patients with the R allele of the FcγRIIa receptor may be able to reverse monocyte deactivation in the presence of acute phase levels of CRP, while those with the H allele lack this ability. Further studies are necessary to delineate the specific mechanism by which CRP may reverse deactivation in these patients.
This study is limited by the number of patients included in our analysis as well as the convenience sampling enrollment which introduces a possible selection bias. However, we confirmed the effect of several independent factors that have previously been associated with an increase in post-traumatic sepsis, giving support to our findings (7,27,33,34). We propose that a combination of clinical variables, early biochemical markers, and genetic factors may aid the clinician in early identification of trauma patients at risk for septic complications.
Supplementary Material
Acknowledgments
Supported By: This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number KL2RR031976. Statistical assistance was provided by the Biostatistics Core at the University of New Mexico Clinical and Translational Science Center through DHHS/NIH/NCRR Grant Number 1UL1RR031977-01
Footnotes
Author Contribution Statement: Dr. West was responsible for conception and design, analysis and interpretation, writing the article and obtaining funding. Dr. Ziegler, Dr. Brooks and Mr. Krencicki were responsible for data analysis and interpretation as well as data collection. Dr. Myers provided analyses and interpretation, assisted in development, review and revision of manuscript. Dr. Mold assisted with conception and design, analysis and interpretation and provided a critical revision of the article.
References
- 1.Osborn TM, Tracy JK, Dunne JR, Pasquale M, Napolitano LM. Epidemiology of sepsis in patients with traumatic injury. Crit Care Med. 2004;32:2234–2240. doi: 10.1097/01.ccm.0000145586.23276.0f. [DOI] [PubMed] [Google Scholar]
- 2.Ingraham AM, Xiong W, Hemmila MR. The attributable mortality and length of stay of trauma-related complications: A matched cohort study. Ann Surg. 2010;252:358–362. doi: 10.1097/SLA.0b013e3181e623bf. [DOI] [PubMed] [Google Scholar]
- 3.Kisat M, Villegas CV, Onguti S, Zafar SN, Latif A, Efron DT, Haut ER, Schneider EB, Lipsett PA, Zafar H, Haider AH. Predictors of sepsis in moderately severely injured patients: An analysis of the National Trauma Data Bank. Surg Inf. 2013;14:62–68. doi: 10.1089/sur.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wafaisade A, Lefering R, Bouillon B, Sakka SG, Thamm OC, Paffrath T, Neugebauer E, Maegele M Trauma Registry of the German Society for Trauma Surgery. Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39:621–628. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and the associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21:128–140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 7.Cheron A, Foloccard B, Allaouchiche B, Guignant C, Poitevin F, Malcus C, Crozon J, Faure A, Guillaume C, Marcotte G, Vulliez A, Monneuse O, Monneret G. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care. 2010;14:R208. doi: 10.1186/cc9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC., Jr Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 9.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: Restoration by IFN-γ treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 10.West SD, Mold C. Monocyte deactivation correlates with Injury Severity Score, but not with heme oxygenase-1 levels in trauma patients. J Surg Res. 2012;172:5–10. doi: 10.1016/j.jss.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 12.García-García E, Rosales C. Fc receptor signaling in leukocytes: Role in host defense and immune regulation. Current Immunology Reviews. 2009;5:227–242. [Google Scholar]
- 13.Gillis C, Gouel-Chéron A, Jönsson F, Bruhns P. Contribution of Human FcγRs to Disease with Evidence from Human Polymorphisms and Transgenic Animal Studies. Frontiers in Immunology. 2014;5:1–13. doi: 10.3389/fimmu.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Pol WL, Van de Winkel JGJ. IgG receptor polymorphisms: risk factor for disease. Immunogenetics. 1998;48:222–32. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 15.Du Clos TW, Mold C. C-Reactive Protein: Structure, synthesis and function. Immunobiology of Carbohydrates. 2003:39–55. [Google Scholar]
- 16.Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, Du Clos TW. C-reactive protein binding to FcgRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest. 2000;105:369–376. doi: 10.1172/JCI7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, Sun P. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci U S A. 2011;108:4974–4979. doi: 10.1073/pnas.1018369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is Fcγreceptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan FF, Tanner J, Chan PKS, Biffin S, Dyer WB, Geczy AF, Tang JW, Hui DSC, Sung JJY, Sullivan JS. Influence of FcγRIIa and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens. 2005;66:291–296. doi: 10.1111/j.1399-0039.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West SD, Goldberg D, Ziegler A, Krencicki M, Du Clos TW, Mold C. Transforming Growth Factor-β, Macrophage Colony-Stimulating Factor and C-Reactive Protein Levels Correlate with CD14highCD16+ Monocyte Induction and Activation in Trauma Patients. PLOS One. 2012;7(12):e52406. doi: 10.1371/journal.pone.0052406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Femling J, West SD, Hauswald E, Gresham H, Hall P. Nosocomial infections after severe trauma are associated with lower apolipoproteins B and AII. J Trauma Acute Care Surg. 2013;74:1067–1073. doi: 10.1097/TA.0b013e3182826be0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working Group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Edberg JC, Wainstein E, Wu J, Csernok E, Sneller MC, Hoffman GS, Keystone EC, Gross WL, Kimberly RP. Analysis of Fc gamma RII gene polymorphisms in Wegener's granulomatosis. Exp Clin Immunogenet. 1997;14:183–195. [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- 26.Papia G, McLellan BA, El-Helou P, Louie M, Rachlis A, Szalai JP, Simor AE. Infection in hospitalized trauma patients: incidence, risk factors and complications. J Trauma. 1999;47:923–927. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Gouel-Chéron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G for AzuRea Group. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLOS One. 2012;7:e33095. doi: 10.1371/journal.pone.0033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu W, Zeng L, Zhang LY, Jiang DP, Du DY, Hu P, Wang HY, Liu Q, Huang SN, Jiang JX. Association of interleukin 4 -589T/C polymorphism with T(H)1 and T(H)2 bias and sepsis in Chinese major trauma patients. J Trauma. 2011;71:1583–1587. doi: 10.1097/TA.0b013e3182115034. [DOI] [PubMed] [Google Scholar]
- 29.Croce MA, Brasel K, Coimbra R, Adams CA, Jr, Miller PR, Pasquale MD, McDonald CS, Vuthipadadon S, Fabian TC, Tolley EA. National Trauma Institute prospective evaluation of the ventilator bundle in trauma patients: does it really work? J Trauma Acute Care Surg. 2013;74:354–360. doi: 10.1097/TA.0b013e31827a0c65. [DOI] [PubMed] [Google Scholar]
- 30.Torrance HD, Brohi K, Pearse RM, Mein CA, Wozniak E, Prowle JR, Hinds CJ, O'dwyer MJ. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Ann Surg. 2014;00:1–9. doi: 10.1097/SLA.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 31.Piatkowski A, Grieb G, Das R, Bokkurt A, Ulrich D, Pallua N. Soluble CD163: A novel biomarker for the susceptibility to sepsis in severe burn injuries. Indian Journal of Plastic Surgery. 2011;44:118–124. doi: 10.4103/0970-0358.81454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmon JE, Edberg JC, Brogle NL, Kimberly RP. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. Journal of Clinical Investigation. 1992;89:1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hranjec T, Swenson BR, Dossett LA, Metzger R, Flohr TR, Popovsky KA, Bonatti HJ, May AK, Sawyer RG. Diagnosis-dependent relationships between cytokine levels and survival in patients admitted for surgical critical care. J Am Coll Surg. 2010;210:833–846. doi: 10.1016/j.jamcollsurg.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets-an updated view. Mediators Inflamm. 2013 doi: 10.1155/2013/165974. 165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.