Synopsis
Interventional MR uses rapid imaging to guide diagnostic and therapeutic procedures. One of the attractions of MR-guidance is the abundance of inherent contrast mechanisms available. Dynamic procedural guidance with real-time imaging has pushed the limits of MR technology, demanding rapid acquisition and reconstruction paired with interactive control and device visualization. This article reviews the technical aspects of real-time MR sequences that enable MR-guided interventions.
Keywords: Real-time MR imaging, MR image reconstruction, parallel imaging, non-Cartesian imaging
Introduction
Interventional MR imaging is valuable for real-time dynamic procedural guidance and intra-procedural imaging during diagnostic or therapeutic procedures, including surgery, tissue biopsy, ablation therapy, endovascular procedures, and device placement. The flexibility of MR image contrast is appealing for procedural guidance; however, the demands of MR-guided interventions are unique and require a specialized environment.
Diagnostic MR imaging is well established in the clinic to provide high-resolution images with excellent soft tissue contrast, designed to assess pathological tissue and derive quantitative metrics. Typically, diagnostic MR imaging uses long scan times to generate the desired image contrast, and may require offline image reconstruction or processing.
Interventional MR imaging, on the other hand, demands much faster image acquisition, reconstruction, and processing. Furthermore, procedural guidance employs interactive parameter control and requires the simultaneous visualization of tissue and interventional devices (e.g., biopsy needles, guidewires, catheters, stents, occluders, forceps). Table 1 summarizes the differing demands of diagnostic and interventional MR imaging.
Table 1.
Differences between diagnostic and interventional MRI
| Diagnostic MRI | Interventional MRI |
|---|---|
| Scans run in “batch mode” | Interactive environments are used to modify real-time imaging on-the-fly |
| Long scan times | Short scan times |
| Offline reconstruction and post processing is possible |
Reconstruction and processing must be performed with low latency |
| Image quality is of utmost importance | Imaging speed is of utmost importance |
| Used for anatomical imaging | Used for anatomical imaging and device imaging |
| Cardiac and respiratory gating and/or breath holding can be used to compensate for motion |
Real-time MRI is not gated or breath-held |
IMAGING PROTOCOLS
Interventional MR imaging encompasses pre-procedural imaging for planning, intra-procedural imaging to assess progress, and real-time imaging for dynamic procedural guidance. The preprocedural and intraprocedural imaging is used to assess anatomy, physiology, or pathology relevant to the procedure. Here, we focus primarily on the technical details of the fast real-time imaging used during dynamic procedural guidance.
Real-time imaging
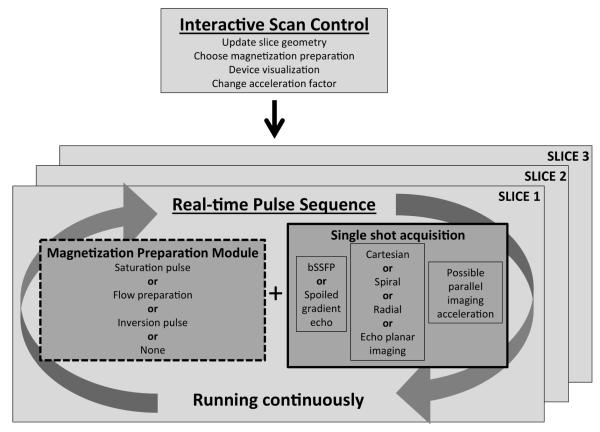
Imaging efficiency is crucial during MR-guided interventions, especially when competing against established interventional modalities such as radiography and ultrasonography. Radiography generates approximately 15 frames/s with a pixel matrix of 1024 × 1024. In comparison, 5-10 frames/s are used for MR imaging during procedural guidance with a much smaller pixel matrix of 128 × 128 or 144 × 192. Real time imaging is not gated nor breath held, and the entire image is acquired in a single shot. Figure 1 shows the single shot real-time image acquisition running continuously, with multiple slices updating in rapid succession. Throughout an interventional procedure, slice geometry and image contrast are interactively controlled, either by interventionists in the MR suite or by operators in the control room.
Figure 1.
Diagram of real-time acquisition running continuously with multiple slices updating in rapid succession. Image contrast is changed using an optional magnetization preparation module and interactive parameter control. bSSFP, balanced steady-state free precession.
For real-time procedural guidance, the challenge is to generate adequate tissue contrast and sufficient image quality in terms of signal-to-noise ratio (SNR) and artifact, while also maintaining short imaging times. Typically, balanced steady state free precession imaging (bSSFP) is used to accomplish this1,2. bSSFP uses magnetization efficiently to provide high SNR with short repetitions times and the resulting images have T2/T1 weighted contrast with bright blood and fat signal and darker muscle tissue. Fully sampled bSSFP images can achieve a temporal resolution of 377 ms/image or 2.6 frames/s (echo time [TE]/repetition time [TR] = 1.27/2.62 ms, matrix = 192 × 144). Using parallel imaging, the temporal resolution can be pushed to 94 ms/image or 10.6 frames/s (acceleration factor 4, see “Parallel Imaging” section below). Banding artifacts in bSSFP are not usually a concern for these short TR sequences; however, real-time bSSFP does suffer from undesirable bright signal from fat. If needed, fat suppression for bSSFP can be accomplished using radiofrequency (RF) cycling and TR alternation3-5. Real-time imaging can also be achieved using spoiled gradient echo sequences6, though used less frequently due to the lower SNR.
A magnetization preparation module can be programed into the pulse sequence such that it is toggled on/off interactively while the single shot acquisition runs continuously. For example, non-selective saturation pre-pulses can be added to the sequence to enhance gadolinium contrast while suppressing tissue signal (see “Device visualization” section below). Furthermore, flow-sensitive saturation preparations can produce dark-blood images to enhance the gadolinium contrast and preserve tissue signal7. Inversion pulses can be inserted into the real-time pulse sequence for infarct imaging8. Interactive color flow MRI using phase contrast has been implemented to rapidly visualize cardiac and vascular flow9. Virtual dye angiography uses volume selective saturation pulses that can be turned on/off interactively to provide flow visualization mimicking contrast angiography during endovascular procedures10 (Figure 2). These interactive magnetization preparation modules modify image contrast, as needed, throughout the procedure.
Figure 2.
Virtual dye angiography uses volume selective-saturation pulses to saturated blood signal locally. The difference images between saturation off/on (black and white images) are used to produce a color-flow map. Ao, aorta; LA, left atrium; LV, left ventricle. (From George AK, Faranesh AZ, Ratnayaka K, et al. Virtual dye angiography: flow visualization for MRI-guided interventions. Magn Reson Med 2012;67(4):1013–21; with permission.)
Parallel imaging
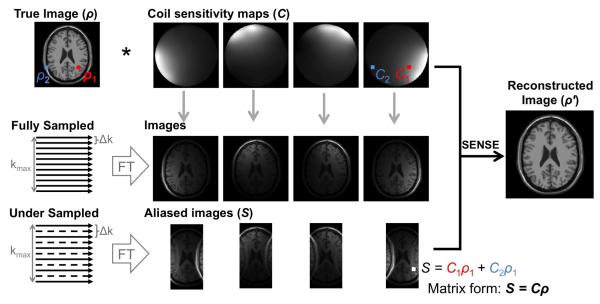
Parallel imaging can be used to accelerate real-time imaging by skipping some phase encoding lines throughout the acquisition and exploiting multi-channel signal receiver arrays during reconstruction for added spatial encoding. By eliminating some phase encoding steps, the resulting images are aliased. Parallel imaging describes a family of reconstruction techniques that allows us to recover images from the aliased ones. The two most common methods used in the clinic are SENSE11 and GRAPPA12. Excellent reviews of parallel imaging techniques are available13,14.
Each point in an aliased single-coil image is a linear signal superposition with weights according to the coil sensitivity profile (Figure 3). SENSE reconstruction unambiguously unfolds aliased images by solving a linear system using knowledge of coil sensitivity maps calibrated at the beginning of the examination. TSENSE15 is an alternative algorithm which uses interleaved undersampling to integrate the coil sensitivity maps with the image acquisition. TSENSE is specifically designed for dynamic imaging and is critical for high frame rate imaging.
Figure 3.
Illustration of SENSE reconstruction using undersampled aliased images and coil sensitivity maps. (Data from Collins DL, Zijdenbos AP, Kollokian V, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging 1998;17(3):463–8; and BrainWeb: Simulated Brain Database. Available at: http://brainweb.bic.mni.mcgill.ca/brainweb/.)
GRAPPA aims to regenerate the missing phase encoding lines from the raw k-space data using information about a given data point contained within the neighboring points in k-space. GRAPPA requires a fully sampled region of k-space known as the autocalibration signal that is used to calculate a kernel to regenerate all missing k-space points. GRAPPA is robust in cases in which sensitivity maps are difficult to generate, e.g., when the prescribed field of view is too small for the imaged object and there are regions with aliasing in the calibration data.
In general, acceleration rates of four are robustly used in a clinical setting, and both SENSE and GRAPPA reconstructions are available with vendor-supplied reconstruction software.
Compressed sensing algorithms show potential for further accelerating image acquisition16, but are currently limited in their application to interventional MR imaging by prohibitively long reconstruction times.
Efficient k-space trajectories
More efficient k-space trajectories can also be used to speed up acquisitions (Figure 4). Echo planar imaging (EPI) is an accelerated Cartesian acquisition whereby multiple phase encoding steps are acquired following an RF pulse. Using single shot EPI, the entire image can be acquired following a single RF pulse. EPI has found clinical utility for interventional and real-time applications17-20.
Figure 4.

Spin warp Cartesian imaging (a) compared to more efficient k-space trajectories: echo planar imaging (b), spiral (c), and radial k-space trajectories (d).
Spiral imaging21 and radial imaging22 are examples of non-Cartesian acquisitions used for MR-guided interventions23-26. Spiral imaging is particularly attractive for high frame rate applications in the interventional MR imaging environment. Oversampling of the k-space center in non-Cartesian sampling patterns results in flow and motion insensitivity and robustness to aliasing artifacts. Either spoiled gradient echo or bSSFP contrast can be achieved with spiral and radial trajectories.
Spiral and radial k-space trajectories do not lie on a Cartesian grid and therefore, require samples to be interpolated onto a grid during image reconstruction in a process called “regridding”27. Typically, regridding uses a Kaiser-Bessel kernel28 or, in the case of nonuniform fast Fourier transformation29, using least-squares design of interpolation coefficients. Since sampling density is nonuniform over k-space, density compensation is applied before regridding30. Alternatively, radial acquisitions can be reconstructed using back projection methods originally designed for computed tomography reconstruction.
Parallel imaging can be combined with non-Cartesian acquisitions. Undersampling is achieved by omitting spiral interleaves or radial projections from the acquisition. For non-Cartesian trajectories, the resulting aliasing is irregular and classic SENSE unfolding is impossible. Instead, an iterative method, namely, conjugate gradient SENSE31,32, is utilized to solve the linear system. GRAPPA reconstruction can also be performed with undersampled non-Cartesian data sets33-37; however, non-Cartesian GRAPPA schemes are currently incompatible with the interventional environment because of the large number of fully sampled calibration frames required. Approaches to reduce the number of calibration scans are under development38. Readers are directed to reviews on non-Cartesian imaging for further details39,40.
Keyhole imaging
Keyhole imaging increases apparent frame rate by reconstructing consecutive images combining newly acquired data with data acquired from previous frames23,41,42. Non-Cartesian sampling patterns are better suited to keyhole reconstruction because the center of k-space is reacquired with each interleaf/projection and a full range of spatial frequencies is contained in both new and old data.
Fast reconstruction
For interventional applications, fast acquisition is only valuable if it is paired with fast reconstruction. MR system vendors provide the capability to reconstruct Cartesian images with standard parallel imaging in real-time. However, for more complicated reconstructions (e.g., non-Cartesian imaging, complex parallel imaging schemes, or iterative reconstructions), additional reconstruction tools are necessary.
Imaging with large coil arrays is computationally costly. A number of algorithms have been proposed for coil selection43-45 to choose the most suitable subset of coils for reconstruction and array compression46-49 to combine channels for reducing reconstruction time. Most notably, principle component analysis has been used for array compression without the need for coil sensitivity maps47.
Graphics processing unit (GPU) accelerated computing has been used for significant improvements in reconstruction speed, has been applied to advanced 3D reconstructions50, parallel imaging51,52, and nonuniform FFT53. An 85-fold acceleration compared with a state-of-the-art 64 bit central processing unit53 has been reported. GPU-accelerated computing requires additional hardware that may not be available on the vendor-supplied reconstruction system.
An open source software package (Gadgetron, http://gadgetron.github.io54) for medical image reconstruction has recently been made available. The software contains standard reconstruction tools, iterative solvers, and GPU components. It is designed to run on either the local MR computer, an external workstation, or on multiple nodes in a distributed computing environment55. Images may be piped directly to the host computer for online display. This framework permits complicated reconstructions in a reasonable time frame with image display on the MR imaging host computer.
Device Visualization
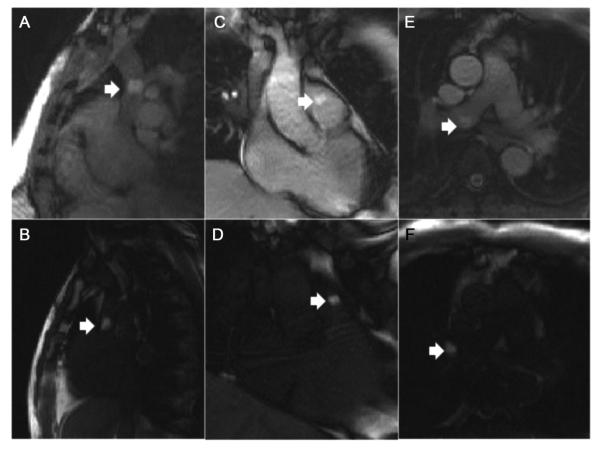
Real-time device visualization is also necessary during MR imaging-guided. Passive visualization uses the intrinsic material properties. For example, metallic devices (e.g., biopsy needles, guidewires, stents, occluders) create a signal void on real-time MR images because they distort the local magnetic field, leading to local signal dephasing56. Signal voids can be emphasized using long TE gradient echo sequences. Alternatively, positive contrast techniques in which the metallic device appears bright compared to the background signal can be used for real-time visualization57,58. Non-metallic devices, such as plastic catheters, can be made visible on real-time imaging by filling the lumen with gadolinium59,60 although it restricts use of the lumen to deliver other devices or agents. Air, gadolinium, or carbon dioxide filled balloon catheters have been used to guide right heart catheterization under MR-guidance61,62. Saturation pre-pulse modules are used to enhance gadolinium contrast of catheter devices (Figure 5).
Figure 5.
Right heart catheterization using real-time MR imaging to navigate a gadolinium-filled balloon (arrows) between cardiac chambers. Standard real-time bSSFP (A, C, E) and real-time bSSFP with saturation prepulses (B, D, F) are depicted. Saturation prepulses are used to isolate the gadolinium-filled balloon signal. (From Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J 2013;34(5):380–9; with permission.)
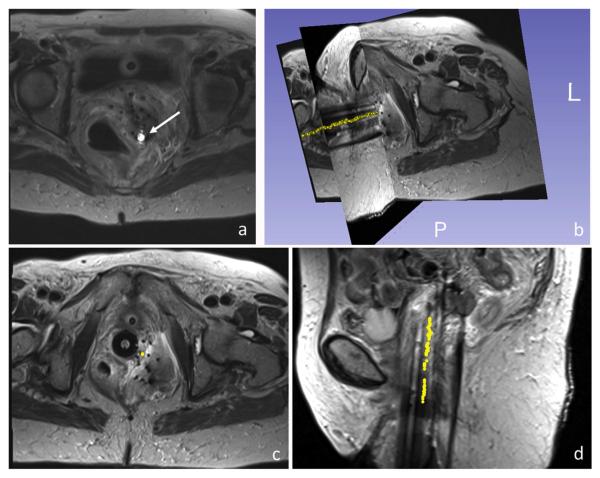
Active visualization involves the embedding of receiver electronics into the device such that a unique device signal can be collected separately from the other receiver coils. This unique signature can be overlaid in color onto anatomical images in real-time63. Alternatively, device tracking uses fast device localization and graphically represents the device position on previously acquired images64 (Figure 6). Point source coils can be localized rapidly using three orthogonal echoes65 whereas spatially extensive coils can be localized using three orthogonal projection images (non-slice-selective). The unique signature of active devices can also be exploited for automatic repositioning of the imaging slice when the tip moves out of plane66,67. Accurate localization of devices is essential for effective procedural guidance.
Figure 6.
(A–D) Active microcoil (arrow) tracking of brachytherapy stylet. The trajectory (yellow points) represents consecutive tracking positions overlaid on previously acquired images. (From Wang W, Dumoulin CL, Viswanathan AN, et al. Real-time active MR-tracking of metallic stylets in MR-guided radiation therapy. Magn Reson Med 2015;73(5):1810; with permission.)
Interactive Environments
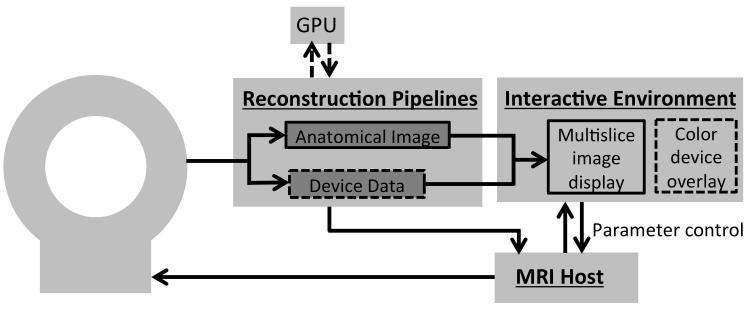
Interactive environments permit graphical control for real-time imaging, allowing flexibility to modify imaging parameters according to the needs of the procedure without stopping to change the pulse sequences or imaging protocol.68 The entire infrastructure of the MR imaging system, reconstruction pipeline, and interactive control is outlined in Figure 7. Features found in interactive environments are:
Graphical slice positioning
Multi-slice display
Real-time modification of scan parameters (eg, slice thickness, acceleration factor, magnetization preparation modules)
Roadmap visualization in combination with real-time imaging or device tracking
Color visualization of device channels and device tracking
Figure 7.
Overview of the imaging infrastructure that could be used in the interventional MR imaging environment. The device data can be isolated during reconstruction to permit the color overlay of the device signal in the interactive environment. Dashed lines represent optional components. GPU, graphics processing unit.
Video 1 demonstrates the modification of imaging parameters in an interactive environment. Interactive imaging environments are available from each of the major MR system vendors: Siemens Medical Solutions offers the Interactive Front End (Siemens Corporate Research, Princeton, NJ) for investigational use; General Electric Medical Systems (Waukesha, WI) offers MR Echo; and Philips Healthcare (Best, Netherlands) offers eXTernal Control (XTC)69. In addition, third party software can provide interactive imaging and visualization. RT Hawk (HeartVista, Menlo Park, CA)70 runs from a “stub” pulse sequence and offers dynamic switching between pulse sequences and reconstruction algorithms. Vurtigo (Sunnybrook Health Sciences Centre, Toronto, ON, Canada)71,72 is open source software for visualization of interventional procedures.
Furthermore, automatic device-scanner interaction has been demonstrated, where temporal resolution and field of view are automatically increased when fast device motion is detected73. Automatic slice positioning for device visualization can also be incorporated in the interactive environments66,67.
PEARLS, PITFALLS AND VARIANTS
Artifacts
Pushing the limits of temporal resolution can result in unwelcome artifacts in images. Parallel imaging acceleration comes at the expense of a loss in image SNR:
where R is the acceleration factor and g (g ≥ 1) is the coil geometry factor (or “g-factor”) related to the properties of the coil receiver array. The coil geometry in relation to the slice position is constantly changing throughout an MR-guided intervention; therefore, it is useful to interactively modify acceleration factor to tradeoff image quality and imaging speed for a given slice geometry.
EPI, spiral imaging, and radial imaging methods are also susceptible to image artifacts. Specifically, ghosting artifacts are common in EPI images caused by system imperfection which result in the misalignment of odd and even echoes during the bipolar readout. Standard reference lines are used to correct this by default, but users should be aware of the potential for residual ghosting. EPI is also susceptible to distortions caused by magnetic field inhomogeneity.
Off-resonance manifests as blurring in non-Cartesian images that can be eliminated in a composite image from multi-frequency reconstruction74. Non-Cartesian k-space trajectories are also sensitive to distortions caused by errors in gradient waveforms. A number of methods have been developed to retrospectively measure the true spiral k-space trajectories for distortion correction during image reconstruction75,76. Similarly, gradient delays can be measured and compensated for within the pulse sequence for correction of radial images77. Unfortunately, these additional measurements are impractical in the interventional MR imaging setting. Recently, the gradient system impulse response function (GIRF) has been demonstrated for predicting true gradient waveforms from the nominal waveforms prescribed in the pulse sequence78, and a real-time framework using GIRF trajectory correction and interactive off-resonance reconstruction for de-blurring has been applied for distortion correction of spiral images79.
RF-induced heating
Device safety can also be a concern in the interventional MR imaging environment. Energy deposited by the RF pulses used for imaging can cause heating in conductive devices that may lead to tissue damage. Eddy currents in conductive devices will create slight heating80. Resonating RF waves along long metallic guidewires can generate dangerous levels of heating (up to 70°C)81-84. RF induced heating is inversely proportional to TR and is proportional to the square of the flip angle. bSSFP imaging uses flip angles of 40°- 60° and short TRs. Gradient echo (low flip angle) and non-Cartesian (long TR) imaging methods use lower RF energy. Patient safety must be considered when using metallic devices in the MR imaging environment.
POINTS FOR THE REFERRING PHYSICIAN
Interventional MR imaging is different from diagnostic MR imaging and the successful implementation of MRI-guided interventions requires a highly specialized environment and trained staff beyond that requisite for a standard diagnostic MR imaging85. Features found in the interventional MRI environment are:
A real-time interactive scanning environment
Devices that are visible and safe with MR imaging
In-room image display used during dynamic procedural guidance
Audio communication between interventionists and scanner control room
Physiology and hemodynamic monitoring (higher quality than that used for diagnostic MR imaging) to monitor the patient throughout the procedure.
An emergency patient bailout strategy (usually to an adjoining suite using an intermodality transfer table).
SUMMARY
A wide variety of different pulse sequences may be used during MR-guided interventions. The choice among these is dictated by the pathology and type of procedure. One common requirement for all MR-guided interventions is that both imaging and reconstruction must be fast. The capability now exists to perform very fast imaging with interactive control, permitting real-time procedural guidance with MRI. With the technology in place, many more clinical applications of this promising tool can be expected in the future.
Supplementary Material
Video 1: Real-time imaging in an interactive environment (Interactive Front End, Siemens Corporate Research, Princeton, NJ) with reconstruction using the Gadgetron. Interactive modification of slice orientation, parallel imaging acceleration, slice thickness and flow-sensitive saturation pre-pulse is demonstrated in a swine.
Key Points.
Interventional MR imaging requires a specialized environment and work-flow.
Rapid image acquisition and rapid image reconstruction are a prerequisite for all MR-guided interventions.
High frame-rate real-time imaging can be achieved for dynamic procedural guidance.
Imaging can be accelerated using parallel imaging or efficient k-space trajectories.
MR imaging can enable simultaneous device and tissue visualization.
Acknowledgments
The authors disclose that this work was supported by the National Heart, Lung, and Blood Institute Division of Intramural Research (Z01-HL006039, Z01-HL005062). The National Heart, Lung, and Blood Institute and Siemens Medical Systems have a Cooperative Research and Development Agreement (CRADA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adrienne E Campbell-Washburn, Division of Intramural Research, Cardiovascular and Pulmonary Branch National Heart Lung and Blood Institute, National Institutes of Health Address: National Institutes of Health, 9000 Rockville Pike, Building 10 Room B1D416 Bethesda MD 20892, USA Tel: 301-402-1032
Anthony Z Faranesh, Division of Intramural Research, Cardiovascular and Pulmonary Branch National Heart Lung and Blood Institute, National Institutes of Health Address: National Institutes of Health, 9000 Rockville Pike, Building 10 Room 2C713, Bethesda MD 20892, USA Tel: 301-451-4928 faranesa@nhlbi.nih.gov
Robert J Lederman, Cardiovascular and Pulmonary Branch, Division of Intramural Research National Heart Lung and Blood Institute, National Institutes of Health Address: National Institutes of Health, 9000 Rockville Pike, Building 10 Room 2C713, Bethesda MD 20892, USA Tel: 301-402-6769 lederman@nih.gov
Michael S Hansen, Division of Intramural Research, Cardiovascular and Pulmonary Branch National Heart Lung and Blood Institute, National Institutes of Health Address: National Institutes of Health, 9000 Rockville Pike, Building 10 Room B1D416, Bethesda MD 20892, USA Tel: 301-496-1457 michael.hansen@nih.gov
REFERENCES
- 1.Duerk JL, Lewin JS, Wendt M, Petersilge C. Remember true FISP? A high SNR, near 1-second imaging method for T2-like contrast in interventional MRI at .2 T. J Magn Reson Imaging. 1998;8(1):203–208. doi: 10.1002/jmri.1880080134. [DOI] [PubMed] [Google Scholar]
- 2.Yutzy SR, Duerk JL. Pulse sequences and system interfaces for interventional and real-time MRI. J Magn Reson Imaging. 2008;27(2):267–275. doi: 10.1002/jmri.21268. [DOI] [PubMed] [Google Scholar]
- 3.Leupold J, Hennig J, Scheffler K. Alternating repetition time balanced steady state free precession. Magn Reson Med. 2006;55(3):557–565. doi: 10.1002/mrm.20790. [DOI] [PubMed] [Google Scholar]
- 4.Cukur T, Nishimura DG. Fat-water separation with alternating repetition time balanced SSFP. Magn Reson Med. 2008;60(2):479–484. doi: 10.1002/mrm.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derbyshire JA, Herzka DA, McVeigh ER. S5FP: spectrally selective suppression with steady state free precession. Magn Reson Med. 2005;54(4):918–928. doi: 10.1002/mrm.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rempp H, Loh H, Hoffmann R, et al. Liver lesion conspicuity during real-time MR-guided radiofrequency applicator placement using spoiled gradient echo and balanced steady-state free precession imaging. J Magn Reson Imaging. 2014;40(2):432–439. doi: 10.1002/jmri.24371. [DOI] [PubMed] [Google Scholar]
- 7.Faranesh AZ, Hansen MS, Rogers T, Lederman RJ. Interactive black blood preparation for interventional cardiovascular MRI. Journal of Cardiovascular Magnetic Resonance. 2014;16(Suppl 1):P32. [Google Scholar]
- 8.Guttman MA, Dick AJ, Raman VK, Arai AE, Lederman RJ, McVeigh ER. Imaging of myocardial infarction for diagnosis and intervention using real-time interactive MRI without ECG-gating or breath-holding. Magn Reson Med. 2004;52(2):354–361. doi: 10.1002/mrm.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak KS, Pauly JM, Kerr AB, Hu BS, Nishimura DG. Real-time color flow MRI. Magn Reson Med. 2000;43(2):251–258. doi: 10.1002/(sici)1522-2594(200002)43:2<251::aid-mrm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.George AK, Faranesh AZ, Ratnayaka K, Derbyshire JA, Lederman RJ, Hansen MS. Virtual dye angiography: flow visualization for MRI-guided interventions. Magn Reson Med. 2012;67(4):1013–1021. doi: 10.1002/mrm.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 12.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 13.Larkman DJ, Nunes RG. Parallel magnetic resonance imaging. Phys Med Biol. 2007;52(7):R15–55. doi: 10.1088/0031-9155/52/7/R01. [DOI] [PubMed] [Google Scholar]
- 14.Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging. 2012;36(1):55–72. doi: 10.1002/jmri.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45(5):846–852. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 16.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 17.Golby AJ, Kindlmann G, Norton I, Yarmarkovich A, Pieper S, Kikinis R. Interactive diffusion tensor tractography visualization for neurosurgical planning. Neurosurgery. 2011;68(2):496–505. doi: 10.1227/NEU.0b013e3182061ebb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimsky C, Ganslandt O, Hastreiter P, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130–137. doi: 10.1227/01.neu.0000144842.18771.30. discussion 138. [DOI] [PubMed] [Google Scholar]
- 19.Kim YC, Nielsen JF, Nayak KS. Automatic correction of echo-planar imaging (EPI) ghosting artifacts in real-time interactive cardiac MRI using sensitivity encoding. J Magn Reson Imaging. 2008;27(1):239–245. doi: 10.1002/jmri.21214. [DOI] [PubMed] [Google Scholar]
- 20.Dragonu I, de Senneville BD, Quesson B, Moonen C, Ries M. Real-time geometric distortion correction for interventional imaging with echo-planar imaging (EPI) Magn Reson Med. 2009;61(4):994–1000. doi: 10.1002/mrm.21903. [DOI] [PubMed] [Google Scholar]
- 21.Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28(2):202–213. doi: 10.1002/mrm.1910280204. [DOI] [PubMed] [Google Scholar]
- 22.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med. 1992;28(2):275–289. doi: 10.1002/mrm.1910280209. [DOI] [PubMed] [Google Scholar]
- 23.Terashima M, Hyon M, de la Pena-Almaguer E, et al. High-resolution real-time spiral MRI for guiding vascular interventions in a rabbit model at 1.5 T. J Magn Reson Imaging. 2005;22(5):687–690. doi: 10.1002/jmri.20409. [DOI] [PubMed] [Google Scholar]
- 24.Rasche V, Holz D, Köhler J, Proksa R, Röschmann P. Catheter tracking using continuous radial MRI. Magn Reson Med. 1997;37(6):963–968. doi: 10.1002/mrm.1910370623. [DOI] [PubMed] [Google Scholar]
- 25.Peters DC, Lederman RJ, Dick AJ, et al. Undersampled projection reconstruction for active catheter imaging with adaptable temporal resolution and catheter-only views. Magn Reson Med. 2003;49(2):216–222. doi: 10.1002/mrm.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters DC, Guttman MA, Dick AJ, Raman VK, Lederman RJ, McVeigh ER. Reduced field of view and undersampled PR combined for interventional imaging of a fully dynamic field of view. Magn Reson Med. 2004;51(4):761–767. doi: 10.1002/mrm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan JD. A fast sinc function gridding algorithm for fourier inversion in computer tomography. IEEE Trans Med Imaging. 1985;4(4):200–207. doi: 10.1109/TMI.1985.4307723. [DOI] [PubMed] [Google Scholar]
- 28.Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application] IEEE Trans Med Imaging. 1991;10(3):473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 29.Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. J Magn Reson. 2007;188(2):191–195. doi: 10.1016/j.jmr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoge RD, Kwan RK, Pike GB. Density compensation functions for spiral MRI. Magn Reson Med. 1997;38(1):117–128. doi: 10.1002/mrm.1910380117. [DOI] [PubMed] [Google Scholar]
- 31.Hestenes MR, Stiefel E. Methods of conjugate gradients for solving linear systems. Journal of Research of the National Bureau of Standards. 1952;49(6):409–436. [Google Scholar]
- 32.Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 33.Seiberlich N, Breuer F, Blaimer M, Jakob P, Griswold M. Self-calibrating GRAPPA operator gridding for radial and spiral trajectories. Magn Reson Med. 2008;59(4):930–935. doi: 10.1002/mrm.21565. [DOI] [PubMed] [Google Scholar]
- 34.Seiberlich N, Ehses P, Duerk J, Gilkeson R, Griswold M. Improved radial GRAPPA calibration for real-time free-breathing cardiac imaging. Magn Reson Med. 2011;65(2):492–505. doi: 10.1002/mrm.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seiberlich N, Lee G, Ehses P, Duerk JL, Gilkeson R, Griswold M. Improved temporal resolution in cardiac imaging using through-time spiral GRAPPA. Magn Reson Med. 2011;66(6):1682–1688. doi: 10.1002/mrm.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griswold MA, Heidemann RM, Jakob PM. Direct parallel imaging reconstruction of radially sampled data using GRAPPA with relative shifts. Proceedings of the 11th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Toronto. July 10–16, 2003.p. 2349. [Google Scholar]
- 37.Heidemann RM, Griswold MA, Seiberlich N, et al. Direct parallel image reconstructions for spiral trajectories using GRAPPA. Magn Reson Med. 2006;56(2):317–326. doi: 10.1002/mrm.20951. [DOI] [PubMed] [Google Scholar]
- 38.Seiberlich N, Griswold MA. Self-calibrating through-time spiral GRAPPA for real-time CMR. Journal of Cardiovascular Magnetic Resonance. 2013;15(Supp 1):E28. [Google Scholar]
- 39.Delattre BM, Heidemann RM, Crowe LA, Vallée JP, Hyacinthe JN. Spiral demystified. Magn Reson Imaging. 2010;28(6):862–881. doi: 10.1016/j.mri.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-Cartesian parallel imaging reconstruction. J Magn Reson Imaging. 2014;40(5):1022–1040. doi: 10.1002/jmri.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaranarayanan A, Wendt M, Aschoff AJ, Lewin JS, Duerk JL. Radial keyhole sequences for low field projection reconstruction interventional MRI. J Magn Reson Imaging. 2001;13(1):142–151. doi: 10.1002/1522-2586(200101)13:1<142::aid-jmri1022>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 42.Duerk JL, Lewin JS, Wu DH. Application of keyhole imaging to interventional MRI: a simulation study to predict sequence requirements. J Magn Reson Imaging. 1996;6(6):918–924. doi: 10.1002/jmri.1880060613. [DOI] [PubMed] [Google Scholar]
- 43.Müller S, Umathum R, Speier P, et al. Dynamic coil selection for real-time imaging in interventional MRI. Magn Reson Med. 2006;56(5):1156–1162. doi: 10.1002/mrm.21028. [DOI] [PubMed] [Google Scholar]
- 44.Feng S, Zhu Y, Ji J. Efficient large-array k-domain parallel MRI using channel-by-channel array reduction. Magn Reson Imaging. 2011;29(2):209–215. doi: 10.1016/j.mri.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Doneva M, Börnert P. Automatic coil selection for channel reduction in SENSE-based parallel imaging. MAGMA. 2008;21(3):187–196. doi: 10.1007/s10334-008-0110-x. [DOI] [PubMed] [Google Scholar]
- 46.Huang F, Lin W, Duensing GR, Reykowski A. A hybrid method for more efficient channel-by-channel reconstruction with many channels. Magn Reson Med. 2012;67(3):835–843. doi: 10.1002/mrm.23048. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Vijayakumar S, Li Y, Hertel S, Duensing GR. A software channel compression technique for faster reconstruction with many channels. Magn Reson Imaging. 2008;26(1):133–141. doi: 10.1016/j.mri.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. Magn Reson Med. 2007;57(6):1131–1139. doi: 10.1002/mrm.21237. [DOI] [PubMed] [Google Scholar]
- 49.Beatty PJ, Chang S, Holmes JH, et al. Design of k-space channel combination kernels and integration with parallel imaging. Magn Reson Med. 2014;71(6):2139–2154. doi: 10.1002/mrm.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone SS, Haldar JP, Tsao SC, Hwu WM, Sutton BP, Liang ZP. Accelerating Advanced MRI Reconstructions on GPUs. J Parallel Distrib Comput. 2008;68(10):1307–1318. doi: 10.1016/j.jpdc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen MS, Atkinson D, Sorensen TS. Cartesian SENSE and k-t SENSE reconstruction using commodity graphics hardware. Magn Reson Med. 2008;59(3):463–468. doi: 10.1002/mrm.21523. [DOI] [PubMed] [Google Scholar]
- 52.Sørensen TS, Atkinson D, Schaeffter T, Hansen MS. Real-time reconstruction of sensitivity encoded radial magnetic resonance imaging using a graphics processing unit. IEEE Trans Med Imaging. 2009;28(12):1974–1985. doi: 10.1109/TMI.2009.2027118. [DOI] [PubMed] [Google Scholar]
- 53.Sorensen TS, Schaeffter T, Noe KO, Hansen MS. Accelerating the nonequispaced fast Fourier transform on commodity graphics hardware. IEEE Trans Med Imaging. 2008;27(4):538–547. doi: 10.1109/TMI.2007.909834. [DOI] [PubMed] [Google Scholar]
- 54.Hansen MS, Sørensen TS. Gadgetron: an open source framework for medical image reconstruction. Magn Reson Med. 2013;69(6):1768–1776. doi: 10.1002/mrm.24389. [DOI] [PubMed] [Google Scholar]
- 55.Xue H, Inati S, Sørensen TS, Kellman P, Hansen MS. Distributed MRI reconstruction using gadgetron-based cloud computing. Magn Reson Med. 2014 doi: 10.1002/mrm.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halabi M, Faranesh AZ, Schenke WH, et al. Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. J Cardiovasc Magn Reson. 2013;15(1):61. doi: 10.1186/1532-429X-15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seppenwoolde JH, Viergever MA, Bakker CJ. Passive tracking exploiting local signal conservation: the white marker phenomenon. Magn Reson Med. 2003;50(4):784–790. doi: 10.1002/mrm.10574. [DOI] [PubMed] [Google Scholar]
- 58.Campbell-Washburn AE, Rogers T, Xue H, Hansen MS, Lederman RJ, Faranesh AZ. Dual echo positive contrast bSSFP for real-time visualization of passive devices duringmagnetic resonance guided cardiovascular catheterization. J Cardiovasc Magn Reson. 2014;16:88. doi: 10.1186/s12968-014-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omary RA, Unal O, Koscielski DS, et al. Real-time MR imaging-guided passive catheter tracking with use of gadolinium-filled catheters. J Vasc Interv Radiol. 2000;11(8):1079–1085. doi: 10.1016/s1051-0443(07)61343-8. [DOI] [PubMed] [Google Scholar]
- 60.Unal O, Korosec FR, Frayne R, Strother CM, Mistretta CA. A rapid 2D time-resolved variable-rate k-space sampling MR technique for passive catheter tracking during endovascular procedures. Magn Reson Med. 1998;40(3):356–362. doi: 10.1002/mrm.1910400304. [DOI] [PubMed] [Google Scholar]
- 61.Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34(5):380–389. doi: 10.1093/eurheartj/ehs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razavi R, Hill DL, Keevil SF, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362(9399):1877–1882. doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- 63.Sonmez M, Saikus CE, Bell JA, et al. MRI active guidewire with an embedded temperature probe and providing a distinct tip signal to enhance clinical safety. J Cardiovasc Magn Reson. 2012;14:38. doi: 10.1186/1532-429X-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, Dumoulin CL, Viswanathan AN, et al. Real-time active MR-tracking of metallic stylets in MR-guided radiation therapy. Magn Reson Med. 2014 doi: 10.1002/mrm.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumoulin CL, Souza SP, Darrow RD. Real-time position monitoring of invasive devices using magnetic resonance. Magn Reson Med. 1993;29(3):411–415. doi: 10.1002/mrm.1910290322. [DOI] [PubMed] [Google Scholar]
- 66.George AK, Derbyshire JA, Saybasili H, et al. Visualization of active devices and automatic slice repositioning (“SnapTo”) for MRI-guided interventions. Magn Reson Med. 2010;63(4):1070–1079. doi: 10.1002/mrm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wacker FK, Elgort D, Hillenbrand CM, Duerk JL, Lewin JS. The catheter-driven MRI scanner: a new approach to intravascular catheter tracking and imaging-parameter adjustment for interventional MRI. AJR Am J Roentgenol. 2004;183(2):391–395. doi: 10.2214/ajr.183.2.1830391. [DOI] [PubMed] [Google Scholar]
- 68.Guttman MA, Ozturk C, Raval AN, et al. Interventional cardiovascular procedures guided by real-time MR imaging: an interactive interface using multiple slices, adaptive projection modes and live 3D renderings. J Magn Reson Imaging. 2007;26(6):1429–1435. doi: 10.1002/jmri.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smink J, Häkkinen M, Holthuizen R, et al. eXTernal Control (XTC): a flexible, real-time, low-latency, bi-directional scanner interface. Proceedings of the 19th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Montreal. May 7–13 2011.p. 1755. [Google Scholar]
- 70.Santos JM, Wright GA, Pauly JM. Flexible real-time magnetic resonance imaging framework. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1048–1051. doi: 10.1109/IEMBS.2004.1403343. [DOI] [PubMed] [Google Scholar]
- 71.Pintilie S, Biswas L, Oduneye SO, Anderson KA, Wright GA, Radau PE. Visualization platform for real-time, MRI-guided cardiac interventions. Proceedings of the 19th Annual Meeting of the International Society for Magnetic Resonance in Medicine.2011. p. 3735. [Google Scholar]
- 72.Radau PE, Pintilie S, Flor R, et al. VURTIGO: Visualization platform for real-time, MRI-guided cardiac electroanatomic mapping. Proceedings of Statistical Atlases and Computational Models of the Heart workshop (The Medical Image Computing and Computer Assisted Intervention Society workshop); Toronto. September 22 2011. [Google Scholar]
- 73.Elgort DR, Wong EY, Hillenbrand CM, Wacker FK, Lewin JS, Duerk JL. Real-time catheter tracking and adaptive imaging. J Magn Reson Imaging. 2003;18(5):621–626. doi: 10.1002/jmri.10402. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Meyer CH. Semiautomatic off-resonance correction in spiral imaging. Magn Reson Med. 2008;59(5):1212–1219. doi: 10.1002/mrm.21599. [DOI] [PubMed] [Google Scholar]
- 75.Duyn JH, Yang Y, Frank JA, van der Veen JW. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson. 1998;132(1):150–153. doi: 10.1006/jmre.1998.1396. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Hetherington HP, Stokely EM, Mason GF, Twieg DB. A novel k-space trajectory measurement technique. Magn Reson Med. 1998;39(6):999–1004. doi: 10.1002/mrm.1910390618. [DOI] [PubMed] [Google Scholar]
- 77.Peters DC, Derbyshire JA, McVeigh ER. Centering the projection reconstruction trajectory: reducing gradient delay errors. Magn Reson Med. 2003;50(1):1–6. doi: 10.1002/mrm.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vannesjo SJ, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn Reson Med. 2013;69(2):583–593. doi: 10.1002/mrm.24263. [DOI] [PubMed] [Google Scholar]
- 79.Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med. 2015 doi: 10.1002/mrm.25788. doi: 10.1002/mrm.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buchli R, Boesiger P, Meier D. Heating effects of metallic implants by MRI examinations. Magn Reson Med. 1988;7(3):255–261. doi: 10.1002/mrm.1910070302. [DOI] [PubMed] [Google Scholar]
- 81.Armenean C, Perrin E, Armenean M, Beuf O, Pilleul F, Saint-Jalmes H. RF-induced temperature elevation along metallic wires in clinical magnetic resonance imaging: influence of diameter and length. Magn Reson Med. 2004;52(5):1200–1206. doi: 10.1002/mrm.20246. [DOI] [PubMed] [Google Scholar]
- 82.Konings MK, Bartels LW, Smits HF, Bakker CJ. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12(1):79–85. doi: 10.1002/1522-2586(200007)12:1<79::aid-jmri9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 83.Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13(1):105–114. doi: 10.1002/1522-2586(200101)13:1<105::aid-jmri1016>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 84.Yeung CJ, Susil RC, Atalar E. RF safety of wires in interventional MRI: using a safety index. Magn Reson Med. 2002;47(1):187–193. doi: 10.1002/mrm.10037. [DOI] [PubMed] [Google Scholar]
- 85.Ratnayaka K, Faranesh AZ, Guttman MA, Kocaturk O, Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Reson. 2008;10:62. doi: 10.1186/1532-429X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Real-time imaging in an interactive environment (Interactive Front End, Siemens Corporate Research, Princeton, NJ) with reconstruction using the Gadgetron. Interactive modification of slice orientation, parallel imaging acceleration, slice thickness and flow-sensitive saturation pre-pulse is demonstrated in a swine.