Abstract
The G-protein coupled protease-activated receptor 2 (PAR2) plays an important role in the pathogenesis of various inflammatory and auto-immune disorders. In airway epithelial cells (AECs), stimulation of PAR2 by allergens and proteases triggers the release of a host of inflammatory mediators to regulate bronchomotor tone and immune cell recruitment. Activation of PAR2 turns on several cell signaling pathways of which the mobilization of cytosolic Ca2+ is likely a critical but poorly understood event. Here, we show that Ca2+ release-activated Ca2+ (CRAC) channels encoded by STIM1 and Orai1 are a major route of Ca2+ entry in primary human AECs and drive the Ca2+ elevations seen in response to PAR2 activation. Activation of CRAC channels induces the production of several key inflammatory mediators from AECs including TSLP, IL-6 and PGE2, in part through stimulation of gene expression via NFAT (nuclear factor of activated T-cells). Furthermore, PAR2 stimulation induces the production of many key inflammatory mediators including PGE2, IL-6, IL-8 and GM-CSF in a CRAC-channel dependent manner. These findings indicate that CRAC channels are the primary mechanism for Ca2+ influx in AECs and a vital checkpoint for the induction of PAR2-induced proinflammatory cytokines.
INTRODUCTION
The epithelial cells of the lung are directly exposed to inhaled air and form the first line of defense against environmental hazards (1–3). In addition to serving as a physical barrier to protect the lung, airway epithelial cells (AECs) play an active role in orchestrating inflammatory effector responses to inhaled substances through the production of a wide array of secreted cytokines and through their interactions with many immune cells (3, 4). Effector responses in AECs are coordinated through a multitude of interactions between extrinsic signaling molecules and intrinsic signal transduction programs activated within the AECs (1, 3). In this signaling repertoire, protease-activated receptor 2 (PAR2) is of particular importance in regulating allergic inflammatory responses that are characteristic of diseases like asthma. PAR2 receptors belong to a family of seven-transmembrane G-protein coupled receptors (GPCRs) that are widely expressed in a variety of cell types and are activated by cleavage of the extracellular N-terminus through the serine protease activity of PAR2 proteolytic agonists. In the airway epithelium, PAR2 receptors are activated by several types of allergens derived from dust mites, cockroach and fungi, all well-known triggers of asthma, and also by endogenous protease molecules such as trypsin and mast-cell tryptase (5–7). PAR2 activation in AECs stimulates the production of several proinflammatory cytokines (IL-6, GM-CSF and TSLP) and chemokines (IL-8 and eotaxin) (8–10). Moreover, asthmatic patients show increased expression of PAR2 receptors in their airway epithelium and PAR2 lacking mice show reduced eosinophilic infiltration and airway hyper-responsiveness (11, 12). These findings underscore the importance of PAR2 proteins in mediating allergic inflammatory responses in the airway.
Despite the well-defined importance of PAR2 receptors in driving inflammatory responses, the signal transduction mechanisms involved in PAR2-mediated effector responses are not well-understood. PAR2 activation stimulates a multi-component signal transduction cascade within which the mobilization of Ca2+ by phospholipase-C (PLCβ) activation and subsequent IP3-mediated release of Ca2+ from endoplasmic reticulum (ER) Ca2+ stores is a key signaling process (13, 14). As a multifunctional second messenger, Ca2+ activates distinct genetic programs and enzymatic cascades to regulate many processes in the immune system including lymphocyte activation, mast-cell degranulation and neutrophil mediated bacterial killing (15–18). There is growing interest in the role of cellular Ca2+ as a key second messenger regulating effector responses in the airway (19–21). Yet the functional architecture of the Ca2+ signaling network: the molecular entities and their organization, and how this machinery regulates Ca2+ signaling and PAR2 evoked effector responses remains poorly understood in airway epithelial cells.
In many non-excitable cells, mobilization of cellular Ca2+ signaling occurs through the opening of store-operated Ca2+ release-activated Ca2+ (CRAC) channels (17, 18). These highly Ca2+ selective ion channels are encoded by the Orai genes (Orai1–3) and activated through direct physical interactions with the ER Ca2+ sensors, STIM1 and STIM2 (22). Mechanistically, it is now known that STIM1 and STIM2 sense the [Ca2+]ER, and, in response to ER Ca2+ store-depletion, translocate to the junctional ER to interact with Orai channels (22, 23). In immune cells, previous studies have established that CRAC channels encoded by STIM1/Orai1 proteins play a central role in driving Ca2+ signaling that controls the function of T-cell, mast cells, B-cells and neutrophils (15–18). However, the role of CRAC channels in regulating immune functions of the airway epithelium, and in particular, their contributions to the production and release of inflammatory mediators, are unknown.
In this study, we show that primary human AECs show robust store-operated Ca2+ entry (SOCE) mediated by the CRAC channel proteins STIM1 and Orai1. Activation of CRAC channels stimulates several critical effector functions in AECs including gene transcription and production of a range of inflammatory modulators. We further find that stimulation of PAR2 receptors produces intracellular Ca2+ elevations that are critically dependent on CRAC channel activity. In turn, CRAC channel-mediated Ca2+ signals induce the production of several pro-inflammatory mediators IL-8, IL-6, GM-CSF and PGE2 in part through stimulation of gene transcription via the NFAT-calcineurin signaling axis. These results demonstrate that CRAC channels are a major route of Ca2+ entry in AECs and serve as a key check-point for PAR2 mediated generation of inflammatory mediators.
MATERIALS AND METHODS
Cells and media
NHBE (Normal human bronchial epithelial) cells were purchased from Lonza, Walkersville, MD and grown in bronchial epithelial cell growth medium (BEGM) containing various growth factors. Cells from passages 2–3 were used for experiments. BEAS-2B, a bronchial epithelial cell-line was a kind gift from Curtis Harris, National Cancer Institute and were cultured in DMEM/F12 medium containing 5% FBS (cell passages 44–49 were used for experiments). A549, an alveolar epithelial cell line was a kind gift of Dr. Jacob Sznajder, Northwestern University (cell passages 12–18 were used for experiments). 1Haeo- cells, a bronchial epithelial cell-line was a kind gift from Dr. Alice Prince, Columbia University. Both A549 and 1Haeo- cells were cultured in DMEM containing 10% FBS. All the cells were maintained at 37°C and in 5% CO2.
Plasmids and transfections
The plasmids and RNAi constructs used in this study were transfected into primary cells and cell-lines using Lipofectamine2000 (Invitrogen) according to manufacturer instructions. E106A Orai1-YFP plasmid has been described previously (24). Human NFATc3-GFP construct was a kind gift of Dr. Anjana Rao (UC San Diego). NFAT-luciferase and renilla luciferase plasmids were obtained from Dr. Richard Lewis (Stanford University). RNAi constructs used to down regulate Stim1 and Orai1 protein expression as well as scrambled siRNA control was purchased from Ambion, Life Technologies (SilencerSelect predesigned siRNA). Transfected cells were used for experiments 24–36 hours after transfection for cDNA constructs and 48–72 hours for RNAi treated cells.
Reagents and chemicals
The standard extracellular Ringers solution had the following composition (in mM): 150 NaCl, 4.5 KCl, 10 D-glucose, 1 MgCl2, 2 CaCl2 and 5 Na-HEPES. pH was adjusted to 7.4 using NaOH. For the Ca2+ free Ringers solution, CaCl2 was excluded from the above composition and MgCl2 was increased to 3mM. The 20 mM Ca2+ solution used for electrophysiological recordings contained 20 mM CaCl2 and 130 mM NaCl, other components of this solution were identical to the standard Ringer’s solution. The divalent-free (DVF) Ringer’s solution contained (in mM): 150 NaCl, 10 HEDTA, 1 EDTA and 10 Hepes (pH 7.4). Stock solutions of 2-APB (2-Aminoethoxydiphenylborane), BTP2 (3,5-bis (trifluroromethyl) pyrazole) , thapsigargin (TG), RO2959, Cyclosporin A (CsA) and FK-506 were dissolved in DMSO. PAR2-agonists including type IX trypsin (Sigma) as well as the agonistic peptides SLIGRL and SLIGKV, control peptide LRGILS and the PAR1 peptide TFLLR (all from Tocris Bioscience) were constituted in water. 2-APB, CsA and U73122 were from Sigma Aldrich. RO2959 (Difluoro-N-(5-(4-methyl-1-(5-methyl-thiazol-2-yl)-1,2,5,6-tetrahydro-pyridin-3-yl)-pyrazin-2-yl)-benzamide) was from Synta Pharmaceuticals.
Intracellular Ca2+ measurements
NHBE cells and various cell lines were grown on poly-L lysine coated glass bottom dishes (MatTek corp). Cells were loaded with 2.5 µM Fura-2 AM (Invitrogen) in dark for 40 minutes at room temperature in the appropriate culture medium containing 5–10% FBS. After washing away excess dye, cells were incubated in media for an additional 10 minutes before initiating Ca2+ imaging. Single cell [Ca2+]cyt measurements were done according to the protocol described previously (25). Image acquisition and analysis was performed using IPLab (Scanalytics, Rockville, MD, USA) and Slidebook (Denver, CO, USA). For data analysis, regions of interest (ROIs) were drawn around single cells, background subtracted, and the F340/ F380 intensity ratios were determined for each time point. The F340/ F380 intensity ratios were converted to [Ca2+]cyt using the formula:
where R is the F340/ F380 fluorescence intensity ratio and Rmax (=9.645) and Rmin (=0.268) were determined by in-vitro calibration of fura-2. β (=20.236) was determined from the Fmin/Fmax ratio at 380 nm and Kd is the apparent dissociation constant of fura-2 binding to Ca2+ (135 nM). Where applicable, rate of SOCE, “Δ[Ca2+]cyt/Δt” was calculated as the slope of a line fitted to the 1st three points following addition of 2 mM Ca2+ in the Ca2+ imaging trace. Slope was averaged over several cells in the imaging field to generate the relevant bar graphs.
Western blots
NHBE and BEAS-2B cells were cultured in 6-well plates. At ~70–80% confluency, cells were washed with cold PBS following which cell lysis and Western blotting was done using protocols described previously (25). Orai1 and STIM1 proteins were detected using affinity purified polyclonal antibodies and peroxidase labelled secondary antibodies (26, 27).
Patch clamp measurements
Patch clamp recordings and analysis of CRAC currents were performed using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) using standard methods as previously described (28). The holding potential was +30 mV. The standard voltage stimulus consisted of a 100-ms step to −100 mV followed by a 100 ms ramp from −100 to +100 mV applied at 1-s intervals. Cells were pretreated with 1 µM thapsigargin (TG) prior to establishment of the seal to deplete stores and activate CRAC channels.
NFAT-luciferase assay
Endogenous NFAT activity in cells was measured using the PGL3-NFAT luciferase reporter construct (29). NFAT activity was normalized to the activity of Renilla luciferase (pRL-Tk-luc). Plasmids were transfected into AECs in a ratio of 20:1 (NFAT: Renilla). 24 hours after transfection, cells were pre-treated with the CRAC channel inhibitors BTP-2 and RO2959 for 1 hour followed by treatment with 0.5 µM TG and 50 nM PDBu for 8–10 hours. Cells were lysed and the luciferase activity was determined using the Dual-luciferase Reporter Assay Kit (Promega) and a single tube luminometer (Berthold Instruments, Germany).
NFAT translocation assay
BEAS-2B cells were transfected with NFATc3-GFP construct and imaged for GFP 24- 36 hours later. Images were taken in resting 2 mM Ca2+ solution and after depleting ER Ca2+ stores with TG (1 µM, 20 min). The percentage of cells showing NFAT localization to the nucleus was calculated and averaged between several fields and reported as a bar graph.
Analysis of cytokine secretion
AECs (NHBE, BEAS, A549 and 1Haeo- cells) were cultured in 24 well plates. Twelve hours before treatment, media was changed to a 2 mM Ca2+ media without growth factors. Cells were treated with specific CRAC channel activating stimuli (either TG and PDBu or PAR2 agonists: SLIGRL and SLIGKV) for 2- 24 hours and the levels of various inflammatory mediators was measured using custom made ELISA kits (RayBiotech for TNFα, IL-6, IL1α and GM-CSF, Cayman chemicals for PGE2, R&D for TSLP, LifeTechnologies for IL-8 and IL1β).
Data analysis
All bar-graphs summarizing the data are reported as mean ± SEM. For data sets involving more than two groups, initial statistical analysis was performed using ANOVA with a confidence interval of 5%. This was followed by two-tailed paired student t-test for comparing different treatment conditions within the set. For data sets with only two groups, two-tailed paired student t-test was used to compare between control and test conditions.
RESULTS
A screen for GPCR agonists identifies PAR2 receptors as activators of store-operated Ca2+ entry in the airway epithelium
In what context would CRAC channels be relevant for Ca2+ signaling in AECs? CRAC channels are voltage independent and require depletion of ER Ca2+ stores for activation (17). There are numerous extracellular stimuli (e.g., growth factors, pathogens, GPCR ligands, etc.) that act in this manner and could be regarded as potential candidates that stimulate CRAC channels in AECs. To examine this, we screened several ligands that are coupled to the generation of IP3 in NHBE cells. Although several receptors coupled to PLC-IP3 signaling are thought to be expressed in AECs (30), this screen revealed that only a subset of ligands, including agonists for PAR2 and purinergic P2Y receptors (and to a smaller extent, thrombin and bradykinin) evoked Ca2+ signals consistent with store-operated Ca2+ entry (Table I). PAR2 receptors are important for the ability of AECs to sense allergens and endogenous proteases and for mediating the subsequent inflammatory responses in the lung (31). Moreover, aberrant PAR2 signaling has been implicated in several allergic respiratory disorders including asthma (31). We therefore sought to define the mechanisms and functional consequences of CRAC channel signaling in AECs following PAR2 activation.
Table I.
Effect of GPCR and Toll-Like Receptor (TLR) agonists on SOCE in AECs.
| LIGAND | KNOWN RECEPTOR |
RECEPTOR TYPE | SOCE ACTIVATION? |
|---|---|---|---|
| PROTEASE ACTIVATED RECEPTORS | |||
| Type IX Trypsin | PAR2 | GPCR | ++ |
| SLIGKV, SLIGRL | PAR2 | GPCR | ++ |
| TFLLR | PAR1 | GPCR | − |
| Thrombin | PAR1, 3, 4 | GPCR | + |
| PATHOGENS | |||
| PamC3, LTA | TLR2 | TLR | − |
| pI:C | TLR3 | TLR | − |
| LPS | TLR4 | TLR | − |
| PURINERGIC AGONISTS | |||
| ATP | P2X, P2Y | GPCR | ++ |
| UTP | P2Y2, P2Y4 | GPCR | ++ |
| UDP-glucose | P2Y14 | GPCR | − |
| OTHER LIGANDS | |||
| Bradykinin | BK1, 2 | GPCR | + |
| PGE2 | EP1 | GPCR | − |
| PAF (platelet activating factor) | PAFR | GPCR | − |
| EGF (epidermal growth factor) | EGFR | GPCR | − |
| Salbutamol | β2 adrenergic | GPCR | − |
| Denatonium | Bitter taste receptors | GPCR | − |
| tBHP (oxidative stress) | − | STIM1, IP3 | − |
Table I: NHBE cells were treated with the indicated ligands in a Ca2+-free Ringer’s solution. Extracellular Ca2+ (2 mM) was then restored and the amplitude of the [Ca2+]cyt rise was examined. Cells were considered responders if the [Ca2+]cyt elevation was > 2 x SEM above the resting [Ca2+]cyt.
PAR2 activation stimulates Ca2+ signaling in AECs by activating CRAC channels
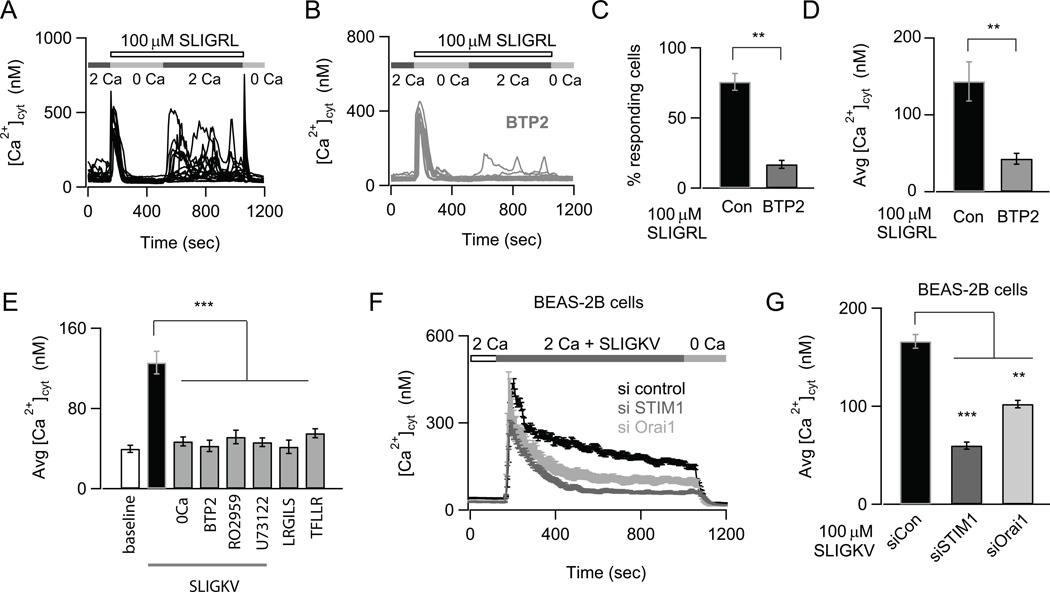
PAR2 activation initiates many cell signaling cascades including IP3 mediated mobilization of [Ca2+]cyt (13). However the pathways involved in generating PAR2 mediated [Ca2+]cyt elevations and the functional significance of these Ca2+ elevations in AECs are unclear. To address the potential role of store-operated CRAC channels in generating PAR2 mediated Ca2+ signals in AECs, we examined the ability of PAR2 ligands to elicit SOCE by imaging cytosolic Ca2+ transients using the Ca2+ indicator, fura2. Activation of PAR2 by the PAR2 activating peptides, SLIGRL and SLIGKV, caused a biphasic [Ca2+]cyt elevation that included a rapid initial rise in [Ca2+]cyt followed by sustained Ca2+ oscillations in NHBE cells (Fig. 1A, E and data not shown). The rise in [Ca2+]cyt following add-back of extracellular Ca2+ indicates that Ca2+ entry across the plasma membrane is necessary for sustaining PAR2-mediated Ca2+ elevations (Fig. 1A). The Ca2+ response was specific to PAR2 activation, since administration of a control peptide LRGILS or a PAR1-specific peptide TFLLR, failed to elicit Ca2+ signaling (Fig. 1E). Response to SLIGKV was also abolished when cells were pre-treated with the phospholipase C (PLC) inhibitor U73122 consistent with PAR2 activating a Gq mediated PLC signaling pathway (Fig. 1E). Importantly, the CRAC channel inhibitors BTP2 and RO2959 (32, 33) attenuated both the number of oscillations and the amplitude of the Ca2+ response to PAR2 peptides (Fig. 1B–E). These results indicate that PAR2 stimulation evokes a biphasic Ca2+ signal whose sustained component arises from CRAC channel activation.
FIGURE 1.
Store-operated CRAC channels regulate Ca2+ signals induced by PAR2 activation. (A) The PAR2-agonist SLIGRL induces oscillatory Ca2+ signals due to SOCE. NHBE cells were treated with 100 µM SLIGRL in a nominally Ca2+ free solution to deplete internal stores. 2 mM Ca2+ was re-added to the external bath solution to activate SOCE. The Ca2+ traces show responses of individual cells in the imaging field. (B) Pre-treatment with the CRAC channel inhibitor BTP2 does not affect store-release but abrogates SOCE. Cells were treated with 500 nM BTP2 for 1.5 hours (A, B; data are mean ± SEM of N=18–21 cells; representative of 3 independent experiments). (C) Bar-graph summarizing the percentage of cells showing Ca2+ responses after re-addition of external Ca2+. (D) Summary of the amplitude of Ca2+ response at 840 sec (data are mean ± SEM of N=38–45 cells; 3 independent experiments). (E) Summary of the average elevation in [Ca2+]cyt in response to the PAR2 activating peptide SLIGKV in Ca2+ and nominally Ca2+ free solutions, control peptide LRGILS and PAR1 activating peptide TFLLR. The effects of CRAC channel inhibitor RO2959 and PLC inhibitor U73122 on SLIGKV mediated Ca2+ elevation is also indicated. The [Ca2+]cyt was measured 156–192 sec after addition of various peptides to NHBE cells with 2mM Ca2+ in the external bath (data are mean ± SEM of N=12–23 cells; 3 independent experiments). (F) Effects of siRNA knockdown of STIM1 and Orai1 on SLIGKV-induced Ca2+ signals in BEAS-2B cells (data are mean ± SEM of N=24–31 cells; representative of 3 independent experiments). The traces reflect the mean Ca2+ concentration of all cells in the imaging field. (G) Summary of mean [Ca2+]cyt levels 748 sec after addition of ligand. Statistical significance was determined by unpaired two-sample student t-test). The following standardized notation for statistical significance (P-values) is used in all the figures, * P< 0.05, ** P< 0.01, *** P< 0.001, n.s. (not significant).
Previous evidence indicates that the CRAC channel proteins Orai1 and STIM1 are expressed in the lung (21, 34), raising the possibility that these proteins may contribute to Ca2+ signaling in AECs. We therefore sought to investigate the contribution of STIM1 and Orai1 to PAR2 evoked Ca2+ signals. siRNA mediated knock-down of STIM1 and Orai1 proteins significantly reduced their expression and inhibited the sustained Ca2+ signals in response to the PAR2 activating peptide SLIGKV in the bronchial cell-line BEAS-2B (Figs. 1F and G, Supplemental Fig. 1A). Similarly over-expression of a dominant negative pore mutant of CRAC channels, E106A Orai1, (35), significantly reduced SLIGKV activated Ca2+ response (data not shown). These results indicate that CRAC channels encoded by STIM1 and Orai1 are essential for mobilizing PAR2 evoked Ca2+ signals.
PAR2 activation by the serine protease trypsin stimulates CRAC channel mediated Ca2+ signals in AECs
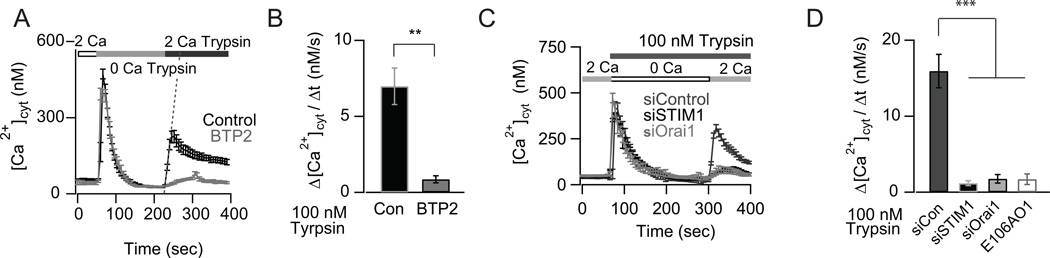
In the airway epithelium, PAR2 is activated by endogenous proteases including trypsin, mast cell tryptase and human airway trypsin-like proteases that are released during inflammation and injury (31). We therefore tested the effects of trypsin on Ca2+ signaling in AECs. Administration of type IX trypsin produced a sustained Ca2+ signal only when Ca2+ was present in the external bath solution. The sustained component of the [Ca2+]cyt elevation was abrogated by the CRAC channel inhibitor BTP2 (Figs. 2A and B) without affecting store-release. Likewise, over-expression of E106A Orai1, which functions as a dominant negative inhibitor of CRAC channels (35) as well as the knockdown of CRAC channel proteins STIM1 and Orai1 attenuated trypsin induced Ca2+ signal (Figs. 2C and D). Taken together, these results show that PAR2 activation in response to both synthetic peptides and to enzymatic cleavage by trypsin mobilizes Ca2+ signaling in primary human AECs through activation of CRAC channels.
FIGURE 2.
The PAR2 agonist, type IX trypsin, evokes Ca2+ elevations by activating CRAC channels. (A) Type IX trypsin (100 nM) activates SOCE in NHBE cells. SOCE was activated using the same protocol as in Figure 1A. The CRAC channel inhibitor, BTP2, abrogates trypsin induced Ca2+ elevations in NHBE cells (mean ± SEM of N= 17–25 cells, 3 independent experiments). Slope of the line (used to calculate rate of SOCE) following addition of 2 mM Ca2+ is indicated for the control condition. (B) Summary of the rate of Ca2+ influx for SOCE shown in Fig. 2A. (C) siRNA knockdown of STIM1 or Orai1 inhibits trypsin mediated SOCE in BEAS-2B cells. (D) Summary of the rates of trypsin mediated Ca2+ influx shown in Fig. 2C and in cells transfected with dominant negative E106A Orai1 (mean ± SEM of N=16–26 cells; 3 independent experiments).
STIM1 and Orai1 contribute to SOCE in human AECs
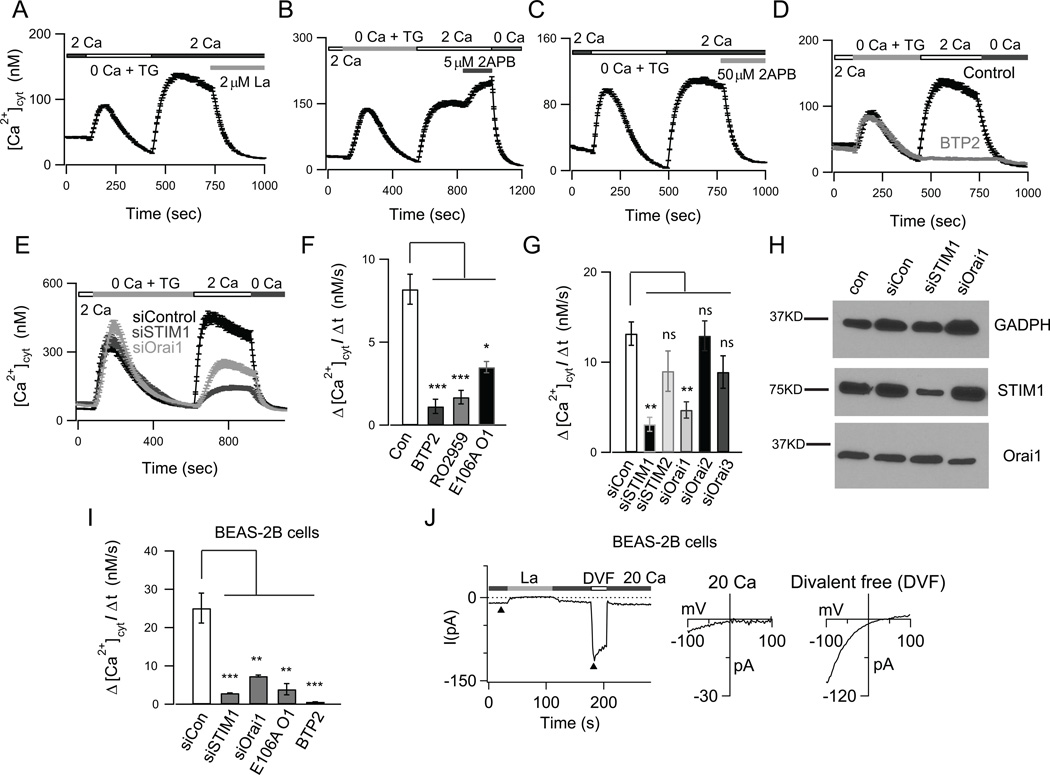
The ability of CRAC channel antagonists to suppress PAR2-evoked Ca2+ signals implies that human AECs express the machinery for CRAC channels. To directly address this issue, we next examined the pharmacology, biophysical characteristics, and molecular basis of CRAC channels in AECs. Following the depletion of ER Ca2+ stores with the SERCA pump inhibitor thapsigargin (TG) in a nominally Ca2+ free solution, robust Ca2+ influx was seen when Ca2+ was added to the external bath in primary bronchial (NHBE) cells (Fig. 3A). SOCE in NHBE cells exhibited pharmacological properties consistent with CRAC channels including acute inhibition by 2 µM La3+, potentiation by 5 µM 2-APB and inhibition by a higher dose of 2-APB (50 µM) (Fig. 3A to 3C). Moreover, SOCE was significantly inhibited by the CRAC channel antagonists BTP2 and RO2959 (Fig. 3D and 3F). Similar results were obtained in the bronchial cell lines BEAS-2B and 1Haeo- as well as the alveolar epithelial cell line A549, suggesting that SOCE is widespread in lower airway epithelial cells (Fig. 3I, Supplemental Fig. 1B and C). The rates of Ca2+ influx following Ca2+ add-back reflect store-operated channel activity and are summarized in Figure 3E. These findings are consistent with recent reports indicating presence of CRAC channels in the bronchial cell lines 16HBE and CFBE410− (21, 34).
FIGURE 3.
Characterization of store-operated CRAC channels in human airway epithelial cells. (A–C) ER Ca2+ stores were depleted with 1 µM TG in a nominally Ca2+ free solution. Re-addition of 2 mM Ca2+ to the external bath solution evoked SOCE. (A) SOCE is acutely blocked by 2 µM La3+. (B) A low dose of (5 µM) 2-APB facilitated SOCE. (C) A high dose of 2-APB (50 µM) inhibited SOCE. (D) Pre-treatment with the CRAC channel inhibitor BTP2 (500 nM) abolished SOCE in NHBE cells (A-D; mean ± SEM of N=18–29; representative of 3 independent experiments). (E) Knockdown of STIM1 and Orai1 expression in NHBE cells significantly inhibited SOCE (N=21–26 cells; 3 independent experiments). (F–G) Summary of the rates of SOCE in NHBE cells following treatment with the CRAC channel inhibitors BTP2 (500 nM), RO2959 (500 nM) and expression of dominant negative E106A Orai1 (N=21–42 cells; 3 experiments) (F), and following siRNA mediated knockdown of STIM1–2, Orai 1–3 (mean ± SEM of N=22- 34; 3 experiments) (G). (H) Western blots showing expression of Orai1 and STIM1 in NHBE cells. (I) Summary of rates of SOCE in BEAS-2B cells (N=31–48 cells; 3 independent experiments). (J) Whole-cell patch-clamp recordings of ICRAC in BEAS-2B cells. Cells were pretreated with TG to deplete ER Ca2+ stores. The external solution was periodically switched between 20 mM Ca2+ and a divalent-free (DVF solution). In 20 mM Ca2+o, the current exhibits a current-voltage (I-V) relationship with strong inward rectification and positive reversal potential (>+60 mV), consistent with the known biophysical hallmarks of CRAC channels.
We next sought to resolve the molecular machinery of CRAC channels in AECs. CRAC channels are activated by STIM proteins (STIM1 and 2) and encoded by the Orai proteins (Orai1, 2 and 3) (36). Western blots showed the expression of both STIM1 and Orai1 proteins in NHBE and BEAS-2B cells (Fig. 3H and Supplemental Fig. 1A). Over-expression of dominant negative E106A Orai1 significantly abrogated SOCE in various AECs (Figs. 3F and 3I). Moreover, knockdown of STIM1 and Orai1 using siRNA significantly reduced the protein expression and inhibited both the rate and the amplitude of SOCE in NHBE cells and various cell-lines (Figs. 3E, 3G and 3I, Supplemental Fig. 1B, C and data not shown), indicating that these isoforms do not appreciably contribute to SOCE in human AECs. siRNA mediated knockdown of Orai2, Orai3 and STIM2 did not significantly affect SOCE (Fig. 3G). We were unable to directly study the expression of Orai2 and Orai3 isoforms due to non-specificity of the commercially available antibodies we tested (data not shown). Nevertheless, together with the functional evidence presented in the previous section, these results indicate that the CRAC channel proteins STIM1 and Orai1 are essential for conferring SOCE in human AECs. We therefore focused our studies on the functional roles of STIM1 and Orai1 in the airway epithelium.
CRAC channels have a distinct electrophysiological profile characterized by an inwardly rectifying current-voltage relationship, low permeability to large monovalent cations such as Cs+ and depotentiation in divalent free solutions (37). Patch-clamp recordings of store-operated currents in BEAS-2B cells following treatment with TG to deplete ER Ca2+ stores showed the presence of a cation current consistent with these well-known properties of CRAC channels (17, 25) (Fig. 3J). In 20 mM Ca2+o, the current-voltage (I-V) relationship showed strong inward rectification and an extremely positive reversal potential. In divalent-free solution, the I-V relationship revealed a reversal potential of ~+50 mV, indicating low permeability to internal Cs+ (Fig. 3J). Similar results were seen in the airway epithelial cell line A549 (data not shown). These results indicate that SOCE in airway cells exhibits the canonical biophysical properties of CRAC channels encoded by STIM1 and Orai1.
CRAC channel activation stimulates cytokine and chemokine production in AECs
AECs play a vital role in host defense by producing inflammatory mediators such as TSLP, IL-6, PGE2, IL-33, IL-8 and RANTES in response to a variety of stimuli including infectious agents, allergens and other bioactive molecules (38–41). Although Ca2+ signals have been implicated in the generation of inflammatory mediators from AECs, the Ca2+ signaling pathways that mediate this process remain poorly understood (7, 20, 42).
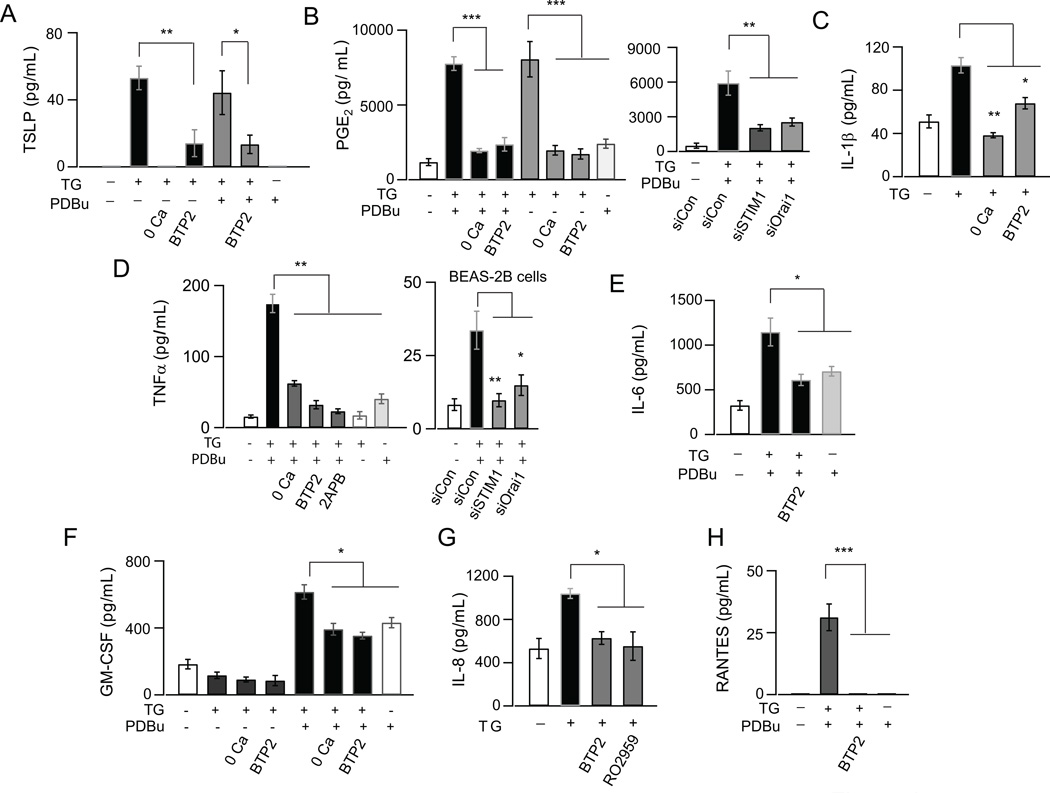
To address a potential role for CRAC channels in the production of inflammatory mediators from AECs, we initially performed a multiplex cytokine screen in NHBE cells following activation of CRAC channels by the SERCA inhibitor TG (Supplemental Fig. 2). Previous studies have shown that Ca2+ signaling pathways in immune cells including T-cells often work in synergy with protein kinase-C (PKC) dependent signaling to induce cytokines (43). Therefore, in these experiments, we also co-stimulated PKC by administering the phorbol ester, PDBu. Key hits from this screen were further examined by individual ELISA kits. These tests revealed that several key inflammatory mediators are regulated by CRAC channel activation in NHBE cells, including TSLP, IL-6, TNFα, IL1β and GM-CSF (Fig. 4A, 4C to 4F). CRAC channel activation also induced the production of the arachidonic acid metabolite PGE2 (Prostaglandin E2) from AECs (Fig. 4B). We also confirmed IL-6, TNFα and PGE2 production in various airway epithelial cell lines (Fig. 4D, Supplemental Fig. 3A–C). The production of these inflammatory mediators was prevented in Ca2+ free medium and by the CRAC channel inhibitor BTP2 (Fig. 4A to 4F). Furthermore, knock down of STIM1 and Orai1 inhibited CRAC channel induced production of TNFα and PGE2 (Fig. 4B and C). Thus, these results implicate a causal role for Ca2+ influx through CRAC channels in the production of inflammatory cytokines from AECs. Interestingly, while maximal induction of TNFα and IL-6 required co-stimulation of NHBE cells with both TG and PDBu, TSLP and PGE2 were induced maximally by TG alone, suggesting that Ca2+ signals differentially regulate cytokine production depending on the presence of specific co-stimulatory signals.
FIGURE 4.
CRAC channel activation induces production of inflammatory mediators in human airway epithelial cells. (A-H) Production of cytokines and chemokines as detected by ELISA. AECs were stimulated with either TG alone (0.25 µM) or TG (0.25 µM) and PDBu (50 nM), in the absence or presence of the CRAC channel inhibitor BTP2 (250 nM) and in nominally Ca2+ free media. Cells treated with solvent alone (DMSO) were used as control. Cell-culture supernatants were collected 8–12 hours after treatment for all cytokines and after 48 hours for TSLP. N=3–4 replicates; representative of 3 independent experiments. Unless otherwise noted, all experiments were in NHBE cells.
In addition to the cytokines, CRAC channel activation also caused significant increase in the production of chemokines IL-8 and RANTES which was blocked by the CRAC channel inhibitors BTP2 and RO2959 (Figs. 4G and H). The multiplex cytokine screen further revealed that while AECs also produce IFNγ and CXCL1, these factors are not induced by CRAC channels, indicating that only a subset of inflammatory mediators produced by AECs are regulated by CRAC channels. Collectively, these results establish a key role for CRAC channels as an important route of Ca2+ entry for the production of cytokines and chemokines in the airway epithelium.
PAR2 agonists activate CRAC channels to induce cytokine production in AECs
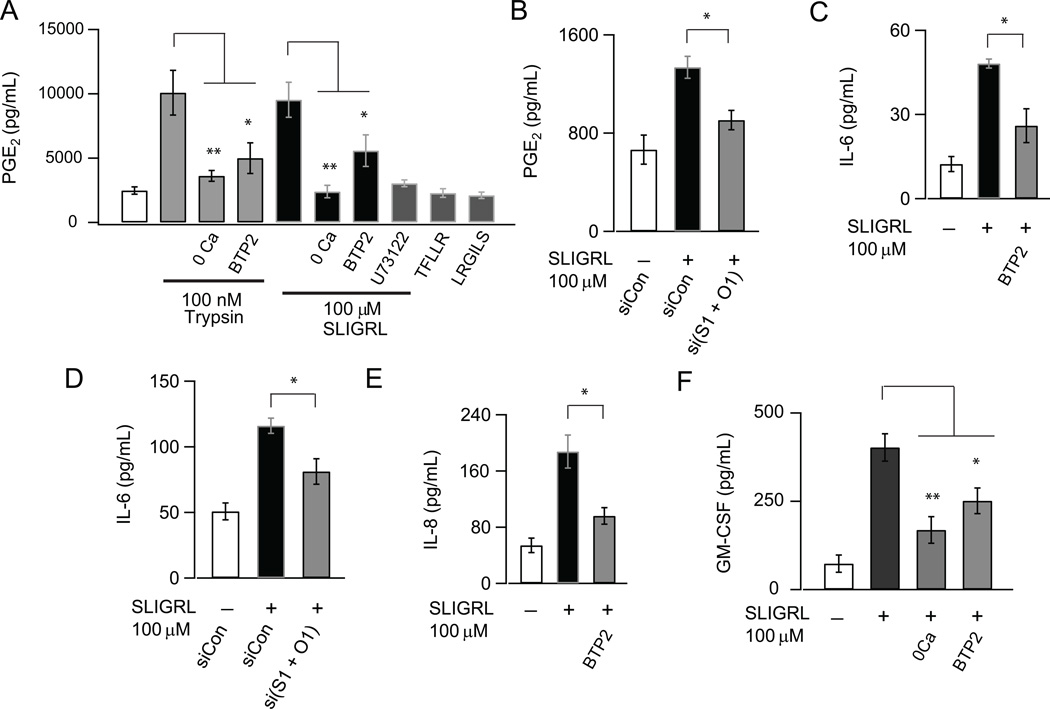
Previous studies have established that PAR2 activation in AECs induces the production of a wide range of cytokines including IL-6, GM-CSF, TSLP and chemokines IL-8 and eotaxin, the growth factor PDGF, arachidonic metabolites PGE2 and PGD2 as well as the enzyme MMP-9 (8–10, 40, 44). The dual findings that PAR2 activation mobilizes Ca2+ signals through CRAC channels (Fig. 1 and 2) and that CRAC channel activation with TG stimulates cytokine production (Fig. 4 and 5) therefore led us to consider a role for CRAC channels in the production of inflammatory mediators by PAR2 agonists. These tests revealed that administration of the PAR2 agonist peptide SLIGRL resulted in a significant increase in the production of PGE2, IL-6, GM-CSF and IL-8 (Fig. 5A, C, E and F). PGE2 was induced in 2 hours while induction of IL-6, GM-CSF and IL-8 occurred after 8- 24 hours of PAR2 activation. The PAR2 mediated production of these factors was significantly inhibited by the CRAC channel antagonist, BTP2 (Figs. 5A, C, E and F). Interestingly, PAR2 activation by SLIGRL did not evoke TNFα induction (not shown). This is in contrast to cell stimulation with TG/PDBu, which was very effective in evoking TNFα production (Figure 4D). The reasons for this difference need to be further investigated, but could be because the amplitude and duration of Ca2+ elevations in response to PAR2 activation are insufficient to induce TNFα. PGE2 and IL-6 induction in response to SLIGRL were significantly inhibited by combined knockdown of STIM1 and Orai1 (Figs. 5B and D). Knockdown of STIM1 or Orai1 alone, however, did not significantly diminish the production of these cytokines (data not shown). We suspect that this is due to the low knockdown efficiency of protein expression in NHBE cells (~50% for both proteins as seen by Western Blot, Fig. 3H) which would be expected to only partially inhibit SOCE. Similarly, trypsin produced a significant increase in PGE2 production that was attenuated by the CRAC channel blocker BTP2 (Fig. 5A). Thus, these findings strongly suggest that CRAC channels contribute to the production and release of inflammatory modulators in response to PAR2 activation.
FIGURE 5.
CRAC channels regulate generation of inflammatory mediators in response to PAR2 activation in human airway epithelial cells. NHBE cells were stimulated with the PAR2-activating peptide SLIGRL (100 µM) in the absence or presence of BTP2 or following knockdown of both STIM1 and Orai1. Cell-culture supernatants were collected after 2 hours (for PGE2) or 24 hours (for IL-6, IL-8 and GM-CSF measurements) and levels of PGE2, IL-6, IL-8 and GM-CSF were determined by cytokine-specific ELISA kits. (A) PGE2 induction by SLIGRL and Type IX trypsin (100 nM) is inhibited by BTP2 and U73122. A scrambled peptide (LRGILS) and a PAR1-specific peptide (TFLLR) had no effect on PGE2 induction. (B) Inhibition of PGE2 secretion by knockdown of STIM1 and Orai1. (C) Inhibition of IL-6 secretion by BTP2 and (D) siRNA mediated knockdown of STIM1 and Orai1. (E-F) Effect of BTP2 on IL-8 and GM-CSF induction (N=3 replicates, 3 independent experiments).
PAR2 receptor stimulation results in IL-6 and IL-8 production through the activation of NFAT
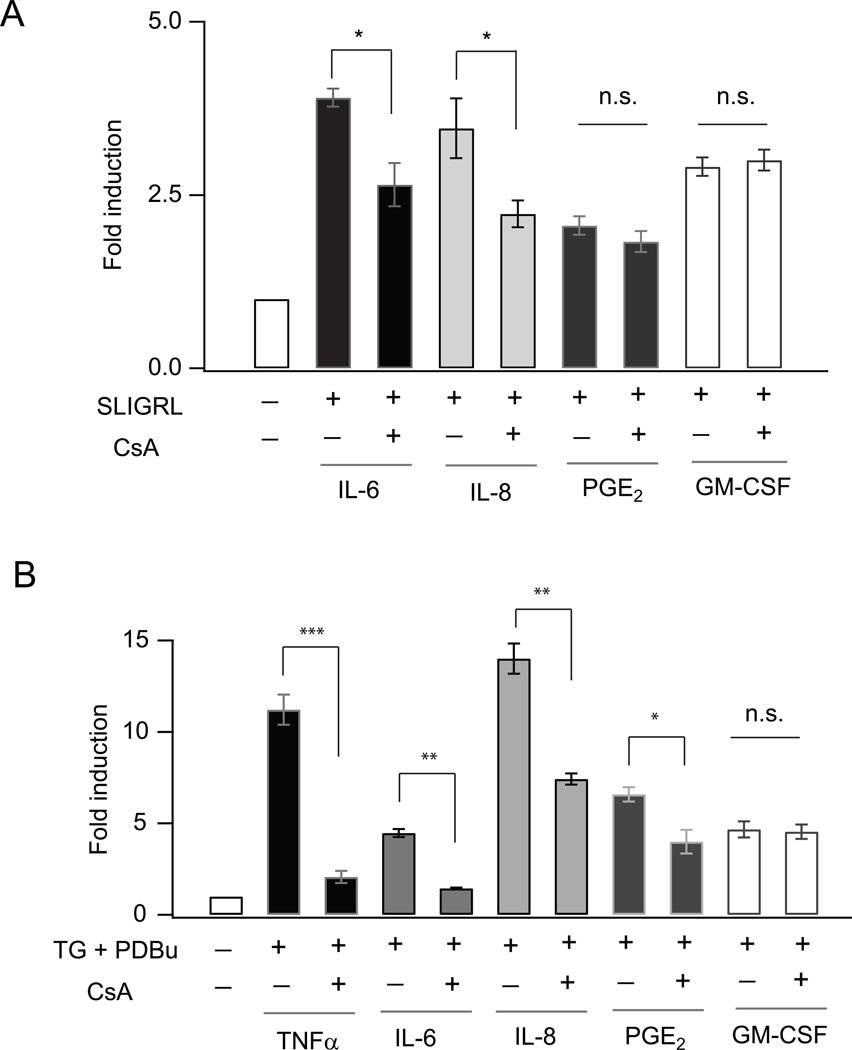
By what mechanism does Ca2+ influx through CRAC channels regulate PAR2 mediated production of cytokines? In many immune cells, cytokine induction is regulated transcriptionally by the transcription factor NFAT (nuclear factor of activated T-cells) (45, 46). Ca2+ elevations activate NFAT through the protein phosphatase, calcineurin, which then causes NFAT to move into the nucleus and bind to target DNA sequences (43). While AECs express NFAT proteins (47), the specific targets of NFAT and the physiological relevance of this signaling remains unknown. To explore whether induction of cytokines by PAR2 activation occurs through the calcineurin-NFAT pathway, we measured PAR2 induced cytokine levels in the presence of the calcineurin inhibitor Cyclosporin A (CsA), a widely used small molecule inhibitor of calcineurin/NFAT signaling (48). Induction of both IL-6 and IL-8 was significantly inhibited by CsA (Fig. 6A). By contrast, the NF-kB inhibitor, caffeic acid, had no effect on induction of IL-6 or IL-8 indicating that under these experimental conditions, the induction of the cytokines primarily occurs through NFAT-dependent gene transcription (Supplemental Fig. 3D). Moreover, induction of PGE2 and GM-CSF was not affected by CsA, suggesting that the induction of these cytokines in response to PAR2 activation is not significantly regulated by calcineurin/NFAT signaling. These findings highlight a novel role for NFAT dependent gene expression in the induction of specific cytokines and chemokines in response to PAR2 signaling.
FIGURE 6.
Calcineurin-NFAT signaling regulates PAR2 and CRAC channel mediated induction of inflammatory mediators from AECs. (A) Summary of the effect of calcineurin inhibitor CsA (500 nM) on IL-6, IL-8, PGE2 and GM-CSF induction in response to the PAR2 agonistic peptide SLIGRL (100 µM) in NHBE cells. Cells were stimulated for 2 hours for PGE2 induction, 8 hours for IL-6 and IL-8 induction and 24 hours for GM-CSF induction. Data is shown as fold change in cytokine production (actual concentrations: IL-6: 18–70 pg/mL; IL-8: 55–200 pg/mL; PGE2: 2300–4500 pg/mL; GM-CSF: 75–220 pg/mL). (B) Summary of the change in cytokine levels in NHBE cells (12 hour stimulation) evoked by CRAC channel activation. NHBE cells were treated with TG (250 nM) and PDBu (50 nM) in the absence and presence of CsA (250 nM). N=3 replicates, 3 independent experiments.
NFAT regulates cytokine and chemokine production in response to CRAC channel activation
To explore the role of NFAT in CRAC-channel mediated cytokine production more directly, we stimulated NHBE cells with TG and PDBu for 12 hours in the presence or absence of CsA and evaluated inflammatory mediator production by ELISA. CsA caused an almost complete inhibition of TNFα and IL-6 production, implicating a key role for NFAT in induction of these cytokines (Fig. 6B). CsA also partially inhibited production of PGE2 and IL-8, suggesting that NFAT contributes to the production of these mediators (Fig. 6B). The partial inhibition of PGE2 induction in this case stands in contrast to the lack of CsA effect seen following two hours of PAR2 stimulation (Fig. 6A), suggesting that at longer time points, PGE2 production is influenced by NFAT-mediated gene expression, possibly through transcriptional regulation of the enzymes mediating PGE2 generation (49). Interestingly, although the promoter elements of GM-CSF is believed to exhibit NFAT binding, the induction of this cytokine in response to both PAR2 and TG/ PDBu was unaffected by CsA, suggesting that not all cytokines induced by CRAC channels are regulated through the calcineurin- NFAT pathway (Fig. 6B).
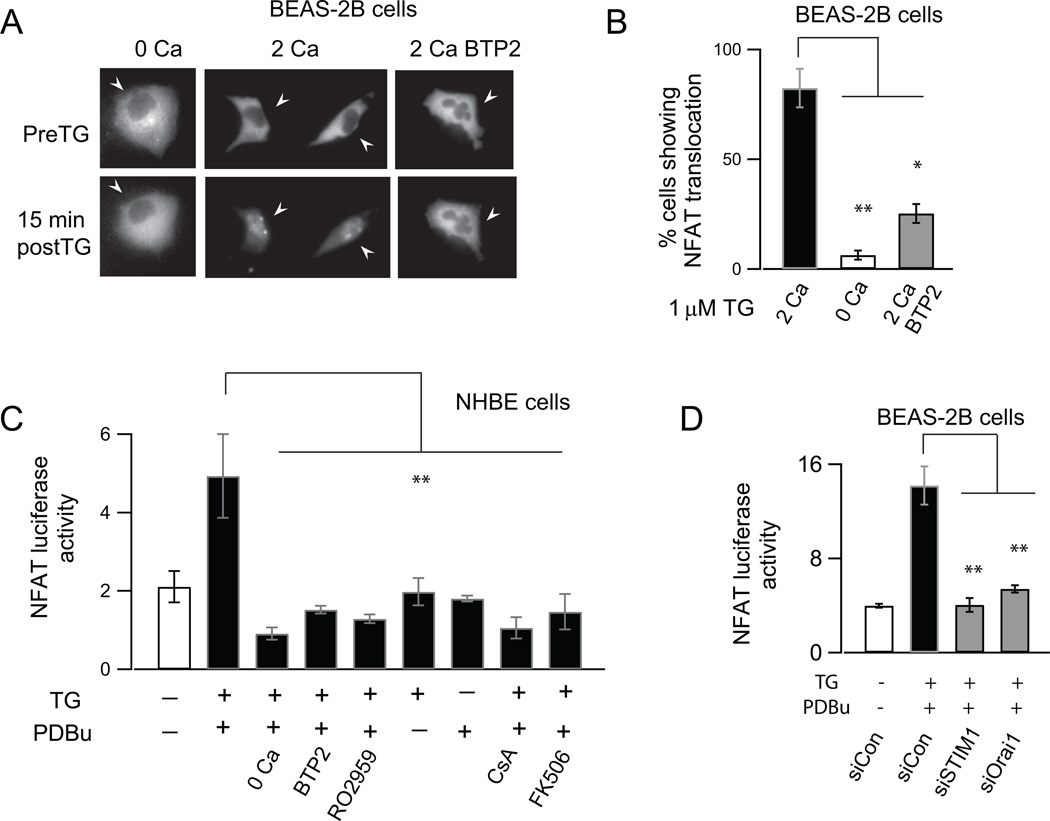
NFAT is normally phosphorylated at rest and resides in the cytoplasm in the inactive state. Ca2+ elevations mobilize NFAT activation through dephosphorylation of conserved serine/threonine residues by the Ca2+-activated protein phosphatase, calcineurin, which causes NFAT to move into the nucleus and bind to target DNA sequences (43). Consistent with this property of NFAT, examination of a fluorescently tagged NFAT isoform (GFP-NFATc3) revealed that activation of CRAC channels by TG resulted in robust NFAT import into the nucleus (Figs. 7A and B). The import was blocked in a nominally Ca2+ free solution and by BTP2 indicating that Ca2+ entry through CRAC channels was required to trigger nuclear translocation of NFAT (Figs. 7A and B). Moreover, examination of NFAT-dependent gene transcription using a luciferase reporter assay indicated that CRAC channel activation stimulated NFAT-dependent luciferase expression in NHBE cells (Fig. 7C). Luciferase activity was abrogated in the absence of external Ca2+, and by the CRAC channel antagonists BTP2 and RO2959 (Fig. 7C) without affecting cell viability (Supplemental Fig. 4). Importantly, siRNA mediated suppression of STIM1 and Orai1 expression significantly inhibited NFAT-dependent gene transcription (Fig. 7D). The calcineurin inhibitors CsA and FK506 also strongly inhibited induction of NFAT-luciferase activity consistent with the well-known role of calcineurin in controlling NFAT activation (Fig. 7C). Thus, these results highlight a broader role for NFAT in the generation of inflammatory mediators from the airway epithelium.
FIGURE 7.
CRAC channels activate NFAT dependent gene expression in AECs. (A) Wide-field images showing the localization of NFATc3-GFP protein under resting conditions and after CRAC channel activation with TG (1 µM, 20 min.) in the presence or absence of Ca2+ in the external bath and in the presence of BTP2 (500 nM). In each case, the pre- and post-TG images show the same cells. (B) Summary of the percentage of cells showing NFAT translocation in BEAS-2B cells following CRAC channel activation. (mean ± SEM of N=12–15 cells from 3 separate coverslips for each condition). (C) Summary of NFAT-luciferase activity in the presence and absence of CRAC channel (BTP2 and RO2959: 500 nM) and calcineurin inhibitors (CsA 500 nM, FK506 1 µM. Data is mean ± SEM of 4 replicates; 3 independent experiments in NHBE cells). NFAT-luciferase activity was measured using a pGL3 NFAT luciferase reporter construct (29). Cells were stimulated with TG and PdBu to activate CRAC channels and PKC, respectively. (D) siRNA knockdown of STIM1 and Orai1 significantly inhibits NFAT luciferase activity in BEAS-2B cells (mean ± SEM of 3 replicates; 3 independent experiments). NFAT luciferase activity normalized to renilla luciferase in order to correct for variations in cell density as described in the methods.
DISCUSSION
Ca2+ signals regulate many key physiological processes in airway epithelial cells (AECs) including activation of Ca2+ activated Cl− conductances, ciliary beat frequency, and mucous and surfactant production (19, 50, 51). There is now growing evidence that Ca2+ signals also play an important role in the induction of inflammatory mediators from the airway epithelium in response to pathogens and allergens and may be involved in orchestrating inflammatory responses in several airway diseases including cystic fibrosis, asthma, and acute lung injury (7, 20, 52). Despite these observations, our understanding of how Ca2+ signals are generated in human AECs and how they are linked to effector functions remains incomplete. Knowledge of molecules that comprise the functional architecture of the Ca2+ signaling network and the mechanisms by which these proteins shape Ca2+ signals is important to gain a full understanding of how Ca2+ signals modulate downstream inflammatory responses in human AECs. In this study we show that CRAC channels encoded by Orai1 and STIM1 mediate a critical role in generating Ca2+ signals in response to PAR2 activation and regulate the production of several key inflammatory mediators in the airway epithelium, in part by activating NFAT dependent gene expression. These results highlight CRAC channels as a key checkpoint for transducing responses from proteases and for the generation of inflammatory mediators from the airway epithelium and identify CRAC channels as a potential target for therapeutic treatment of allergic and inflammatory airway diseases.
CRAC channels are a major route of Ca2+ entry in AECs
We show that primary human AECs exhibit SOCE and store-operated currents that share the pharmacological and biophysical hallmarks of CRAC channels (37). These features include block by low concentrations of (1–2 µM) La3+, modulation by 2-APB, inhibition by the CRAC channel inhibitors BTP2 and RO2959, an inwardly rectifying I-V, fast inactivation, and depotentiation of the Na+ CRAC current (Fig. 3). Analysis of the underlying molecular machinery further reveals that the CRAC channel proteins, STIM1 and Orai1 make essential contributions to SOCE in primary human AECs (Figs. 3E to G). Both bronchial and alveolar epithelial cells exhibited STIM1-Orai1 dependent SOCE, suggesting that this Ca2+ influx pathway is broadly conserved throughout the lower airway epithelium (Fig. 3 and Supplemental Fig. 1). These findings confirm and broaden observations from past studies that have described STIM1 and Orai1 mediated SOCE in various airway epithelial cell-lines and corroborate the emerging viewpoint that CRAC channels are a well conserved mechanism for Ca2+ influx in the lower airways (21, 34, 53). Our data however do not rule out a role for other CRAC channel isoforms. Indeed, SOCE was not fully abolished by STIM1 and Orai1 knockdown. Moreover, STIM2 and Orai3 knockdown resulted in a modest inhibition of SOCE (Fig. 3G), raising the possibility that these proteins may be expressed and make additional contributions to SOCE in AECs. More studies using isoform specific antibodies and knockout mice would be needed to test this issue. These issues notwithstanding, our findings unequivocally show that STIM1 and Orai1 are essential for conferring SOCE in human AECs.
CRAC channels mediate the downstream effects of PAR2 signaling
A screen for ligands that activate SOCE revealed that only a very specific set of receptors stimulate CRAC channel activation in AECs (Table 1). A prominent hit in this screen was PAR2, an important sensor of allergens and endogenous proteases in the airway epithelium (5, 6, 9). While many elements of the PAR2 signaling pathway have been studied in AECs, it is unclear whether PAR2 activation evokes CRAC channel-mediated Ca2+ signals. Here, we find that Ca2+ influx through CRAC channels encoded by STIM1 and Orai1 is needed to maintain the sustained Ca2+ signal that arises from PAR2 activation (Fig. 1 and 2). Furthermore, we find that PAR2 activation induces production of PGE2, IL-6, IL-8 and GM-CSF in AECs in a CRAC channel-dependent manner. These findings highlight a novel role for CRAC channels as key control element in the PAR2 signaling response. It is worth noting that while PGE2 production is seen following brief activation of PAR2 receptors (2 hours), IL-6, IL-8 and GM-CSF production required prolonged stimulation of PAR2 (8–24 hours). Because PGE2 evokes bronchodilation through effects on the airway smooth muscle, these results are consistent with the emerging idea that acute responses to PAR2 tend to be protective largely, while more sustained responses to PAR2 activation result in induction of cytokines that are pro-inflammatory thereby contributing to the pathogenesis of diseases like asthma (12, 54).
CRAC channels induce the production of inflammatory mediators from AECs
In the repertoire of inflammatory mediators secreted by AECs, several including PGE2, TSLP, IL-6, IL-8 and GM-CSF are thought to be induced by cytosolic Ca2+ elevations (7, 55, 56). However, the source of Ca2+ signals driving the induction of these proinflammatory mediators remains unclear. We find that CRAC channel activation induces the production of a host of inflammatory mediators from primary bronchial cells including the cytokines IL-6, TSLP, TNFα and IL1β, the arachidonic acid metabolite PGE2, the chemokines IL-8 and RANTES, and the growth factor GM-CSF (Fig. 6). Further examination shows that TNFα, IL-6 and GM-CSF were specifically secreted only by co-stimulation of CRAC channels with protein-kinase C (PKC) activation while TSLP, PGE2 and IL1β are maximally induced by CRAC channel stimulation alone (Figs 6 A-F). These results indicate that cross-talk between signaling pathways can change the specific repertoire of inflammatory mediators released from AECs.
Among the inflammatory mediators that regulate airway inflammation, TSLP and PGE2 are produced primarily by the airway epithelium. TSLP plays a vital role in effecting a Th2 type airway inflammatory response that is characteristic of allergic diseases like asthma and is known to be induced by various bacterial, viral and fungal products (1). On the other hand, the arachidonic acid metabolite PGE2 has immunoprotective effects in the airway that includes attenuation of broncho-constriction in exercise and allergen induced asthma, and inhibition of lymphocytic proliferation (57, 58). Here, we find that CRAC channel activation induces both TSLP and PGE2 in AECs but at very different time scales: PGE2 is induced in less than 2 hours while TSLP induction requires at least 48 hours of AEC stimulation. These temporal differences indicate that CRAC channels regulate multiple stages of the inflammatory response in AECs and the net consequences for airway function will depend on balance between the ensuing pro- and anti-inflammatory effector responses. Curiously, while CRAC channel activation produced PGE2, we saw no induction of the leukotrienes (LTB4, LTC4-E4) which are pro-inflammatory products of the arachidonic acid pathway (Fig. 5 and data not shown). This is in contrast to findings in mast cells, where CRAC channel activation is a potent and specific trigger for the induction of LTC4 production (46). CRAC channels may thus activate different arachidonic acid metabolites and produce distinct biological responses depending on the cell type.
CRAC channels activate NFAT dependent gene-expression in AECs
Induction of IL-6, TNFα, PGE2 and IL-8 in response to CRAC channel activation was suppressed by CsA (Fig. 6), indicating that these cytokines are transcriptionally regulated by the calcineurin-NFAT pathway. This is consistent with observations that the promoter regions of a number of cytokine genes including IL-6, IL-8 and TNFα harbor NFAT binding sites (43) and reveals an important role for NFAT as a CRAC channel dependent regulator of cytokine production in AECs. In line with this interpretation, PAR2 mediated induction of IL-6 and IL-8 was also significantly inhibited by blocking the NFAT signaling pathway. Interestingly, while CsA inhibited IL-8 production in response to both PAR2 agonists and TG/PDBu to the same extent, the induction of IL-6 by PAR2 activation was only partially inhibited by CsA. We speculate that this difference may reflect the possibility that additional NFAT independent mechanisms activated by PAR2 signaling come into play to drive IL-6 induction. Moreover, not all inflammatory mediators activated by PAR2 signaling require NFAT gene expression (e.g., PGE2 and GM-CSF; Fig. 6A) suggesting that the induction of these mediators occurs through alternate Ca2+-regulated pathways. One possibility is that Ca2+-mediated stimulation of cPLA2 may drive the induction of PGE2 (59), something that remains to be tested. Taken together, our results highlight a hitherto unappreciated role for NFAT as an important mediator of downstream PAR2 signaling in the airway epithelium.
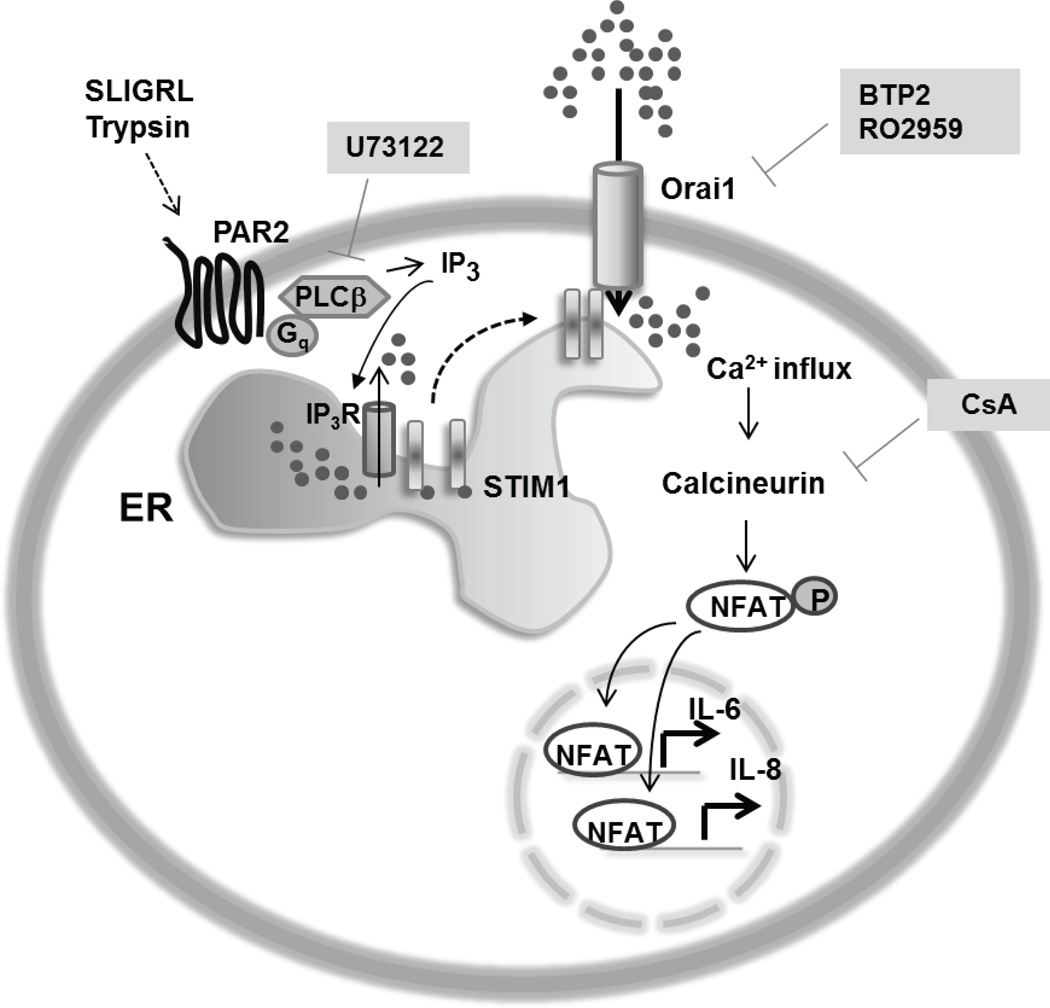
Based on these results, we propose a model for PAR2 receptor signaling in the airway epithelium wherein activation of these receptors, either by peptides or endogenous proteases, causes Gq-PLCβ-IP3 mediated store-release, leading to the generation of long-lasting Ca2+ signals through the activation of store-operated CRAC channels. The ensuing Ca2+ influx triggers the activation of transcription factor NFAT which then translocates to the nucleus and drives expression of IL-6 and IL-8 genes in conjunction with other signaling mechanism (Fig. 8). In conclusion, our study reports a novel Ca2+ signaling pathway in the airway epithelium mediated by CRAC channels which likely constitute an important regulatory element in the induction of inflammatory mediators from the airway epithelium.
FIGURE 8.
Model for the Ca2+ dependent signaling pathway activated by PAR2. Activation of PAR2 receptors by serine proteases (trypsin) and peptide agonists (SLIGRL and SLIGKV) evokes phospholipase C (PLC) stimulation leading to IP3 production and ER store-release through the opening of IP3 receptors. Release of Ca2+ from intracellular stores leads to activation of STIM1 which opens CRAC channels comprised of Orai1 subunits. Ca2+ influx through CRAC channels activates calcineurin-NFAT signaling to transcriptionally induce IL-6 and IL-8 production, likely in conjunction with other pathways. CsA, Cyclosporin A; U73122, PLCβ inhibitor; BTP2 and RO2959, CRAC channel inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH Grant NS057499) and a grant from the Skin Disease Research Center of Northwestern University.
Abbreviations used
- AEC
airway epithelial cells
- BTP2
3,5-bis(trifluoromethyl)pyrazole
- CRAC
Calcium release-activated Ca2+
- ER
endoplasmic reticulum
- GPCR
G-protein coupled receptor
- IP3
Inositol triphosphate
- NFAT
nuclear factor of activated T-cells
- PAR
Protease-activated receptor
- PKC
protein kinase C
- PLC
phospholipase C
- SOCE
store-operated Ca2+ entry
- STIM
Stromal interaction molecule
- TSLP
thymic stromal lymphopoietin
REFERENCES
- 1.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell. Mol. Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 4.Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66:579–587. doi: 10.1111/j.1398-9995.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 5.de Boer JD, Van’t Veer C, Stroo I, van der Meer AJ, de Vos AF, van der Zee JS, Roelofs JJ, van der Poll T. Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate. Immun. 2013;20:618–625. doi: 10.1177/1753425913503387. [DOI] [PubMed] [Google Scholar]
- 6.Hong JH, Hong JY, Park B, Lee SI, Seo JT, Kim KE, Sohn MH, Shin DM. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2008;39:530–535. doi: 10.1165/rcmb.2007-0410OC. [DOI] [PubMed] [Google Scholar]
- 7.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int. Arch. Allergy Immunol. 2012;158(Suppl 1):19–29. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000;105:1185–1193. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 10.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. quiz 1009. [DOI] [PubMed] [Google Scholar]
- 11.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J. Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 12.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J. Allergy Clin. Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 13.Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R. Selected contribution: tryptase-induced PAR-2-mediated Ca(2+) signaling in human airway smooth muscle cells. J. Appl. Physiol. 2001;91:995–1003. doi: 10.1152/jappl.2001.91.2.995. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol. Pharmacol. 2009;76:791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw PJ, Weidinger C, Vaeth M, Luethy K, Kaech SM, Feske S. CD4(+) and CD8(+) T cell-dependent antiviral immunity requires STIM1 and STIM2. J. Clin. Invest. 2014;124:4549–4563. doi: 10.1172/JCI76602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Clemens RA, Liu F, Hu Y, Baba Y, Theodore P, Kurosaki T, Lowell CA. STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense. Blood. 2014;123:2238–2249. doi: 10.1182/blood-2012-08-450403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh AB. Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug. Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 19.Antigny F, Norez C, Becq F, Vandebrouck C. CFTR and Ca Signaling in Cystic Fibrosis. Front. Pharmacol. 2011;2:67. doi: 10.3389/fphar.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukocyte. Biol. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samanta K, Bakowski D, Parekh AB. Key role for store-operated Ca2+ channels in activating gene expression in human airway bronchial epithelial cells. PloS one. 2014;9:e105586. doi: 10.1371/journal.pone.0105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita M, Somasundaram A, Prakriya M. Competitive modulation of Ca2+ release-activated Ca2+ channel gating by STIM1 and 2-aminoethyldiphenyl borate. J. Biol. Chem. 2011;286:9429–9442. doi: 10.1074/jbc.M110.189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 30.Chang W, Chen J, Schlueter CF, Hoyle GW. Common pathways for activation of proinflammatory gene expression by G protein-coupled receptors in primary lung epithelial and endothelial cells. Exp. Lung. Res. 2009;35:324–343. doi: 10.1080/01902140802712738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawabata A, Kawao N. Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: cellular signaling and physiological/pathological roles. J. Pharmacol. Sci. 2005;97:20–24. doi: 10.1254/jphs.fmj04005x4. [DOI] [PubMed] [Google Scholar]
- 32.Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Panicker S, Lau KY, Apparsundaram S, Patel VA, Chen SL, Soto R, Jung JK, Ravindran P, Okuhara D, Bohnert G, Che Q, Rao PE, Allard JD, Badi L, Bitter HM, Nunn PA, Narula SK, DeMartino JA. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Mol. Immunol. 2013;54:355–367. doi: 10.1016/j.molimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Balghi H, Robert R, Rappaz B, Zhang X, Wohlhuter-Haddad A, Evagelidis A, Luo Y, Goepp J, Ferraro P, Romeo P, Trebak M, Wiseman PW, Thomas DY, Hanrahan JW. Enhanced Ca2+ entry due to Orai1 plasma membrane insertion increases IL-8 secretion by cystic fibrosis airways. FASEB J. 2011;25:4274–4291. doi: 10.1096/fj.11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 36.Prakriya M, Lewis R. Store-operated calcium channels. Physiological Reviews. 2015 doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNally BA, Prakriya M. Permeation, selectivity and gating in store-operated CRAC channels. J. Physiol. 2012;590:4179–4191. doi: 10.1113/jphysiol.2012.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Churchill L, Chilton FH, Resau JH, Bascom R, Hubbard WC, Proud D. Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am. Rev. Respir. Dis. 1989;140:449–459. doi: 10.1164/ajrccm/140.2.449. [DOI] [PubMed] [Google Scholar]
- 39.Cromwell O, Hamid Q, Corrigan CJ, Barkans J, Meng Q, Collins PD, Kay AB. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 40.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J. Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr. Opin. Pharmacol. 2013;13:351–361. doi: 10.1016/j.coph.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Chun J, Prince A. Activation of Ca2+-dependent signaling by TLR2. J. Immunol. 2006;177:1330–1337. doi: 10.4049/jimmunol.177.2.1330. [DOI] [PubMed] [Google Scholar]
- 43.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 44.Kawao N, Nagataki M, Nagasawa K, Kubo S, Cushing K, Wada T, Sekiguchi F, Ichida S, Hollenberg MD, MacNaughton WK, Nishikawa H, Kawabata A. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J. Pharmacol. Exp. Ther. 2005;315:576–589. doi: 10.1124/jpet.105.089490. [DOI] [PubMed] [Google Scholar]
- 45.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. USA. 2012;109:6969–6974. doi: 10.1073/pnas.1201204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dave V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells. J. Biol. Chem. 2004;279:34578–34588. doi: 10.1074/jbc.M404296200. [DOI] [PubMed] [Google Scholar]
- 48.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 49.Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J. Biol. Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- 50.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA. 2012;109:16354–16359. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice WR, Singleton FM. P2Y–purinoceptor regulation of surfactant secretion from rat isolated alveolar type II cells is associated with mobilization of intracellular calcium. Br. J. Pharmacol. 1987;91:833–838. doi: 10.1111/j.1476-5381.1987.tb11282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs. R&D. 2006;7:17–31. doi: 10.2165/00126839-200607010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Usmani SM, von Einem J, Frick M, Miklavc P, Mayenburg M, Husmann M, Dietl P, Wittekindt OH. Molecular basis of early epithelial response to streptococcal exotoxin: role of STIM1 and Orai1 proteins. Cell. Microbiol. 2012;14:299–315. doi: 10.1111/j.1462-5822.2011.01724.x. [DOI] [PubMed] [Google Scholar]
- 54.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 55.Marcet B, Libert F, Boeynaems JM, Communi D. Extracellular nucleotides induce COX-2 up-regulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. Eur. J. Pharmacol. 2007;566:167–171. doi: 10.1016/j.ejphar.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am. J. Respir. Crit. Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 58.Hartney JM, Coggins KG, Tilley SL, Jania LA, Lovgren AK, Audoly LP, Koller BH. Prostaglandin E2 protects lower airways against bronchoconstriction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L105–L113. doi: 10.1152/ajplung.00221.2005. [DOI] [PubMed] [Google Scholar]
- 59.Grossman EM, Longo WE, Mazuski JE, Panesar N, Kaminski DL. Role of cytoplasmic and secretory phospholipase A2 in intestinal epithelial cell prostaglandin E2 formation. Int. J. Surg. Ivestig. 2000;1:467–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.