Abstract
The aim of this study was to evaluate whether technetium 99m hydrazinonicotinamide (99mTc-HYNIC)-annexin V single-photon emission computed tomography (SPECT) would detect dose-dependent doxorubicin (DOX)-mediated cell death in the heart compared with functional echocardiography. Adult female Sprague-Dawley rats were treated with DOX (cumulative dose of 15 or 7.5 mg/kg) or saline (n = 7) and monitored by echocardiography. Rats were injected with 7 to 8 mCi 99mTc–HYNIC-annexin V and imaged 1 hour postinjection using a small animal dual-head SPECT/computed tomography (CT) system with multipinhole technology. Two regions of interest were drawn in the myocardium and soft tissue regions to calculate the cardiac uptake ratio (CUR) of reconstructed images. Myocardium and blood were harvested for radioactivity measurements or TUNEL assay. Biodistribution of 99mTc-HYNIC-annexin V uptake, CUR from SPECT/CT fused cardiac images, and TUNEL of myocardium demonstrated a dose-dependent toxicity response, with the cumulative 15 mg/kg DOX treatment showing the greatest degree of cell death. In contrast, echocardiography detected functional deficits only at the highest DOX dose. In vivo molecular imaging of DOX-induced cardiac toxicity with 99mTc-HYNIC-annexin V detects dose-dependent cell death before ventricular deficits are observed with echocardiography. 99mTc-HYNIC-annexin V SPECT-based molecular imaging may provide an attractive new technique for assessing early changes in myocardial function in patients undergoing DOX therapy.
DOXORUBICIN (DOX) is one of the most widely used agents for cancer treatment, with first- or second-line applications for treatment of lymphoma, leukemia, Wilms tumor, and ovarian and breast cancer,1,2 yet cardiac injury is probably the most feared complication of this important drug. DOX-induced cardiac toxicity can appear during or shortly after treatment or even months or years later.3 Cardiac toxicity limits DOX treatment to patients even if their tumors are responding favorably. Consequently, there is a need for noninvasive cardiovascular monitoring to assess early cell death before a threshold of toxicity is reached and irreversible damage occurs.
Radionuclide ventriculography and echocardiography are two methods performed before and during DOX treatment to assess ventricular function.4–6 However, it is likely that a threshold of cell death must occur before significant functional deficits are detected by either method. Endomyocardial biopsy is a third method used to detect anthracycline-mediated cardiac toxicity.7 Electron microscopy of the biopsied heart is required to evaluate ultrastructural damage to the heart. The invasiveness of biopsy makes it less desirable; thus, the development of a noninvasive method to follow the health of the heart is sought.
Cardiac cell death induced by DOX has been studied with molecular imaging.8 The uptake of indium 111 (111In)-labeled antimyosin by the myocardium occurs when the integrity of the cell membrane is lost as a result of cell damage. The intensity of the antibody uptake is related to the cumulative dose of DOX. Unfortunately, 111In-labeled antimyosin is no longer commercially available. Thus, an alternative molecular imaging target is needed to provide an effective method to monitor the adverse effects of DOX in vivo before heart function is irreversibly damaged.
Single-photon emission computed tomography (SPECT) using technetium 99m hydrazinonicotinamide (99mTc –HYNIC)-annexin V is a promising new technique for imaging cell death in vivo.9 Annexin V binds to cells that are dying, but it is not known if this binding interaction has the sensitivity to detect a dose-related response in cell death or whether it can outperform echocardiography for this indication. The aims of this study were to evaluate in vivo DOX-induced cell death in rat hearts via 99mTc-annexin SPECT/computed tomography (CT) and ex vivo biodistribution of cardiac 99mTc-annexin V and compare these findings with the TUNEL (cell death) assay and conventional echocardiography.
Material and Methods
Ten-week-old female Sprague-Dawley rats weighing 200 g were acquired from Zivic Miller (Pittsburg, PA) and acclimated for 1 week. All procedures associated with this study were reviewed and approved by the Johns Hopkins University Institutional Animal Care and Use Committee and were performed in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
Study Design
Animals were randomly assigned to one of three treatment groups: DOX treatment of 2.5 mg/kg (Novaplus, Bedford Laboratories, OH) in six weekly doses (cumulative dose of 15 mg/kg; n = 7) or three weekly doses (cumulative dose of 7.5 mg/kg; n = 7) or saline treatment (six weekly doses; n = 7). Rats were monitored for changes in cardiovascular parameters via echocardiography from weeks 8 to 10 postinitiation of treatment. Cardiac toxicity was defined by a decline in cardiac ejection fraction (EF) to ≤ 65%. DOX treatments were given via jugular venous injections under ketamine (75 mg/kg) and xylazine (10 mg/kg) anesthesia.
Transthoracic Echocardiography
Transthoracic echocardiography was performed using a Sequoia–Acuson C256 (Sequoia, Siemevs, Malvern, PA) or Visualsonics Vevo 660 Echocardiography System (Visualsonics, Ontario, Canada). Ultrasound machine operators were blinded to the experimental groups. Rats were habituated before study to immobilization inside a modified disposable rodent restrainer (Decapicone, Braintree Scientific Inc, Braintree, MA). This allowed imaging of an unanesthetized rat in approximately 3 minutes. The heart was first imaged in two-dimensional mode in the parasternal short axis for left ventricle (LV) imaging at a sweep speed of 200 mm/s. The cursor was positioned perpendicular to the interventricular septum diastole (IVSD) and left ventricular posterior wall thickness at end diastole (PWTED) at the level of the papillary muscles of the LV while the two-dimensional image was converted to an M-mode echocardiogram. From the M-mode echocardiogram image, LV chamber diameters were measured at the end of diastole (LVEDD) and systole (LVESD). EF% represents the percent change in LV chamber dimension with systolic contraction. For each rat, three values for each measurement were obtained and averaged.10
Annexin V Labeling
Recombinant human HYNIC annexin V prepared in Escherichia coli was obtained from the National Cancer Institute preclinical reagent program.11 To radiolabel the HYNIC-hydrazinonicotinamide-annexin V conjugate with 99mTc, 0.3 mL of saline containing approximately 1,000 MBq of 99mTc pertechnetate was added. Subsequently, 0.2 mL of freshly prepared stannous-tricine was added to the solution. The reaction vial was incubated for 15 to 20 minutes at room temperature. Radiochemical purity was determined chromatographically with instant thin-layer chromatography paper (Gelman Sciences, Ann Arbor, MI) using an acid citrate dextrose buffer (Sigma-Aldrich, St Louis, MO) as the mobile phase. Labeling efficiency consistently exceeded 92%, providing a specific radioactivity of 7.4 MBq/μg of protein. Under these conditions, labeled annexin V was stable for approximately 6 hours.
SPECT/CT Imaging
In vivo SPECT was performed to quantify the amount of cell death in the heart for the three treatment groups (saline, 7.5 mg/kg cumulative dose, and 15 mg/kg cumulative dose). Rats were injected with 7 to 8 mCi 99mTc-HYNIC-annexin V and imaged using a FLEX XSPECT system (Gamma Medica-Ideas, Northridge, CA) 1 hour postinjection. Two imaging methods were used: high-resolution dual-head single-pinhole imaging with 90 views and 30 s/view (total scanning time 45 minutes) or high detection efficiency five-pinhole imaging, with 90 views and 30 s/view. The data were reconstructed with an OS-EM-based three-dimensional pinhole reconstruction algorithm. CT acquisition (75 kVp, 0.24 mA, 512 projections, 0.1 s/projection) was also performed sequentially after SPECT imaging, and data were then coregistered with the corresponding SPECT images for anatomic delineation. Two regions of interest (ROIs) were drawn in the mediastinal (between the third and fourth rib) and the axillary soft tissues on SPECT images to obtain mean intensities for calculating the cardiac uptake ratio (CUR) as a semiquantitative index of cell death:
Biodistribution studies were performed to validate the SPECT results and confirm the specific uptake of 99mTc-HYNIC-annexin V. After SPECT/CT, rats were euthanized, and LV, right ventricle, blood, muscle, and fat were harvested approximately 1.5 hours after injection of radioactivity. Tissues were weighed and counted in a gamma spectrometer, and the radioactivity values were represented as a percentage of injected dose per gram. Those values were then expressed relative to radioactivity within blood.
TUNEL Assay
Rats were euthanized and received a postmortem examination, and the midwall LV was sectioned and fixed for histology and TUNEL assay evaluation. Apoptotic cells were determined using TUNEL according to the manufacturer's instructions (In Situ Cell Death Detection Kit, Fluorescein, Roche Applied Science, Basel, Switzerland). TUNEL-positive cells were determined by direct fluorescence of nuclei-incorporated deoxyuridine triphosphate in cardiomyocytes. The total number of cells per 20× field was quantified by hematoxylin-eosin staining overlaid with the TUNEL stain. TUNEL-positive nuclei were expressed as a percentage of total cells (nuclei) in each heart with five fields/heart analyzed.
Statistical Analysis
All data were presented as mean ± standard deviation and compared using one-way analysis of variance with Bonferroni correction. A level of p < .05 was accepted as statistically significant.
Results
Noninvasive Echocardiography Demonstrates Systolic Decline in High-Dose DOX Treatment
First, we evaluated ventricular systolic function with echocardiography, the conventional clinical and research method used for cardiac assessment during DOX therapy. Representative M-mode echocardiograms of LV function from each treatment group are shown in Figure 1. They demonstrate that a cumulative dose of 15 mg/kg DOX induces significant contractility deficits at 10 weeks from the initial DOX administration. Note that the 7.5 mg/kg group has normal contractility similar to that of the saline control group at the 10-week time point (see Figure 1). The lower DOX group will develop contractility deficits at 14 weeks despite showing no deficits by echocardiography at 10 weeks. At the 10-week time point, the histogram of EF%, an index of contractility, demonstrates significant loss of contractility only in the high-dose treatment group.
Figure 1.
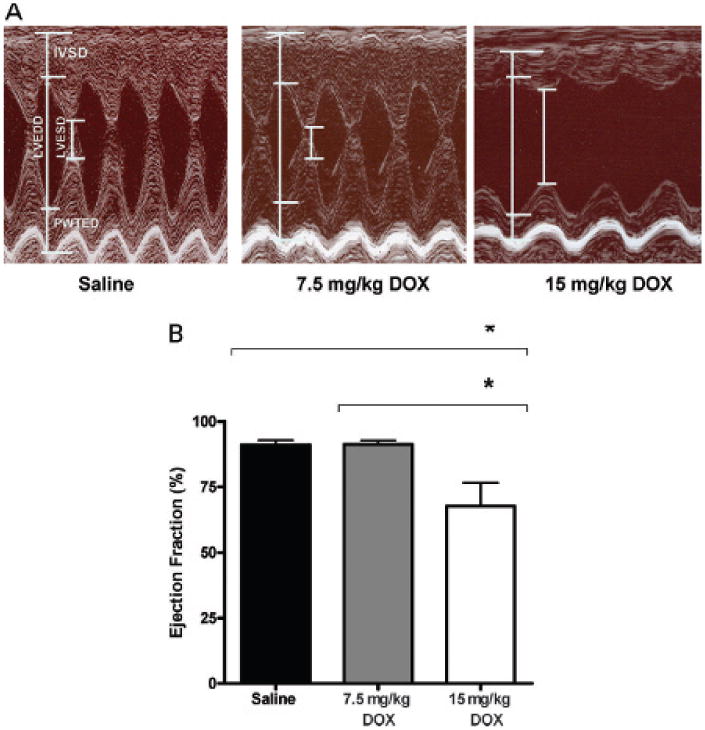
Effect of doxorubicin (DOX) on heart function. A, Representative M-mode echocardiograms of the left ventricle from each treatment group. A cumulative dose of 15 mg/kg DOX induces contractility deficits at 10 weeks from the initial injection. Left ventricle chamber at the end of systole (LVESD) and diastole (LVEDD) and the interventricular septum (IVSD) and left ventricular posterior wall (PWTED) are labeled on the M mode of the left ventricle. B, Histogram of ejection fraction percentage (index of contractility). Columns = mean (n = 7 per group); bars = standard deviation. *p < .001.
The septal and posterior ventricular wall thicknesses were also measured in representative M-mode echocardiograms and are presented as mean ± standard deviation. The wall thickness of the septum (IVSD) was 2.010 ± 0.136 mm for the saline group, 2.062 ± 0.090 mm for the 7.5 mg/kg DOX group, and 1.863 ± 0.182 mm for the 15 mg/kg DOX group and were not significantly different. The wall thickness means of the posterior wall (PWTED) were 1.998 ± 0.113 mm for the saline group, 2.020 ± 0.086 mm for the 7.5 mg/kg DOX group, and 1.815 ± 0.148 mm for the 15 mg/kg DOX group. PWTED means were significantly different between the saline and the 15 mg/kg group (p < .01) and the 7.5 and 15 mg/kg groups (p < .05).
Specific Uptake of 99mTc-HYNIC-Annexin V Is Dose Dependent
After the echocardiography, rats were next imaged by SPECT/CT. Representative images in Figure 2 demonstrate that uptake of 99mTc-HYNIC-annexin V is more intensely localized to the heart in the DOX-treated rats compared with those treated with saline. A dose-dependent effect was observed, with the highest radiopharmaceutical uptake noted in the hearts of animals receiving the highest (15 mg/kg) dose. SPECT CURs were calculated from the ROIs demonstrated in Figure 2A and are summarized in Figure 2B. The accompanying 99mTc-HYNIC-annexin V biodistribution studies of heart harvested after SPECT validate the SPECT CURs. Figure 3 summarizes the 99mTc-HYNIC-annexin V biodistribution studies. The cumulative dose of 15 mg/kg and 7.5 mg/kg DOX showed a similar dose-responsiveness with significance in the biodistribution of 99mTc-HYNIC-annexin V and CUR measurements from SPECT studies.
Figure 2.
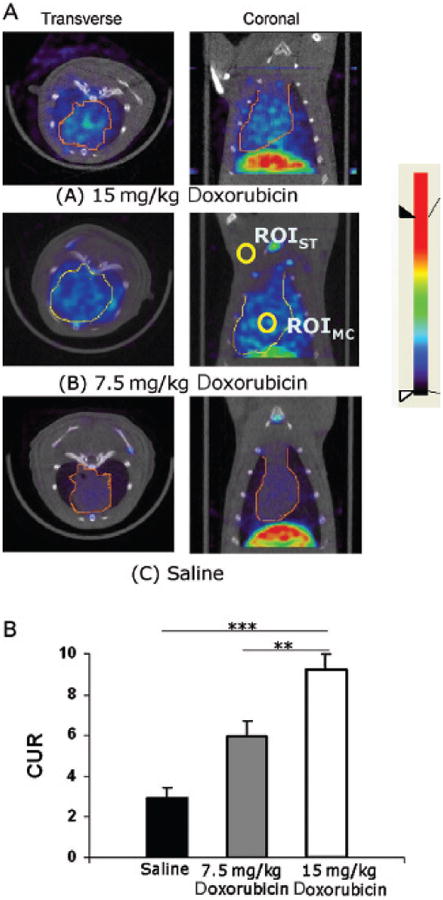
Fused SPECT/CT images delineated the three-dimensional distribution of annexin V uptake. A, From visual assessment, 15 mg/kg doxorubicin-treated rats showed the highest uptake, 7.5 mg/kg doxorubicin-treated rats showed medium uptake, and saline-treated rats expressed the lowest uptake in the heart. All images were displayed with the same color bar. B, Cardiac uptake ratio calculated with chosen regions of interest (ROIs) shown in A. Columns = mean; bars = standard deviation. **p < .01; ***p < .001. ST = soft tissue, MC = myocardium.
Figure 3.
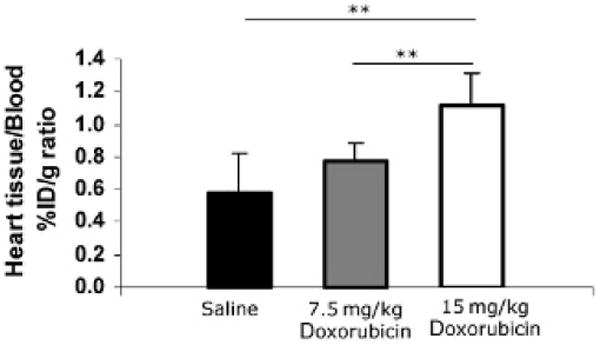
Biodistribution of 99mTc-HYNIC-annexin V in doxorubicin-induced cell death in the heart. The heart was harvested after imaging rats, and biodistribution of 99mTc-HYNIC-annexin V was measured. The percentage of injected dose per gram (%ID/g) was calculated for heart tissues (combine left ventricle and right ventricle) and normalized with the %ID/g of whole blood. Columns = mean; bars = standard deviation. **p < .01.
TUNEL Assay Identifies a Dose-Dependent Response of Cardiac Cell Death
We next used a second ex vivo method to quantify cell death in heart from the three treatments. Rats were euthanized at 10 weeks from initiation of the study, and formalin-fixed hearts were analyzed by TUNEL. Representative images of cardiac TUNEL fluorescence (converted to black/white) for the saline, 7.5 mg/kg, and 15 mg/kg groups are presented in Figure 4 (top panel), along with a summary histogram (bottom panel). The three treatment groups are significantly different, demonstrating a dose-dependent pattern; that is, the highest cell death is observed in animals receiving the highest dose of DOX.
Figure 4.
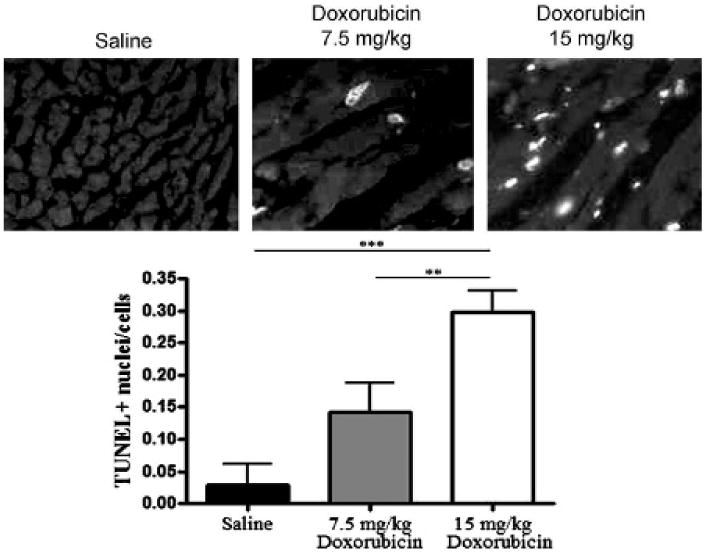
Dose response in TUNEL-positive cell death after doxorubicin. Top panel, Representative images of TUNEL fluorescence (converted to black/white) for the saline-treated and 7.5 mg/kg and 15 mg/kg doxorubicin-treated hearts. Rats were euthanized at 10 weeks, and hearts were analyzed by TUNEL assay. The white nuclei indicate the TUNEL-stained apoptotic nuclei. Bottom panel, Histogram summary of TUNEL-positive nuclei count comparing three treatment groups. Columns = mean; bars = standard deviation. **p < .01; ***p < .001.
Discussion
Cardiac toxicity is the major complication seen in patients undergoing DOX therapy for cancer. To prevent that toxicity, DOX has been prescribed with dexrazoxane or as a liposomal preparation, yet these two preventive methods are neither effective nor widely used.12 Cardiac toxicity also occurs with other cancer therapies, creating the need for methods to monitor cardiac cell death repeatedly and noninvasively.12 Methods to monitor cardiac cell death would also be advantageous in patients with myocardial infarction, diabetes, atherosclerosis, hypertension, or transplant rejection9; however, the sensitivity of detecting cardiac toxicity needs to be investigated in each case. We used a rat model10 to explore whether 99mTc-HYNIC- annexin V SPECT would detect dose-dependent DOX-induced cell death. We also temporally compared the SPECT findings with conventional echocardiography, the current clinical standard.
In this study, radiopharmaceutical-based imaging detected toxicity before ultrasonography. DOX treatment induced a significant decline in heart function measured by transthoracic echocardiography in the 15 mg/kg dose group but not in the 7.5 mg/kg dose group at the 10-week evaluation. Rats receiving the higher dose (15 mg/kg) were euthanized when the EF dropped to 65% or lower, which usually occurred within the tenth week after the first dose of doxorubicin. Rats receiving the lower dose (7.5 mg/kg) do progress to heart failure, but in a delayed manner. In this study, three rats from the 7.5 mg/kg group were not euthanized at 10 weeks but were monitored by ultrasonography for the next 4 weeks. Between weeks 14 and 15, each of these rats progressed to heart failure (EF 55% ± 3). The TUNEL assay, SPECT/CT, and biodistribution studies all demonstrated that the rats receiving the lower dose showed significant cell death compared with controls but significantly less than the higher-dose group. The cell death that occurs in the DOX cardiac toxicity model is reflected in the reduction of wall thickness observed in the 15 mg/kg group. PWTED means were significantly different between the saline and the 15 mg/kg dose group (p < .01) and the 7.5 and 15 mg/kg groups (p < .05). The posterior wall thickness reduction coincides with the increase in cell death. Thus, noninvasive SPECT detected and imaged cell death in rats with normal cardiac function and predicted the development of cardiomyopathy.
Although planar images of DOX-treated rats demonstrate increased uptake of annexin V in the heart, the effect of dose has not yet been evaluated by SPECT molecular imaging.13 A noninvasive method to detect cell death would be useful in screening for drugs that have been shown to induce synergistic cardiac toxicity, for example, anti-erbB2 and DOX.12 In our study, dose-dependent effects in cell death were observed and quantified by SPECT to demonstrate that the high-dose DOX treatment group had significantly higher 99mTc-HYNIC-annexin V uptake compared with the lower-dose group. The saline-treated group had the lowest radiopharmaceutical uptake. Our study establishes that 99mTc-HYNIC-annexin V imaging may be used to evaluate a dose response in cardiac cell death.
The specificity of annexin V binding in this in vivo imaging method is based on the biologic phenomenon that occurs in the early stages of apoptosis, for example, the exposure of phosphatidylserine on the outer surface of the cell.9 Under normal conditions, phosphatidylserine is restricted to the inner leaflet of the plasma membrane and is thus hidden from the extracellular environment. During apoptosis, phosphatidylserine is exposed on the outer cellular surface, and its high affinity for annexin V allows this phenomenon to be imaged with radiolabeled annexin V and SPECT.13 Consequently, to validate that our SPECT results reflected cell death in vivo, ex vivo 99mTc-HYNIC-annexin V biodistribution studies and TUNEL assays were performed on the tissues of interest. The TUNEL assay is a commonly used method to quantify cell death by measuring the number of 3′ ends of nuclear deoxyribonucleic acid (DNA), a phenomenon that occurs in cell death, most notably in apoptosis. We found that results from both the biodistribution of cardiac 99mTc-HYNIC-annexin V and the TUNEL assay demonstrated a significant incremental pattern that paralleled SPECT CUR, validating the in vivo method with the ex vivo methods for detecting cell death.
The dose of DOX used in this model was based on a rat DOX study that used the 2.5 mg/kg dose for 10 consecutive weeks.14 In our early studies, we observed that a treatment schedule of 6 weekly doses of 2.5 mg/kg was sufficient to induce toxicity; thus, we modified this model and decreased the weekly doses from 10 to 6. We further hypothesized that a threshold dose of DOX is needed to induce toxicity. To test this hypothesis, rats were given only three consecutive doses (2.5 mg/kg) of DOX, and, surprisingly, we also observed heart failure. At this lower dose, presentation of heart failure occurred in a delayed manner; for example, heart failure occurred 2 months after the last dose (versus 1 month in the six weekly doses). This suggests that some models of DOX exceed the threshold dose. Since there are no published doses that are subthreshold, we are now in the process of reducing the dose of DOX to observe if a threshold dose exists and if cardiac function deficits are reversible.
Rats are dosed with DOX using the mg/kg method, whereas humans are dosed using the mg/m2 method. A conversion table at <http://www.accessdata.fda.gov/scripts/cder/onctools/animalresults.cfm> demonstrates the conversion between the two dosing methods. Multiply the conversion factor for the species (rat is 6) by the animal dose in mg/kg to obtain the dose in mg/m2 for a human dose equivalent. For example, a cumulative dose of (7.5 mg/kg) (6) = 45 mg/m2 is the estimate dose in mg/m2, human dose equivalent. This cumulative dose of 45 mg/m2 for rats induces cardiac toxicity. This dose is 10 times lower than the cumulative dose given to cancer patients for treatment.
For example, to compare rat cardiac toxicity with human cardiac toxicity, cancer patients given 400 mg/m2 of DOX have an incidence of congestive heart failure between 2 and 5% in follow-up studies. DOX toxicity is exponentially dose dependent and increases dramatically when the cumulative dose reaches 500 mg/m2.1,2 Unfortunately, long-term cardiac follow-ups of cancer patients can be limited, and some patients may die owing to cancer progression. In patients with cancer remission or cancer cure, if heart toxicity develops, it may develop years to decades from the initial exposure to DOX,3 similar to the delayed heart failure we observe in rats 2 months after their last DOX treatment in the 7.5 mg/kg model.
We undertook this study to ask whether molecular SPECT methods would detect DOX toxicity before echocardiography methods. To detect DOX toxicity, planar imaging techniques have been used to differentiate between control and DOX treatment groups. We applied these methods and asked whether a dose-related effect in DOX toxicity could be detected in vivo noninvasively. Using the multipinhole SPECT approach, we were able to detect a dose-dependent effect of cell death induced by DOX. The multiple techniques provide increased sensitivity over the conventional single-pinhole collimator used in small animal imaging studies.15,16 The ability of SPECT to detect toxicity differences between treatment groups allows for future studies in which multidrug protocols can be compared for potentiation or protection from cardiac toxicity. In conclusion, SPECT with 99mTc-HYNIC-annexin V is a promising noninvasive technique for monitoring and quantifying cardiac cell death at a critical stage before functional deficits are observed by conventional echocardiography. With this method, investigators should be able to evaluate other animal models longitudinally to understand the factors that control cardiac cell death. Our results also suggest that molecular imaging of apoptosis should be pursued vigorously in the clinical evaluation of patients undergoing therapy with DOX.
Acknowledgments
We appreciate the experimental assistance of James Fox and Gilbert Green.
This work was supported in part by American Heart Association Scientist Development Grant (AHA SDG) #0335273N, Department of Defense #BC030727, a Spore in Breast Cancer Developmental Research Award #P50CA88843-04, and National Institutes of Health research grants EB1558 and 624 CA92871.
References
- 1.Blum RH, Carter SK. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med. 1974;80:249–59. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- 2.Allen A. The cardiotoxicity of chemotherapeutic drugs. Semin Oncol. 1992;19:529–42. [PubMed] [Google Scholar]
- 3.Bristow MR, Billingham ME, Mason JW, Daniels JR. Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep. 1978;62:873–9. [PubMed] [Google Scholar]
- 4.Mitani I, Jain D, Joska T, et al. Doxorubicin cardiotoxicity: prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10:132–9. doi: 10.1067/mnc.2003.7. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt K, Tulzer G, Merl M, et al. Early detection of doxorubicin and daunorubicin cardiotoxicity by echocardiography: diastolic versus systolic parameters. Eur J Pediatr. 1995;154:201–4. doi: 10.1007/BF01954271. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–18. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 7.Billingham M. Role of endomyocardial biopsy in diagnosis and treatment of heart disease. In: Silver MD, editor. Cardiovascular pathology. New York: Churchill Livingstone; 1991. pp. 1465–86. [Google Scholar]
- 8.Carrio I, Estorch M, Berna L, et al. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med. 1995;36:2044–9. [PubMed] [Google Scholar]
- 9.Korngold EC, Jaffer F, Weissleder R, et al. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev. 2008;13:163–73. doi: 10.1007/s10741-007-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrielson K, Bedja D, Pin S, et al. Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Res. 2007;67:1436–41. doi: 10.1158/0008-5472.CAN-06-3721. [DOI] [PubMed] [Google Scholar]
- 11.Lahorte CM, Vanderheyden J, Steinmetz N, et al. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging. 2004;31:887–919. doi: 10.1007/s00259-004-1555-4. [DOI] [PubMed] [Google Scholar]
- 12.Ng R, Green MD. Managing cardiotoxicity in anthracycline-treated breast cancers. Expert Opin Drug Saf. 2007;6:315–21. doi: 10.1517/14740338.6.3.315. [DOI] [PubMed] [Google Scholar]
- 13.Bennink RJ, Vandenhoff M, Van Hemert F, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45:842–8. [PubMed] [Google Scholar]
- 14.Schwarz ER, Pollick C, Dow J, et al. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res. 1998;39:216–23. doi: 10.1016/s0008-6363(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 15.Beekman FJ, Vander Have F, Vastenhouw B, et al. U-SPECT-I: a novel system for submillimeter-resolution tomography with radiolabeled molecules in mice. J Nucl Med. 2005;46:1194–200. [PubMed] [Google Scholar]
- 16.Pissarek MB, Oros-Peusquens AM, Schramm NU. Challenge by the murine brain: multi-pinhole SPECT of (123)I-labelled pharmaceuticals. J Neurosci Methods. 2008;168:282–92. doi: 10.1016/j.jneumeth.2007.10.011. [DOI] [PubMed] [Google Scholar]


