Abstract
BACKGROUND AND OBJECTIVES:
Although many adults experience resource-intensive and costly health care in the last year of life, less is known about these health care experiences in children with life-threatening complex chronic conditions (LT-CCCs). We assessed hospital resource use in children by type and number of LT-CCCs.
METHODS:
A retrospective analysis of 1252 children with LT-CCCs, ages 1 to 18 years, who died in 2012 within 40 US children’s hospitals of the Pediatric Health Information System database. LT-CCCs were identified with International Classification of Diseases, 9th Revision, Clinical Modification codes. Using generalized linear models, we assessed hospital admissions, days, costs, and interventions (mechanical ventilation and surgeries) in the last year of life by type and number of LT-CCCs.
RESULTS:
In the last year of life, children with LT-CCCs experienced a median of 2 admissions (interquartile range [IQR] 1–5), 27 hospital days (IQR 7–84), and $142 562 (IQR $45 270–$410 087) in hospital costs. During the terminal admission, 76% (n = 946) were mechanically ventilated; 36% (n = 453) underwent surgery. Hospital use was greatest (P < .001) among children with hematologic/immunologic conditions (99 hospital days [IQR 51–146]; cost = $504 145 [IQR $250 147–$879 331]) and children with ≥3 LT-CCCs (75 hospital days [IQR 28–132]; cost = $341 222 [IQR $146 698–$686 585]).
CONCLUSIONS:
Hospital use for children with LT-CCCs in the last year of life varies significantly across the type and number of conditions. Children with hematologic/immunologic or multiple conditions have the greatest hospital use. This information may be useful for clinicians striving to improve care for children with LT-CCCs nearing the end of life.
What’s Known on This Subject:
Children with life-threatening complex chronic conditions (LT-CCCs) experience high hospital use.
What This Study Adds:
Hospital use in the last year of life for these children varies by type and number of LT-CCCs. Most children with ≥3 LT-CCCs are admitted to the hospital for more than 2 months in the last year of life.
Among adults with chronic conditions, health care is most resource-intensive and costly in the last year of life.1,2 Invasive interventions and lengthy hospitalizations are common during this time.1–5 Although helpful in some circumstances to alleviate the burden of illness, this care may be associated with greater patient distress.6 In contrast, less is known about the health care experience of children in the last year of life, especially for those with life-threatening complex chronic conditions (LT-CCCs). With diagnoses such as cerebral palsy and complex congenital heart disease, children with LT-CCCs represent 25% of childhood deaths.7–12 Studies from previous decades report that many children with LT-CCCs rely heavily on hospitals at the end of life, often with admissions lasting weeks.11,13
In recent years, hospital care for children with LT-CCCs has evolved considerably. Children are increasingly admitted to the hospital for invasive procedures and initiation of medical technologies to maintain health.14–17 Consequently, children with LT-CCCs now account for a substantial proportion of pediatric hospital resource use.17–20 Although advancements in hospital care have improved life expectancy for some children with LT-CCCs, many ultimately acquire multiple comorbid conditions as they age. Having multiple conditions may escalate hospital use and heighten health inequities.8,21–24
In this context, current investigation into the hospital experience for children with LT-CCCs in the last year of life is warranted. This multicenter study (1) describes the characteristics, hospital use, and costs for children with LT-CCCs in the last year of life, (2) compares resource use by type and number of conditions, and (3) examines major medical interventions received in the terminal admission.
Methods
Study Design
We conducted a retrospective cohort analysis of the Pediatric Health Information System (PHIS) database. PHIS is an administrative database containing inpatient utilization and cost data from freestanding US children’s hospitals. PHIS is managed by the Children’s Hospital Association, a business alliance of children’s hospitals. Data reliability and validity are monitored by member hospitals, Children’s Hospital Association (Overland Park, KS), and Truven Health Analytics (Ann Arbor, MI). Unique identifiers are assigned to each child in PHIS to follow children across multiple admissions. This study was approved by the institutional review board at Boston Children’s Hospital, with a waiver of informed consent.
Population
Our cohort comprised 1252 children with LT-CCCs, ages 1 to 18 years, who died in 1 of 40 PHIS hospitals between January 1 and December 31, 2012. All children in this study had at least 1 recorded admission to the hospital in the last year of life. Infants <1 year of age at the time of death were excluded to ensure a 1-year retrospective review of hospital use for all children in the study.
To identify children with LT-CCCs, we used Feudtner and colleagues’ complex chronic conditions (CCCs).7 Using diagnosis codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), CCCs are conditions that last at least 1 year, are severe enough to necessitate subspecialty pediatric care, and bear the highest risk of mortality out of all chronic conditions in childhood.7 In this article, we refer to CCCs as life-threatening (ie, LT-CCCs) because all children with them in our cohort died.
There are 9 organ system–based CCC types: cardiovascular, congenital/genetic, gastrointestinal, hematologic/immunologic, malignancy, metabolic, neuromuscular, renal and respiratory. Each CCC type contains relevant diagnoses (eg, heart and great vessel malformations for children with a cardiovascular CCC). Children may carry multiple diagnoses within each CCC, and they may also have multiple types of CCCs, such as coexisting neuromuscular and hematologic/immunologic CCCs. CCCs correlate with hospital resource use.7,9–11,13,15,17,25
Demographic Characteristics
Demographic characteristics analyzed were age, gender, race/ethnicity, and primary health insurance.
Outcome Measures
Outcome measures were hospital resource use and major medical interventions. We characterized hospital resource use by number of hospital admissions, aggregate days in the hospital (ie, total and ICU hospital days), and hospital costs for children in the last year of life by type and number of LT-CCCs. Charges were converted to costs by using an existing cost-to-charge ratio for each hospital, adjusting for inflation via the consumer price index for hospital and related services.14
Major medical interventions included mechanical ventilation, surgery, and new use of medical technology in the terminal admission. Mechanical ventilation was identified if a patient had an ICD-9-CM procedure code for mechanical ventilation (96.70, 96.71, 96.72), or if a patient had a hospital charge for a clinical transaction classification (CTC) code of mechanical ventilation (521166) or other specified ventilation assistance (521169). Surgery was identified if a patient was billed for operating room services with CTC code 611110. We used ICD-9-CM diagnostic and procedure codes described in previous studies to identify new medical technology inserted in the terminal admission.14–16,26,27 Medical technology refers to any device that helps maintain health status and physiologic function (eg, gastrostomy or tracheostomy tube).14
Statistical Analysis
Hospital utilization was reported with median and interquartile range (IQR) because these data were not normally distributed. Generalized linear models were used to assess the effect of type and number of LT-CCCs on hospital days and costs. For all models, we assumed a Poisson distribution for the outcomes and used a random effect for hospital to account for clustering. Statistical significance was defined as P < .05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Univariable models contained only a fixed effect for the variable of interest (eg, number of LT-CCCs). Type III block tests were used in univariable models to assess whether the variable of interest was statistically associated with the outcome. The Kruskal-Wallis test was used to test outcomes between specific pairs of conditions (eg, comparison of hospital days between children with a hematologic/immunologic versus neuromuscular condition).
Multivariable models contained fixed effects for all demographic and clinical characteristics. Two types of multivariable models were derived. The first type, which included all children in the cohort, assessed which specific LT-CCCs, when compared with each other, were associated with the greatest impact on hospital use. The second type assessed which combinations of LT-CCCs were associated with the greatest impact on hospital use. The second type of model was used for the 3 most prevalent types of LT-CCCs in the cohort: neuromuscular, cardiovascular, and malignancy.
Results from multivariable analyses are presented in 2 ways: (1) with rate ratios (95% confidence intervals) to report the relative impact of a particular variable on hospital resource use (eg, rate ratio of hospital days in the presence versus absence of hematologic/immunologic LT-CCCs), and (2) with exponentiation of the model coefficients to report the absolute impact of a particular variable on hospital resource use (eg, how many additional hospital days were experienced when a hematologic/immunologic LT-CCC was present).
Results
Demographic and Clinical Characteristics of the Study Cohort
Median age at in-hospital death for 1252 children with LT-CCCs was 8 years (IQR 3–14 years). Youngest age at death was observed in children with respiratory LT-CCCs (median age 3 [IQR 2–12] years); oldest age at death was observed in children with gastrointestinal LT-CCCs (median age 11 [IQR 3–16] years). Fifty-five percent (n = 693) of children were boys; 44.6% (n = 558) were non-Hispanic white; and 69.8% (n = 874) used public health insurance (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of 1252 Children with LT-CCCs who Died in Children’s Hospitals in 2012
| Characteristic | Finding |
|---|---|
| Age at death, y, median (IQR) | 8 (3–14) |
| Gender,a n (%) | |
| Female | 558 (44.6) |
| Male | 693 (55.4) |
| Race/Ethnicity, n (%) | |
| White, non-Hispanic | 558 (44.6) |
| Hispanic | 276 (22.0) |
| Black, non-Hispanic | 176 (14.1) |
| Asian or Pacific Islander, non-Hispanic | 56 (4.5) |
| Other/Missing | 186 (14.9) |
| Type of primary health insurance, n (%) | |
| Public | 874 (69.8) |
| Commercial | 303 (24.2) |
| Self-pay | 75 (6.0) |
| LT-CCCs: types and diagnoses, n (%) | |
| Neuromuscular | 581 (46.4) |
| Epilepsy | 326 (26.0) |
| Central nervous system degradation/ disease | 285 (22.8) |
| Brain/ spinal cord malformations | 127 (10.1) |
| Infantile cerebral palsy | 160 (12.8) |
| Mental retardation | 79 (6.3) |
| Muscular dystrophies/ myopathies | 17 (1.4) |
| Cardiovascular | 569 (45.4) |
| Conduction disorders/dysrhythmias | 435 (34.7) |
| Heart/ great vessel malformations | 218 (17.4) |
| Cardiomyopathies | 74 (5.9) |
| Malignancy | 455 (36.3) |
| Solid malignancy | 215 (17.2) |
| Hematologic malignancy | 204 (16.3) |
| Secondary malignancy | 146 (11.7) |
| Metabolic | 337 (26.9) |
| Other metabolic disordersb | 295 (23.6) |
| Lipid metabolism | 36 (2.9) |
| Amino acid metabolism | 30 (2.4) |
| Carbohydrate metabolism | 10 (0.8) |
| Storage disorders | 7 (0.6) |
| Congenital/Genetic | 282 (22.5) |
| Bone/ joint anomalies | 132 (10.5) |
| Chromosomal anomalies | 128 (10.2) |
| Other congenital anomaliesc | 52 (4.2) |
| Diaphragm/ abdominal wall | 17 (1.4) |
| Hematologic/Immunologic | 206 (16.5) |
| Hereditary immunodeficiency | 178 (14.2) |
| Sickle cell disease | 18 (1.4) |
| Hereditary anemias | 15 (1.2) |
| Acquired immunodeficiency | 5 (0.4) |
| Respiratory | 124 (9.9) |
| Respiratory malformations | 65 (5.2) |
| Chronic respiratory disease | 51 (4.1) |
| Cystic fibrosis | 21 (1.7) |
| Renal | 99 (7.9) |
| Chronic renal failure | 81 (6.5) |
| Congenital anomalies | 27 (2.2) |
| Gastrointestinal | 93 (7.4) |
| Chronic liver disease/ cirrhosis | 77 (6.2) |
| Inflammatory bowel disease | 9 (0.7) |
| Congenital anomalies | 8 (0.6) |
| Number of LT-CCCs, n (%) | |
| 1 | 425 (34.0) |
| 2 | 396 (31.6) |
| ≥3 | 431 (34.4) |
Gender is missing for 1 child in the cohort.
Other metabolic disorders include disorders of iron, copper, phosphorus, magnesium, purine, and pyrimidine metabolism.
Other congenital anomalies include multiple congenital anomalies, other specified anomalies, congenital anomaly unspecified.
The 3 most prevalent LT-CCC types were neuromuscular (46.4%, n = 581), cardiovascular (45.4%, n = 569), and malignancy (36.3%, n = 455) (Table 1). The most common diagnosis within each of these LT-CCC types were epilepsy (26.0%, n = 326), conduction disorders or dysrhythmias (34.7%, n = 435), and solid malignancies (17.2%, n = 215). Thirty-four percent (n = 425) of children had 1 LT-CCC, 31.6% (n = 396) had 2 LT-CCCs, and 34.4% (n = 431) had ≥3 LT-CCCs (Table 1). Among children with multiple LT-CCCs, the most common combinations of LT-CCCs were neuromuscular and congenital/genetic (4.5%, n = 56), neuromuscular and malignancy (4.4%, n = 55), and neuromuscular and cardiovascular (4.1%, n = 51).
Hospital Utilization and Costs in the Last Year of Life
Overall Cohort
In the last year of life, children with LT-CCCs experienced a median of 2 hospital admissions (IQR 1–5), 27 hospital days (IQR 7–84), and $142 562 (IQR $45 270–$410 087) in hospital costs. Total hospital costs were $392 million, of which 58% ($228 million) occurred in the terminal admission.
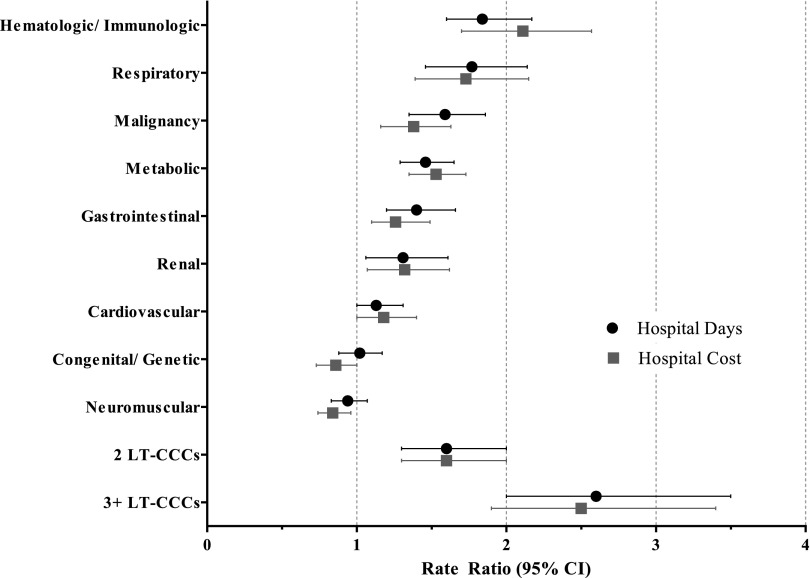
Hospital resource use varied significantly by type of LT-CCC (Table 2). For example, in univariable analyses, children with neuromuscular LT-CCCs had the fewest hospital days (median 24 days [IQR 6–66]), and children with hematologic/immunologic LT-CCCs had the greatest number of hospital days (median 99 days [IQR 51–146]) (P < .001). Immunodeficiencies (eg, severe combined immunodeficiency) were the most common hematologic/immunologic LT-CCCs (Table 1). In multivariable analyses, hematologic/immunologic LT-CCCs had the greatest impact on hospital resource use (Fig 1). When compared with other LT-CCCs, children with a hematologic/immunologic LT-CCC spent 45 (SE 6) more days in the hospital and accumulated $326 844 (SE 56 143) more in hospital costs in their last year of life (P < .001 for both).
TABLE 2.
Hospital Utilization in the Last Year of Life for Children With LT-CCCs Who Died in Children’s Hospitals in 2012
| Type and No. of LT-CCCs | No. of Admissions | Hospital Days | ICU Days | Hospital Cost, $, in Thousands | ||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Pa | Median (IQR) | Pa | Median (IQR) | Pa | Median (IQR) | Pa | |
| Overall cohort | 2 (1–5) | NA | 27 (7–84) | NA | 7 (1–21) | NA | 143 (45–410) | NA |
| Type of LT-CCCb | ||||||||
| Hematologic/ Immunologic | 4 (2–7) | <.001 | 99 (51–146) | <.001 | 12 (2–35) | <.001 | 504 (250–879) | <.001 |
| Gastrointestinal | 4 (2–7) | 92 (29–148) | 14 (6–33) | 483 (200–748) | ||||
| Metabolic | 4 (2–8) | 79 (29–132) | 12 (3–34) | 378 (145–732) | ||||
| Renal | 4 (1–7) | 75 (26–144) | 19 (4–56) | 368 (147–817) | ||||
| Respiratory | 3 (2–6) | 63 (21–126) | 16 (4–44) | 299 (124–588) | ||||
| Malignancy | 5 (2–8) | 59 (22–113) | 6 (1–18) | 278 (106–582) | ||||
| Congenital/ Genetic | 3 (1–5) | 31 (9–74) | 9 (2–26) | 145 (57–322) | ||||
| Cardiovascular | 2 (1–5) | 29 (6–92) | 9 (2–28) | 171 (51–473) | ||||
| Neuromuscular | 2 (1–5) | 24 (6–66) | 7 (2–20) | 109 (38–290) | ||||
| No. of LT-CCCs | ||||||||
| 1 | 1 (1–2) | <.001 | 8 (2–25) | <.001 | 3 (1–11) | <.001 | 60 (24– 152) | <.001 |
| 2 | 2 (1–5) | 27 (8–68) | 6 (1–21) | 129 (48–352) | ||||
| ≥3 | 4 (2–7) | 75 (28–132) | 12 (3–35) | 341 (147–687) | ||||
NA, not applicable.
P values were obtained from Kruskal-Wallis tests; statistical significance is defined as a P < .05.
In this table, LT-CCCs are ranked in order from greatest to least hospital days and cost.
FIGURE 1.
Multivariable analysis of hospital days and costs for children in the last year of life, by type and number of LT-CCCs. Shown are adjusted rate ratios and 95% confidence intervals for hospital resource use by type and number of LT-CCCs. For each type of LT-CCC, the reference group is the absence of that particular condition. For the number of LT-CCCs, the reference group is 1 LT-CCC. Rate ratios are adjusted for demographic (age, race/ethnicity, insurance type) and clinical (mechanical ventilation, surgery, new medical technology) characteristics.
Impact of Multiple Conditions
As children’s total number of LT-CCCs increased from 1 to ≥3, the median number of admissions increased from 1 (IQR 1–2) to 4 (IQR 2–7); median hospital days increased from 8 (IQR 2–25) to 75 (IQR 28–132); and median hospital costs increased from $59 732 (IQR $23 509-$152 399) to $341 222 (IQR $146 698–$686 585) (P < .001 for all) (Table 2). In multivariable analyses, hematologic/immunologic LT-CCCs had the greatest impact on increasing hospital resource use for children with multiple LT-CCCs. Specifically, the presence of a hematologic/immunologic LT-CCC added 58 hospital days (SE 7) in children with a neuromuscular LT-CCC, 47 hospital days (SE 8) in children with a cardiovascular LT-CCC, and 38 hospital days (SE 6) in children with a malignancy LT-CCC (P < .001 for all) (Table 3). In contrast, neuromuscular LT-CCCs reduced hospital resource use among children with multiple LT-CCCs. The presence of a neuromuscular LT-CCC was associated with 29 fewer hospital days (SE 5) in children with a malignancy LT-CCC (P < .001) and 8 fewer hospital days (SE 4) in children with a cardiovascular LT-CCC (P = .03) (Table 3).
TABLE 3.
Effect of Multiple LT-CCCs on Hospital Days in the Last Year of Life for Children With Neuromuscular, Cardiovascular, and Malignancy Conditions
| Type and No. of Additional LT-CCCs | Neuromusculara | Cardiovasculara | Malignancya | |||
|---|---|---|---|---|---|---|
| Hospital Days | Pb | Hospital Days | Pb | Hospital Days | Pb | |
| Median (IQR) hospital days by no. of LT-CCCs | ||||||
| 1 LT-CCC | 5 (2–15) | <.001 | 6 (2–22) | <.001 | 21 (9–51) | <.001 |
| ≥2 LT-CCCs | 32 (11–77) | 46 (11–112) | 75 (31–123) | |||
| Effect of additional LT-CCCs on hospital days (SE)c,d | ||||||
| Hematologic/ Immunologic | +58 (7) | <.001 | +47 (8) | <.001 | +38 (6) | <.001 |
| Gastrointestinal | +50 (9) | <.001 | +35 (9) | <.001 | +18 (8) | .02 |
| Metabolic | +42 (4) | <.001 | +36 (5) | <.001 | +33 (5) | <.001 |
| Renal | +40 (8) | <.001 | +27 (9) | <.01 | +13 (9) | .2 |
| Malignancy | +36 (5) | <.001 | +29 (6) | <.001 | NAe | NA |
| Respiratory | +32 (6) | <.001 | +22 (7) | <.01 | +3 (9) | .8 |
| Cardiovascular | +17 (4) | <.001 | NAe | NA | −9 (6) | .1 |
| Congenital/ Genetic | +5 (4) | .2 | −6 (4) | .1 | −22 (7) | <.001 |
| Neuromuscular | NAe | NA | −8 (4) | .03 | −29 (5) | <.001 |
NA, not applicable.
Neuromuscular, cardiovascular, and malignancy were selected for presentation because they are the 3 most prevalent conditions among children in the cohort.
P values were obtained from a type III block test on the fixed effect of interest (eg, type of LT-CCC) that was included in generalized linear models; statistical significance is defined as a P value of < .05.
Shown are greater (+) or fewer (−) hospital days with SE experienced in the presence of a specific additional LT-CCC. For example, among children with a neuromuscular LT-CCC, the presence of an additional hematologic/immunologic LT-CCC added 58 (SE 7) hospital days in the last year of life. Hospital days and SE were estimated from generalized linear models.
LT-CCCs are ranked in order from greatest to least added hospital days for children with neuromuscular conditions.
Not applicable, as every child in the cohort of interest has this condition.
Health Care Experiences in the Last Year of Life
ICU
Seventy-nine percent (n = 987) of children received care in the ICU in the last year of life, with a median of 7 ICU days (IQR 1–21). The number of ICU days was greatest in children with a renal condition (median 19 days [IQR 4–56]) and fewest in children with a malignancy (median 6 days [IQR 1–18]), P < .001 (Table 2). As children’s total number of LT-CCCs increased from 1 to ≥3, the median number of ICU days increased from 3 (IQR 1–11) to 12 (IQR 3–35) (P < .001).
Terminal Admission
Children spent a median of 8 days (IQR 2–29) in hospital for their terminal admission, with a median of 3 ICU days (IQR 1–13). Hospital days in the terminal admission were greatest among children with a hematologic/immunologic condition (median 40 days [IQR 12–78]) and fewest among children with a neuromuscular condition (median 6 days [IQR 2–20]) (P < .001) (Table 4). According to the recorded admission type, 14% (n = 180) of children were “electively” admitted for their terminal admission.
TABLE 4.
Hospital Resource Use and Interventions in the Terminal Admission for Children With LT-CCCs Who Died in Children’s Hospitals in 2012
| Type of LT-CCCa | Admitted Electively | Hospital Resource Use | Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital Days | ICU Days | Hospital Cost, $, in Thousands | Mechanical Ventilationb | Surgeryc | New Medical Technologyd | ||||||
| n | % | Median (IQR) | Median (IQR) | Median (IQR) | n | % | n | % | n | % | |
| Overall Cohort | 180 | 14.4 | 8 (2–29) | 3 (1–13) | 55 (19–184) | 946 | 75.6 | 453 | 36.2 | 67 | 5.4 |
| Hematologic/ Immunologic | 49 | 31.0 | 40 (12–78) | 8.5 (1–28) | 298 (78–623) | 123 | 77.9 | 89 | 56.3 | 10 | 6.3 |
| Metabolic | 57 | 24.8 | 26 (7–64) | 9.5 (2–26) | 165 (51–479) | 185 | 80.4 | 114 | 49.6 | 18 | 7.8 |
| Gastrointestinal | 15 | 23.8 | 25 (12–50) | 13 (1–23) | 196 (99–439) | 52 | 82.5 | 34 | 54.0 | 5 | 7.9 |
| Renal | 16 | 20.0 | 22 (5–47) | 11 (1–31) | 139 (31–386) | 63 | 78.8 | 38 | 47.5 | 9 | 11.3 |
| Respiratory | 8 | 8.9 | 19 (4–57) | 8 (1–30) | 140 (25–402) | 71 | 78.9 | 42 | 46.7 | 10 | 11.1 |
| Malignancy | 78 | 17.9 | 14 (4–36) | 2 (0–11) | 71 (22–238) | 232 | 53.3 | 154 | 35.4 | 18 | 4.1 |
| Congenital/ Genetic | 30 | 14.5 | 9 (2–31) | 4 (1–14) | 57 (19–184) | 171 | 82.6 | 70 | 33.8 | 12 | 5.8 |
| Cardiovascular | 83 | 17.0 | 8 (2–32) | 5 (1–17) | 69 (24–232) | 426 | 87.1 | 205 | 41.9 | 25 | 5.1 |
| Neuromuscular | 56 | 10.9 | 6 (2–20) | 4 (1–11) | 39 (16–118) | 408 | 79.4 | 155 | 30.2 | 32 | 6.2 |
Conditions are ranked in order from greatest to least hospital days.
Identified by ICD-9-CM procedure and CTC codes for mechanical ventilation or other specified ventilation assistance.
Identified by a CTC code for operating room services.
Identified by ICD-9-CM procedure codes for gastrostomy, tracheostomy, ventriculoperitoneal shunt, baclofen pump, and other technologies inserted in the terminal admission.
Seventy-six percent (n = 946) of children were mechanically ventilated during the terminal admission (Table 4). The highest percentage of mechanical ventilation occurred in children with cardiovascular conditions (87.1%, n = 426); the lowest percentage occurred in children with malignancies (53.3%, n = 232). Thirty-six percent (n = 453) of children underwent a surgery or major procedure in the terminal admission. The highest percentage of surgeries/procedures occurred in children with hematologic/immunologic conditions (56.3%, n = 89); the lowest percentage occurred in children with neuromuscular conditions (30.2%, n = 155). The most common procedure in children with a hematologic/immunologic condition was stem cell transplantation (20.2%, n = 32). Five percent (n = 67) of children with LT-CCCs underwent insertion of a new medical technology.
Discussion
This study suggests that hospital use for children with LT-CCCs in the last year of life varies considerably by type and number of conditions. Across different types of LT-CCCs, the highest and lowest hospital use, respectively, were attributable to children with hematologic/immunologic and neuromuscular conditions. Two-thirds of children had multiple LT-CCCs, which was a major factor contributing to increased hospitalizations, days spent in the hospital, and cost during the last year of life. For example, most children with ≥3 LT-CCCs experienced at least 4 hospitalizations that collectively accounted for >2 months of the last year of life. Clinicians may find this information useful as they strive to optimize care for children with LT-CCCs near the end of life.
Although many children with LT-CCCs in the current study spent a sizeable amount of time in the hospital during the last year of life, we are not positioned to gauge whether this hospital use may have been excessive or insufficient. Some children, families, and clinicians may have perceived the hospital to be the most appropriate setting for care due to illness acuity or complexity of needs, even if months of hospital care were needed.14,15 Admissions may have been attempted or prolonged with the expectation that hospital interventions, such as surgery, might result in a better prognosis or improved symptom management.26,27 Additional investigation is needed to explore these situations in detail and to improve our understanding of how the hospital can be best utilized near the end of life.
When interpreting the influence of specific types of LT-CCCs on hospital use and cost, variations in illness trajectory and treatment approach are important to consider.28 For example, children with neuromuscular LT-CCCs may use the hospital less in the last year of life due to an initially stable degree of neurologic impairment (such as from nonprogressive cerebral palsy), before experiencing an unanticipated precipitous event (such as an aspiration pneumonia) that leads to death in the hospital. Many children with neuromuscular LT-CCCs also may have access to outpatient and community resources that facilitate highly technical care out of hospital.29,30 In contrast, children with hematologic/immunologic LT-CCCs may use the hospital more due to a fluctuating decline in health in the setting of recurrent infections or therapeutic interventions, such as stem cell transplantation.31 For many of these children, there may be limited outpatient alternatives to hospital care. The impact of contrasting trajectories on hospital care for children with different LT-CCCs necessitates further inquiry.
There are several possible explanations for the association between multiple LT-CCCs and greater hospital use.9,11,32 For some children, multiple LT-CCCs may indicate severe fragility in physiologic functioning across several organ systems. This fragility could increase vulnerability to recurrent acute-on-chronic health crises. Preventing these crises in the outpatient setting may be challenging, especially when LT-CCCs interact and intensify one another. Once in hospital, having multiple LT-CCCs may render care coordination difficult.21,23,33 For those children able to leave the hospital alive, transitions from hospital to home may be tenuous.34 Unplanned hospital readmissions are greatest among children with multiple chronic conditions.14,17,35 These composite factors potentially lengthen hospitalizations and heighten associated cost.
Importantly, hospital care in the last year of life may contribute to more procedures and greater receipt of highly acute services.36 In our study, rates of mechanical ventilation, surgery, and ICU use in the terminal admission were higher than previously reported.5,10,11 Although not determined in the current study, secular trends in health care for children with LT-CCCs may help explain this finding. First, mechanical ventilation may be used more frequently due to technologic advancements that reduce lung injury and adverse sequelae, enabling children with LT-CCCs to recover from life-threatening illness.37 Second, existing studies report increasing rates of surgeries and invasive procedures over time in children with LT-CCCs. For example, gastrostomy and tracheostomy have increased in children with neuromuscular LT-CCCs,38–40 and indications for stem cell transplantation have broadened to include children with immunodeficiencies and metabolic diseases.41–43 The effect of these treatments on the child and family experience at the end of life merits future exploration.
This study has several limitations. Administrative data in PHIS are not positioned to draw conclusions about care quality, appropriateness of hospital care, quality of life, or comfort at the end of life. The data cannot distinguish expected from unexpected deaths. We were unable to ascertain which children received palliative care consultation or had advance directives, such as do-not-attempt-resuscitation orders. Previous studies used the palliative care ICD-9-CM code (v66.9) with administrative data to identify patients receiving palliative care.25 In PHIS, this code was not sufficiently sensitive (21%) to identify children who received palliative care consultation at one of our hospitals. Therefore, we elected not to use it in the current study. Alternate methods, including chart review, may be necessary to identify whether hospital use in children with LT-CCCs varies by receipt of palliative care.
We were unable to assign a primary diagnosis for children with multiple LT-CCCs because the CCC classification system does not contain this capability. Furthermore, the time frame of 1 year to assess hospital use is arbitrary; subsequent analyses with an extended time frame (eg, beyond 1 year or from the inception of the LT-CCC) may help contextualize the findings. PHIS does not contain information on outpatient or community care, including hospice. It also does not contain data from nonchildren’s hospitals. Therefore, the generalizability of the current study is limited to children with LT-CCCs who died in a children’s hospital.
Despite these limitations, our study is among the first in the past decade to report the magnitude of hospital utilization and costs, by condition, for a multicenter cohort of children with LT-CCCs nearing the end of life. Depending on the type and number of LT-CCCs endured by a child, anticipatory consideration of the time they could spend in the hospital might aid in advance care planning. Care delivery models that integrate primary, specialty, and surgical providers, including the medical and perioperative surgical home, might be important to explore for some children with LT-CCCs.44,45 With a better understanding of the amount of hospital use experienced by children in the current study, clinicians may be better informed to discuss possibilities of future hospital use in the context of prognosis and to ensure that hospital interventions are in accord with child and family wishes.
Acknowledgments
The authors thank Margaret O’Neill, BA, for her significant contributions to the manuscript revision.
Glossary
- CTC
clinical transaction classification
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- IQR
interquartile range
- LT-CCCs
life-threatening complex chronic conditions
- PHIS
Pediatric Health Information System database
Footnotes
Dr Ananth designed and executed the study, drafted and revised the manuscript, and approved the final manuscript as submitted; Ms Melvin carried out the analyses and reviewed the manuscript; Dr Feudtner critically reviewed the draft manuscript and approved the final manuscript as submitted; and Drs Wolfe and Berry conceptualized and designed the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Ananth was supported by the David A. Abraham Fellowship at Dana-Farber Cancer Institute. Dr Berry was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development (K23HD058092) and the Agency for Healthcare Research and Quality (R21HS23092). Dr Feudtner was supported by the Agency for Healthcare Research and Quality (RO11HS018425). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Koroukian SM, Beaird H, Madigan E, Diaz M. End-of-life expenditures by Ohio Medicaid beneficiaries dying of cancer. Health Care Financ Rev. 2006;28(2):65–80 [PMC free article] [PubMed] [Google Scholar]
- 2.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154(4):235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Robert Wood Johnson Foundation ICU End-Of-Life Peer Group . Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–643 [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209 [PubMed] [Google Scholar]
- 8.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/E99 [DOI] [PubMed] [Google Scholar]
- 9.Feudtner C, Silveira MJ, Christakis DA. Where do children with complex chronic conditions die? Patterns in Washington State, 1980–1998. Pediatrics. 2002;109(4):656–660 [DOI] [PubMed] [Google Scholar]
- 10.Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD. Characteristics of deaths occurring in children’s hospitals: implications for supportive care services. Pediatrics. 2002;109(5):887–893 [DOI] [PubMed] [Google Scholar]
- 11.Feudtner C, DiGiuseppe DL, Neff JM. Hospital care for children and young adults in the last year of life: a population-based study. BMC Med. 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feudtner C, Hexem KR, Shabbout M, Feinstein JA, Sochalski J, Silber JH. Prediction of pediatric death in the year after hospitalization: a population-level retrospective cohort study. J Palliat Med. 2009;12(2):160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandon D, Docherty SL, Thorpe J. Infant and child deaths in acute care settings: implications for palliative care. J Palliat Med. 2007;10(4):910–918 [DOI] [PubMed] [Google Scholar]
- 14.Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buescher PA, Whitmire JT, Brunssen S, Kluttz-Hile CE. Children who are medically fragile in North Carolina: using Medicaid data to estimate prevalence and medical care costs in 2004. Matern Child Health J. 2006;10(5):461–466 [DOI] [PubMed] [Google Scholar]
- 19.Neff JM, Sharp VL, Muldoon J, Graham J, Myers K. Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646 [DOI] [PubMed] [Google Scholar]
- 21.Russell CJ, Simon TD. Care of children with medical complexity in the hospital setting. Pediatr Ann. 2014;43(7):e157–e162 [DOI] [PubMed] [Google Scholar]
- 22.Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159(2):284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo DZ, Goudie A, Cohen E, et al. Inequities in health care needs for children with medical complexity. Health Aff (Millwood). 2014;33(12):2190–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keele L, Keenan HT, Sheetz J, Bratton SL. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013;132(1):72–78 [DOI] [PubMed] [Google Scholar]
- 26.Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava R, Berry JG, Hall M, et al. Reflux related hospital admissions after fundoplication in children with neurological impairment: retrospective cohort study. BMJ. 2009;339:b4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feudtner C. Collaborative communication in pediatric palliative care: a foundation for problem-solving and decision-making. Pediatr Clin North Am. 2007;54(5):583–607, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham RJ, Fleegler EW, Robinson WM. Chronic ventilator need in the community: a 2005 pediatric census of Massachusetts. Pediatrics. 2007;119(6). Available at: www.pediatrics.org/cgi/content/full/119/6/e1280 [DOI] [PubMed] [Google Scholar]
- 30.Mah JK, Thannhauser JE, McNeil DA, Dewey D. Being the lifeline: the parent experience of caring for a child with neuromuscular disease on home mechanical ventilation. Neuromuscul Disord. 2008;18(12):983–988 [DOI] [PubMed] [Google Scholar]
- 31.Al-Ghonaium A. Stem cell transplantation for primary immunodeficiencies: King Faisal Specialist Hospital experience from 1993 to 2006. Bone Marrow Transplant. 2008;42(suppl 1):S53–S56 [DOI] [PubMed] [Google Scholar]
- 32.Lindley LC, Lyon ME. A profile of children with complex chronic conditions at end of life among Medicaid beneficiaries: implications for health care reform. J Palliat Med. 2013;16(11):1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toomey SL, Chien AT, Elliott MN, Ratner J, Schuster MA. Disparities in unmet need for care coordination: the national survey of children’s health. Pediatrics. 2013;131(2):217–224 [DOI] [PubMed] [Google Scholar]
- 34. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581. [DOI] [PMC free article] [PubMed]
- 35.Jurgens V, Spaeder MC, Pavuluri P, Waldman Z. Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158 [DOI] [PubMed] [Google Scholar]
- 36.McCallum DE, Byrne P, Bruera E. How children die in hospital. J Pain Symptom Manage. 2000;20(6):417–423 [DOI] [PubMed] [Google Scholar]
- 37.Rose L, Schultz MJ, Cardwell CR, Jouvet P, McAuley DF, Blackwood B. Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children. Cochrane Database Syst Rev. 2014;(6):CD009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox D, Campagna EJ, Friedlander J, Partrick DA, Rees DI, Kempe A. National trends and outcomes of pediatric gastrostomy tube placement. J Pediatr Gastroenterol Nutr. 2014;59(5):582–588 [DOI] [PubMed] [Google Scholar]
- 39.Overman AE, Liu M, Kurachek SC, et al. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1491 [DOI] [PubMed] [Google Scholar]
- 40. Dursun O, Ozel D. Early and long-term outcome after tracheostomy in children. Pediatr Int. 2011;53(2):202–206. [DOI] [PubMed]
- 41.Worth AJ, Booth C, Veys P. Stem cell transplantation for primary immune deficiency. Curr Opin Hematol. 2013;20(6):501–508 [DOI] [PubMed] [Google Scholar]
- 42.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148(3):356–372 [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Liu H. Is perioperative home the future of surgical patient care? J Biomed Res. 2015;29(3):173–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari LR, Antonelli RC, Bader A. Beyond the preoperative clinic: considerations for pediatric care redesign aligning the patient/family-centered medical home and the perioperative surgical home. Anesth Analg. 2015;120(5):1167–1170 [DOI] [PubMed] [Google Scholar]